Abstract
Neutrophils respond rapidly to cerebral ischemia and are thought to contribute to inflammation-mediated injury during stroke. Using myeloid Mcl1 knockout mice as a model of genetic neutrophil deficiency, we investigated the contribution of neutrophils to stroke pathophysiology. Myeloid Mcl1 knockout mice were subjected to transient middle cerebral artery occlusion and infarct size was assessed by MRI after 24 hours reperfusion. Immune cell mobilization and infiltration was assessed by flow cytometry. We found that myeloid Mcl1 knockout mice had significantly reduced infarct size when compared to heterozygous and wild type control mice (MyMcl1+/+: 78.0 mm3; MyMcl1+/−: 83.4 mm3; MyMcl1−/−: 55.1 mm3). This was accompanied by a nearly complete absence of neutrophils in the ischemic hemisphere of myeloid Mcl1 knockout mice. Although myeloid Mcl1 knockout mice were protected from cerebral infarction, no significant differences in neurological deficit or the mRNA expression of inflammatory genes (TNFα, IL-1β, and MCP1) were detected. Inhibition of neutrophil chemotaxis using CXCR2 pepducin treatment partially reduced neutrophil mobilization and recruitment to the brain after stroke, but did not reduce infarct size 24 hours after transient MCA occlusion. These data confirm that neutrophils have an important role in infarct development during stroke pathophysiology, and suggest that complete deficiency, but not partial inhibition, is necessary to prevent neutrophil-mediated injury during stroke.
Keywords: Neutrophil, cerebral ischemia, stroke, inflammation
INTRODUCTION
Immune cells are critical components of stroke pathophysiology where they respond rapidly to cerebral ischemia and have profound effects on stroke outcome. At the onset of ischemia, both protective and harmful inflammatory signaling molecules are released, and resident and blood-borne immune cells are rapidly mobilized and recruited to the site of injury. Innate immune cells comprised of microglia, neutrophils, and monocytes/macrophages are recruited first, followed by an adaptive immune response comprised of lymphocytes and antigen presenting cells. Targeting the inflammatory response has the potential to be an important therapeutic strategy; however, there has been poor clinical translation of successful preclinical therapies likely due to our incomplete understanding of the critical immune cell phenotypes and their complex responses to cerebral ischemia.
Neutrophils rapidly infiltrate the ischemic brain and are thought to have a proinflammatory, detrimental effect during stroke pathogenesis (Jickling et al., 2015). Intravital microscopy imaging has shown that neutrophils can infiltrate the brain within minutes after injury, and although neutrophil numbers are greatest 24–48 hours after stroke, it is likely that they affect stroke pathophysiology much earlier (Roth et al., 2014). Studies have attempted to identify the contribution of neutrophils and other circulating cells during stroke by targeting cellular adhesion molecules and integrins. These studies have shown that inhibition of immune cell recruitment or inhibition of proinflammatory mediators can significantly reduce infarct size and protect against stroke (Iadecola and Anrather, 2011). Inhibition of immune cell recruitment by genetic ablation or antibody neutralization of the adhesion molecules ICAM-1, P-Selectin, and E-Selectin is protective in animal models of ischemic stroke (Bowes et al., 1995; Connolly et al., 1997; Connolly et al., 1996; Goussev et al., 1998; Huang et al., 2000; Suzuki et al., 1999; Zhang et al., 1995; Zhang et al., 1994). Similarly, genetic deficiency in the leukocyte-specific integrin CD18 and Mac-1 (CD11b/CD18), which binds to ICAM-1, also decrease infarct volume during stroke (Prestigiacomo et al., 1999; Soriano et al., 1999).
Blocking leukocyte chemotaxis through inhibition of inflammatory chemokines like Mcp1 and Cx3cl1 and their cognate receptors CCR2 and CX3CR1 has also been shown to be protective during stroke (Denes et al., 2008; Dimitrijevic et al., 2007; Hughes et al., 2002; Kumai et al., 2004; Soriano et al., 2002). In many of these studies, neuroprotection correlates with reduced neutrophil infiltration, but these strategies for immune cell inhibition are non-specific and can also inhibit the recruitment of other circulating leukocytes. Although anti-neutrophil serum can be protective during models of stroke (Connolly et al., 1996; Cuartero et al., 2013; Murikinati et al., 2010), many of these antibodies can result in cross-reactivity and non-specificity leading to question whether the effect is the result of multiple cell types. There is still much controversy regarding neutrophils and whether they have a pathogenic (or possibly beneficial) role during stroke and whether their presence is merely correlative with injury (reviewed in (Emerich et al., 2002)). More recently, genetic models of immune cell deficiency have been useful in delineating the roles of myeloid cells, T cells and B cells during stroke (Gliem et al., 2012; Kleinschnitz et al., 2010; Ren et al., 2011; Shichita et al., 2009; Yilmaz et al., 2006).
Mcl1 is a member of the antiapoptotic Bcl-2 family, which regulates apoptosis in immune cells and it is essential for neutrophil survival (Kozopas et al., 1993; Marsden and Strasser, 2003). It has been previously reported that genetic ablation of Mcl1 in myeloid cells results in neutrophil deficiency while not significantly affecting macrophage or other myeloid cell survival (Dzhagalov et al., 2007; Steimer et al., 2009). In the current study, we investigated the effect that genetic neutrophil deficiency had during cerebral ischemia. We hypothesized that mice lacking neutrophils would have decreased inflammation and would be protected in a model of ischemic stroke.
METHODS
Animals and Treatments
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition) and were approved by the Institutional Animal Care and Use Committee of the University of Michigan. All mice were maintained on standard laboratory chow (5L0D, LabDiet) and water ad libitum. Myeloid Mcl1 knockout mice were on a C57BL/6J background and were generated by crossing floxed Mcl1 mice with LysMCre (Mcl1fl/fl-LysMCre, MyMcl1−/−). Heterozygous myeloid Mcl1 knockout mice (Mcl1fl/wt-LysMCre, MyMcl1+/−) and wild type mice (MyMcl1+/+) were used as controls.
For CXCR2-pepducin experiments, wild type C57BL6/J male mice 8–10 wk old and weighing 22–25 g were were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice received a previously determined dose (2.5 mg/kg) of CXCR2-pepducin (RTLFKAHMGQKHR, palmitoyl N-terminal, amidation C-terminal) or control peptide (TRFLAKMHQGHKR, palmitoyl N-terminal, amidation C-terminal) in sterile saline 3 h before MCAo via intravenous injection (Jamieson et al., 2012; Kaneider et al., 2005). Mice received an additional subcutaneous injection of pepducin 9 h after stroke.
Middle cerebral artery occlusion
Mice were anesthetized with isoflurane (5% induction, 1.5% maintenance) and core body temperature was maintained at 37°C using a temperature controller with heating pad and rectal probe. An 8 mm midline incision was made in the neck and the submaxillary glands were dissected to expose the right common, external and internal carotid arteries. The external carotid artery was ligated and divided, and a 10 mm long 6–0 silicon rubber–coated nylon monofilament (#602156, Doccol Corporation, CA) was inserted into the external carotid stump and advanced into the internal carotid artery. Regional cerebral blood flow was monitored by laser Doppler flowmetry (Transonic BLF22) before and during monofilament insertion to verify MCA occlusion. Occlusion was defined as a reduction in cerebral tissue perfusion to a level <20% of baseline. After 90 minutes, the filament was removed and animals were allowed to recover.
Measurement of Infarct Volume
Infarcts were assessed by magnetic resonance imaging (MRI) after 24 hours reperfusion. Mice were anesthetized with 2% isoflurane/air mixture throughout MRI examination. Mice lay prone, head first in a 7.0T Varian Unity Inova MR scanner (183-mm horizontal bore; Varian, Palo Alto, CA), with the body temperature maintained at 37°C by forced heated air. A double-tuned volume radiofrequency coil was used to scan the head region of the mice. Axial T2-weighted images were acquired through the use of a fast spin-echo sequence with the following parameters: repetition time/effective echo time, 4000/60 ms; echo spacing, 15 ms; number of echoes, 8; field of view, 20×20 mm; matrix, 256×128; slice thickness, 0.5 mm; number of slices, 25; and number of scans, 1 (total scan time = 2.5 minutes). The infarct volumes were analyzed using NIH ImageJ software (version 1.43) by a blinded observer, and infarct volumes were corrected to account for brain swelling.
The following equation was used to calculate the corrected T2-lesion volumes: TV – ((CV + (IV – LV) x ((TV/2)/CV)); TV indicates total volume in both hemispheres; CV, contralateral volume; IV, ipsilateral volume; and LV, lesion volume.
Evaluation of Neurological Deficit
Neurological deficits were determined 24 hours after MCA occlusion. Neurological scores were assigned using the following criteria: 0, no deficit; 1, forelimb flexion and torso turning to the contralateral side when held by tail; 2, circling to contralateral side; 3, unable to bear weight on contralateral side; and 4, no spontaneous locomotor activity.
Flow Cytometry
Cerebral hemispheres were dissected and minced into small pieces with a razor blade in RMPI containing 5% FBS, and the homogenate was passed through a 40 μm cell strainer. Leukocytes were purified by density gradient centrifugation using a 30% percoll solution and washed twice with PBS. Peripheral whole blood was collected, RBCs were lysed by 10 s hypotonic shock using distilled water, and remaining leukocytes were washed with PBS. Cells were incubated in Fc Block for 10 min and then stained on ice for 1 hr with the following antibodies: PE anti-mouse CD45 (BioLegend, cat # 103106), APC anti-mouse CD11b (BioLegend, cat # 101211), FITC anti-mouse Ly6G (BioLegend, cat # 127606), Pacific Blue anti-mouse Ly6C (BioLegend, cat # 128014). Stained cells were washed twice in PBS and fixed in 0.1% paraformaldehyde before analysis. Flow cytometric analysis was performed on a FACSCanto II Flow Cytometer (BD Biosciences) equipped with three lasers (405-nm violet laser, 488-nm blue laser, and 640-nm red laser) and analyzed with FlowJo software (Treestar). Unstained and isotype controls were used for flow analysis.
mRNA Expression Analysis
Relative mRNA expression was determined using quantitative reverse transcription–polymerase chain reaction (qPCR). Total RNA was isolated from whole cerebral hemispheres using TRIzol reagent and purified using an RNeasy Mini Kit (Qiagen) with an on-column DNase digestion. RNA (1 ug) was reverse transcribed to cDNA with an Applied Biosystems kit and qPCR was performed using a 7900HT fast real-time PCR system (Applied Biosystems). The relative mRNA expression was quantified using the comparative method and normalized to the ribosomal housekeeping gene L32.
Statistics
A Shapiro-Wilk normality test was used to determine if data were normally distributed. For normally distributed data with equal variance, statistical comparison of mean values between groups was performed with the Student t test or by a two-way ANOVA with a Bonferroni post-test, and values are presented as mean ± SEM. All statistical analysis of data was performed in GraphPad Prism (version 6; GraphPad Software, Inc). P < 0.05 was considered significant.
RESULTS
Myeloid Mcl1 knockout mice are protected during cerebral ischemia-reperfusion
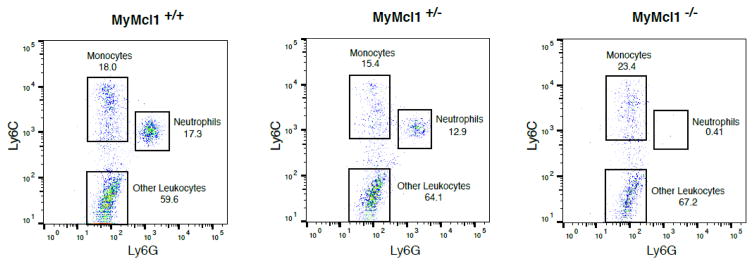
In order to define the contribution of neutrophils during stroke, we used myeloid Mcl1 knockout mice (MyMcl1−/−) that have a neutrophil deficiency. Consistent with previous reports (2001; Dzhagalov et al., 2007; Steimer et al., 2009), homozygous MyMcl1−/− mice had a nearly complete loss of neutrophils from blood when compared to controls where as heterozygous MyMcl1+/− mice were not dramatically affected (Figure 1). In contrast, circulating monocytes were comparatively normal in MyMcl1−/− mice.
Figure 1. Neutrophil deficiency in myeloid Mcl1 knockout mice.
Flow cytometric analysis of blood monocytes and neutrophils from wild type (MyMcl1+/+), heterozygous (MyMcl1+/−), and knockout (MyMcl1−/−) mice. CD45hi leukocytes were gated on monocyte and neutrophil-specific markers, Ly6C and Ly6G, as shown.
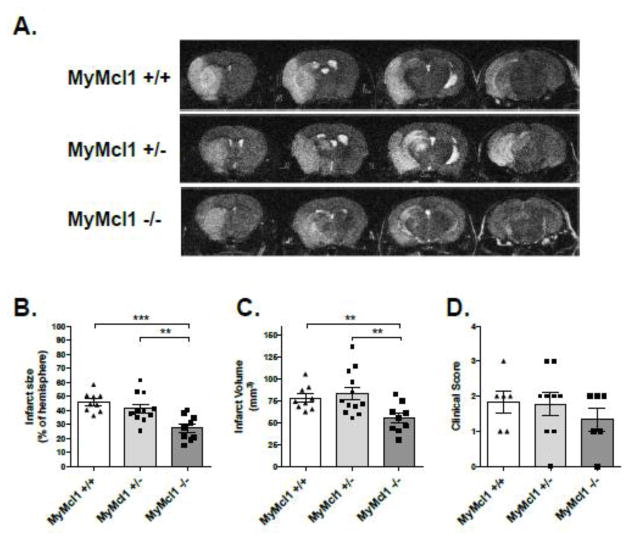
To test whether neutrophil deficiency affected stroke pathophysiology, MyMcl1−/− and control mice were subjected to a 90 minute transient middle cerebral artery occlusion (tMCAo) followed by 24 hours of reperfusion. After 24 hours reperfusion, infarcts assessed by T2-weighted MRI were significantly decreased in the MyMcl1−/− group when compared to both wild type (MyMcl1+/+) and heterozygous (MyMcl1+/−) controls (Figure 2A). Quantification of infarct sizes using ImageJ software revealed a significant decrease in infarct percent in the ipsilateral hemisphere (Figure 2B) with a 29% reduction in the infarct volume (Figure 2C) (MyMcl1+/+: 78.0 mm3; MyMcl1+/−: 83.4 mm3; MyMcl1−/−: 55.1 mm3). No significant difference in infarct size was detected between wild type and heterozygous mice. Neurological deficit was assessed 24 hours after stroke, but there were no significant differences in in MyMcl1−/− mice when compared to controls (Figure 2D).
Figure 2. Myeloid Mcl1 knockout with neutrophil deficiency decreases infarct size during stroke.
(A) Representative MRI sections from MyMcl1+/+, MyMcl1+/−, MyMcl1−/− mice 24 h after transient (90-minute) MCA occlusion. Infarct sizes represented as (B) fraction in ipsilateral hemisphere and (C) total infarct volume. (D) Functional impairment was assessed by scoring neurological deficit in mice after 24 h. Data are expressed as mean ± SEM. n=6–12 per group.
Myeloid Mcl1 knockout prevents neutrophil mobilization and recruitment after stroke without altering inflammatory mRNA gene expression
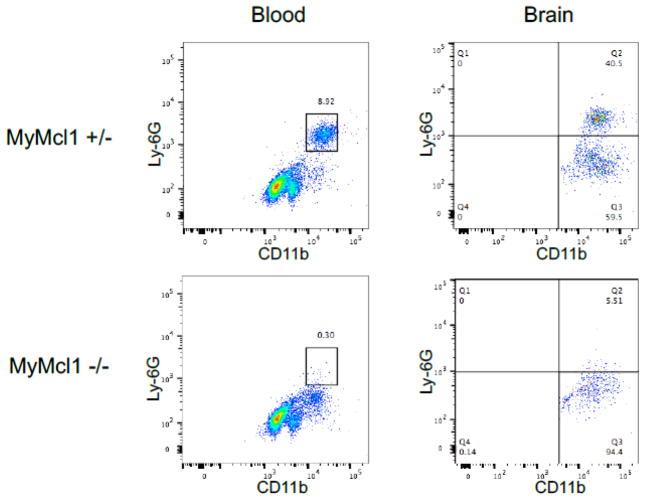
We next determined whether myeloid Mcl1 knockout impaired neutrophil survival, mobilization, and recruitment during ischemic injury. Similar to naïve, uninjured mice, MyMcl1−/− mice had severe neutrophil deficiency in the blood 24 hours after stroke (Figure 3). In addition, MyMcl1−/− mice also showed a dramatic reduction in the percentage of neutrophils in the ipsilateral hemisphere when compared to MyMcl1+/− controls (Figure 3) (MyMcl1+/+: 39.1 ± 8.8, MyMcl1+/−: 43.5 ± 7.6, MyMcl1−/−: 4.9 ± 0.8).
Figure 3. Myeloid Mcl1 deficient mice have impaired neutrophil mobilization and decreased brain infiltration during stroke.
Flow cytometric analysis of neutrophils in blood and brain 24 hr after transient (90-minute) MCA occlusion. CD45hi leukocytes were gated on the myeloid and neutrophil-specific markers, CD11b and Ly6G, as shown.
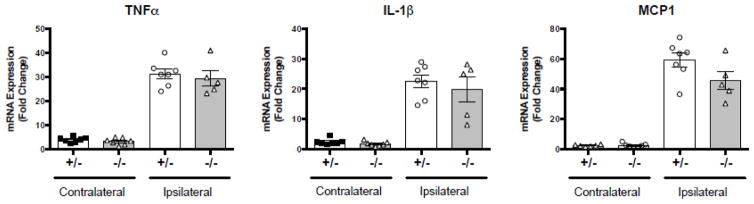
Since neutrophils are major components of the inflammatory response and can contribute to ischemic injury through secretion of inflammatory cytokines, we assessed whether MyMcl1−/− mice had altered inflammatory signaling in the brain. The mRNA expression of proinflammatory cytokines 24 hours after transient MCA occlusion revealed a strong induction of proinflammatory genes in the ischemic, ipsilateral hemisphere when compared to the contralateral hemisphere (Figure 4). Surprisingly, despite the reduced infarct size in MyMcl1−/−, we did not detect any significant differences in the expression of the inflammatory genes TNFα, IL1β and MCP1 in the ischemic hemisphere when compared to controls (Figure 4). This is indicates that inflammatory mRNA gene expression did not correlate with neutrophil infiltration or infarct size, and it suggests that neutrophils affect stroke pathophysiology and infarct development through a different mechanism.
Figure 4. mRNA expression of inflammatory markers in myeloid Mcl1 knockout mice 24 h after stroke.
mRNA expression of proinflammatory genes was measured by qPCR in heterozygous (+/−) and homozygous (−/−) myeloid Mcl1 knockout mice. All genes were normalized to L32. Data are expressed as mean ± SEM. n=5–7 per group.
Inhibition of neutrophil chemotaxis with CXCR2 pepducin does not protect against stroke
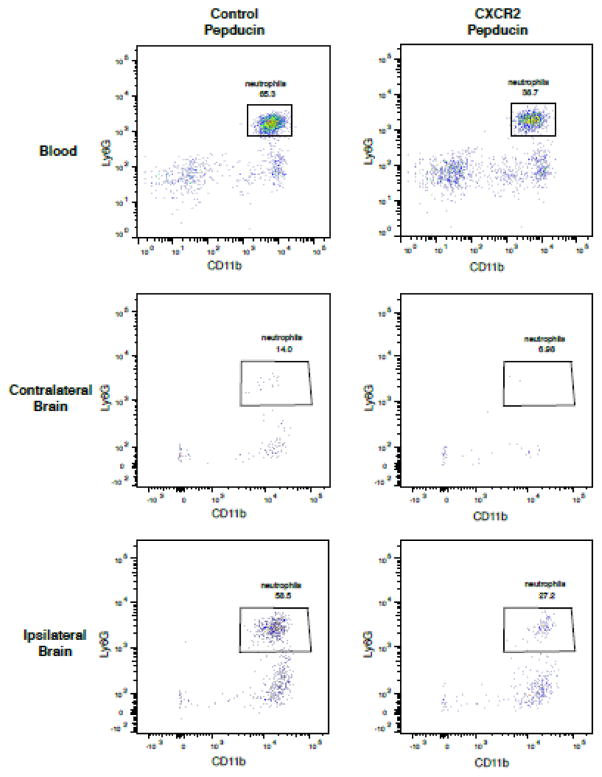
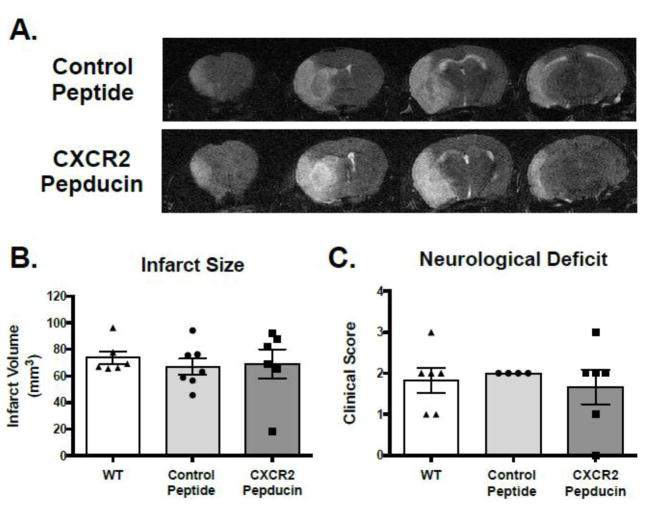
We next sought to determine if a novel CXCR2 pepducin could prevent neutrophil chemotaxis and prevent neutrophil-mediated stroke damage in mice. Pepducins are cell-penetrating lipopeptides that can be designed to specifically target and inhibit receptor signaling. CXCR2 is highly expressed by neutrophils, and CXCR2 pepducin selectively inhibits neutrophil recruitment in other inflammatory disease models (Jamieson et al., 2012; Kaneider et al., 2005). Mice were injected intravenously with CXCR2 pepducin 3 hours before tMCAo and then again 12 hours later and assessed neutrophil mobilization and recruitment by flow cytometry. Compared to nontargeting control peptide treatment, CXCR2 pepducin decreased the number of circulating neutrophils after tMCAo (Figure 5). Similarly, flow cytometric analysis of brain also revealed a reduction in the number of Ly6G+ neutrophils in the ipsilateral hemisphere after stroke. To determine if this decrease in neutrophils affected stroke infarct development, we assessed infarct size by MRI after 24 hours reperfusion. In contrast to genetic neutrophil deficiency, CXCR2 pepducin did not reduce infarct size after tMCAo. CXCR2 pepducin treated mice did not show any significant reductions in infarct size or neurological function when compared to control peptide treated mice (Figure 6).
Figure 5. CXCR2 pepducin partially decreased neutrophil mobilization and recruitment to the ischemic brain.
Flow cytometric analysis of blood and brain neutrophils in control peptide and CXCR2 pepducin treated mice 24 h after transient (90-minute) MCA occlusion. CD45hi leukocytes were gated on the myeloid and neutrophil-specific markers, CD11b and Ly6G, as shown.
Figure 6. Effect of CXCR2 pepducin treatment on infarct size and neurological deficit after stroke.
Wild type mice on a C57BL/6J background received intravenous injection of CXCR2 pepducin (2.5 mg/kg) 3 hours before and 9 hours after stroke. (A) Representative MRI sections from untreated, control peptide and CXCR2 pepducin treated mice 24 h after transient (90-minute) MCA occlusion. (B) Infarct volume in the ipsilateral hemisphere and (C) neurological deficit in mice 24 hr after stroke. Data are expressed as mean ± SEM. n=6–7 per group.
DISCUSSION
Neutrophils have long been thought to be major contributors to inflammation-mediated injury during stroke, and many studies have used antibodies to neutralize neutrophils in order to define their role during stroke (Connolly et al., 1996; Cuartero et al., 2013; Murikinati et al., 2010). In the present study, we used a highly selective genetic model with neutrophil deficiency to delineate the contribution that neutrophils have in cerebral infarct formation and inflammation-mediated injury. Here we have shown that neutrophil deficiency in myeloid Mcl1 knockout mice reduces infarct size after cerebral ischemia-reperfusion injury. We found that myeloid Mcl1 knockout mice were nearly completely devoid of neutrophils in the ischemic hemisphere as well as in the circulation.
Interestingly, we did not detect any differences in the mRNA expression of proinflammatory cytokines TNFα, IL1β and MCP1 indicating that stroke protection did not correlate with inflammatory mRNA gene expression. This is suggestive of an alternate mechanism by which neutrophils contribute to injury and affect infarct formation during stroke. Neutrophils are major producers of reactive oxygen species, and oxidative damage is thought to be a major mechanism of injury by immune cells during ischemia-reperfusion injury. Therefore, it is possible that neutrophil-derived reactive oxygen species are the major pathological mechanism by which neutrophils contribute to stroke damage. In support of this, one study using antibody depletion of neutrophils found protection in a transient, but not permanent occlusive stroke model (Prestigiacomo et al., 1999). Since reactive oxygen species are a thought to be a critical mechanism of reperfusion injury, this would support this hypothesis.
Consistent with the literature, we did not detect any differences in the survival of other immune cell populations in MyMcl1−/− mice. It is possible that while not affecting apoptosis or survival in these other myeloid cell populations, Mcl1 may have significant effects on cell phenotype during cerebral injury. Although Mcl1 knockout does not affect macrophage response to TLR-induced cytokine production (Dzhagalov et al., 2007), it has been shown to be important in macrophage regulation during bacterial clearance (Marriott et al., 2005). Therefore, we cannot rule out the possibility that other myeloid cell populations may be involved. The Mrp8-Cre or Ly6G-Cre are more neutrophil-specific and might provide further support for a specific role for neutrophils (Abram et al., 2014).
Despite a wide body of neutrophil research in stroke over the past several decades, the role of neutrophils in stroke pathogenesis remains controversial. Although numerous studies have found that neutrophil depletion strategies are protective in experimental stroke, there are conflicting results among different studies making it difficult to draw conclusions about the role of neutrophils. For example, there have been several studies that did not detect the presence of neutrophils in the infarct region prior to infarct formation indicating that neutrophil presence may be correlative with damage (Hayward et al., 1996; Lehrmann et al., 1997) and many other studies that have shown beneficial effects failed to assess temporal changes in both infarct and neutrophil infiltration (Emerich et al., 2002). It is also concerning that no studies have shown any type of dose effect with neutrophil depletion methods. Comparison of studies that demonstrate an effect or lack of effect is further complicated by differences in stroke models (permanent v. transient ischemia; intraluminal filament v. ligation v. thrombus) and animal model. In our experiments, we confirmed the absence of neutrophils before and after stroke suggesting a pathological role for neutrophils in our cerebral ischemia-reperfusion stroke model. Although Mcl1 deficiency does not affect the development of other myeloid cell types, it is possible that it might have other unknown roles that could be responsible for the neuroprotection observed in MyMcl1−/− mice.
More recently, the existence of multiple neutrophil phenotypes similar to the spectrum of macrophage activation states has been reported. Although data on these neutrophil phenotypes and their functional roles are unknown, it has been shown that neutrophils can also express the alternatively activated macrophage markers CD206 and Ym1 (Certo et al., 2015; Cuartero et al., 2013). It has been proposed that these CD206 and Ym1-expressing neutrophils may have beneficial effects on resolution of inflammation similar to alternatively activated macrophages. Our data suggest that neutrophils have detrimental effects during early infarct formation since neutrophil deficiency significantly decreased infarct size 24 hours after ischemia-reperfusion injury. However, we did not determine the effect of neutrophil deficiency on infarct size and functional deficit at later time points, and so it is possible that neutrophils contribute significantly to the oxidative damage during early stages of ischemia-reperfusion injury, but may also have important roles during later stages of healing.
In this study, we were unable to induce neuroprotection with intravenous injection of CXCR2 pepducin at a dose similar to what has been reported in the literature to significantly prevent neutrophil mobilization and chemotaxis. Although we did reduce neutrophil mobilization and recruitment to the brain, we only saw an approximate 50% reduction in the total number of neutrophils perhaps suggesting that small numbers of neutrophils can have full effects.
Neutrophils can respond to a various cytokines and chemokines, and there is a wide array of chemotactic signaling molecules released during stroke. Although previous studies have shown CXCR2 pepducin can effectively suppress neutrophil chemotaxis, the milieu of chemotactic molecules during stroke is likely to be dramatically different and may be more profound. A previous report using the CXCR2 antagonist SB225002 found that pharmacological inhibition was only protective in hyperlipidemic mice (Herz et al., 2015). Our data suggest that a greater decrease in neutrophil infiltration is necessary to prevent the detrimental effects of neutrophils, and complete inhibition of neutrophil chemotaxis may not be achievable through inhibition of a single chemokine signaling pathway in our model.
CONCLUSIONS
In this study, we have shown using a highly selective genetic model of neutrophil deficiency, that neutrophils have an important role in infarct development during stroke pathophysiology. We found that neutrophil deficiency decreased infarct size during cerebral ischemia-reperfusion injury independent of inflammatory mRNA gene expression. We further demonstrated that incomplete inhibition of neutrophil chemotaxis with CXCR2 pepducin is not sufficient to reduce the detrimental effects of neutrophils during stroke, indicating that even small neutrophil populations can have substantial effects.
HIGHLIGHTS.
Myeloid Mcl1 knockout mice have a nearly complete loss of neutrophils in naïve mice and lack neutrophil mobilization and recruitment during stroke
Genetic neutrophil deficiency in myeloid Mcl1 knockout mice decreased infarct size during stroke
Genetic neutrophil deficiency did not suppress inflammatory gene mRNA expression during stroke
Inhibition of neutrophil chemotaxis with CXCR2 pepducin was not sufficient to reduce infarct size during stroke
Acknowledgments
FUNDING
These studies were supported in part by grants R01-HL112610 and T32-HL007853 from the National Institutes of Health.
LIST OF ABBREVIATIONS
- MCA
middle cerebral artery
- tMCAo
transient MCA occlusion
- MRI
magnetic resonance imaging
- Mcl1
myeloid cell leukemia sequence 1
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
RAF, RMM, YMS designed the experiments; RAF performed the stroke surgeries; YC performed the flow cytometry; RAF, GG, TV, JS, CGA, YC analyzed the data; RAF wrote the manuscript; All authors discussed the results and provided comments on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 2.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- 4.Certo M, Endo Y, Ohta K, Sakurada S, Bagetta G, Amantea D. Activation of RXR/PPARgamma underlies neuroprotection by bexarotene in ischemic stroke. Pharmacol Res. 2015;102:298–307. doi: 10.1016/j.phrs.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circulation research. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- 6.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbi AL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- 8.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke; a journal of cerebral circulation. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 10.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerich DF, Dean RL, 3rd, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct? Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- 12.Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, Jander S. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Annals of neurology. 2012;71:743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- 13.Goussev AV, Zhang Z, Anderson DC, Chopp M. P-selectin antibody reduces hemorrhage and infarct volume resulting from MCA occlusion in the rat. J Neurol Sci. 1998;161:16–22. doi: 10.1016/s0022-510x(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 14.Hayward NJ, Elliott PJ, Sawyer SD, Bronson RT, Bartus RT. Lack of evidence for neutrophil participation during infarct formation following focal cerebral ischemia in the rat. Exp Neurol. 1996;139:188–202. doi: 10.1006/exnr.1996.0093. [DOI] [PubMed] [Google Scholar]
- 15.Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of Neutrophils in Exacerbation of Brain Injury After Focal Cerebral Ischemia in Hyperlipidemic Mice. Stroke. 2015;46:2916–2925. doi: 10.1161/STROKEAHA.115.010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES., Jr Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke; a journal of cerebral circulation. 2000;31:3047–3053. [PubMed] [Google Scholar]
- 17.Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 22.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 23.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumai Y, Ooboshi H, Takada J, Kamouchi M, Kitazono T, Egashira K, Ibayashi S, Iida M. Anti-monocyte chemoattractant protein-1 gene therapy protects against focal brain ischemia in hypertensive rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1359–1368. doi: 10.1097/01.WCB.0000143534.76388.3C. [DOI] [PubMed] [Google Scholar]
- 25.Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J Comp Neurol. 1997;386:461–476. [PubMed] [Google Scholar]
- 26.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 28.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 29.Prestigiacomo CJ, Kim SC, Connolly ES, Jr, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke; a journal of cerebral circulation. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 30.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metabolic brain disease. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 33.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. Journal of neuroimmunology. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 34.Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 35.Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Hayashi T, Tojo SJ, Kitagawa H, Kimura K, Mizugaki M, Itoyama Y, Abe K. Anti-P-selectin antibody attenuates rat brain ischemic injury. Neuroscience letters. 1999;265:163–166. doi: 10.1016/s0304-3940(99)00229-3. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 38.Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke; a journal of cerebral circulation. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]
- 39.Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, Miyasaka M, Ward PA. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]