Abstract
Purpose
Cognitive dysfunction is a major concern for children with brain tumors. A valid, user-friendly screening tool could facilitate prompt referral for comprehensive neuropsychological assessments and therefore early intervention. Applications of the pediatric perceived cognitive function item bank (pedsPCF) such as computerized adaptive testing can potentially serve as such a tool given its brevity and user-friendly nature. This study aimed to evaluate whether pedsPCF was a valid indicator of cerebral compromise using the criterion of structural brain changes indicated by leukoencephalopathy grades.
Methods
Data from 99 children (mean age =12.6 years) with brain tumors and their parents were analyzed. Average time since diagnosis was 5.8 years; time since last treatment was 4.3 years. Leukoencephalopathy grade (range: 0–4) was based on white matter damage and degree of deep white matter volume loss shown on MRI. Parents of patients completed the pedsPCF. Scores were based on the US general population-based T-score metric (mean=50; standard deviation=10). Higher scores reflect better function.
Results
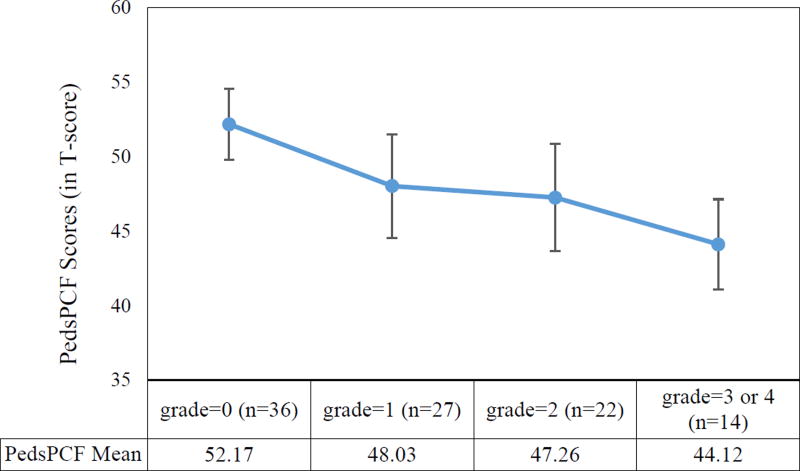
Leukoencephalopathy grade distributions were as follows: 36 grade 0; 27 grade 1; 22 grade 2; 13 grade 3, and 1 grade 4. The mean pedsPCF T-score was 48.3 (SD=8.3; range: 30.5 to 63.7). The pedsPCF scores significantly discriminated patients with different leukoencephalopathy grades, F=4.14, p=0.0084. Effect sizes ranged from 0.09 (grade 0 versus 1) to 1.22 (grade 0 versus 3 / 4).
Conclusions
This study demonstrates that the pedsPCF is a valid indicator of leukoencephalopathy and provides support for its use as a screening tool for more comprehensive neurocognitive testing.
Keywords: Brain Tumor, Pediatrics, Leukoencephalopathy, Perceived Cognitive Function, Patient-centered Outcome
INTRODUCTION
Brain tumor survivors are vulnerable to adverse cognitive outcomes[1; 2] and often demonstrate a myriad of cognitive symptoms such as memory loss, distractibility, and difficulty with multitasking,[3–5] all of which can contribute to poor health-related quality of life.[6–8] Clinicians’ awareness of these adverse effects can facilitate patient surveillance, enhancing early identification of cognitive dysfunction and intervention. However, timely intervention requires regular assessment to identify and monitor the late effects of cancer and its treatment.[9] Neuropsychological testing (NPT) is considered the gold standard for identifying neurocognitive deficits, by measuring how a patient performs on a series of standardized tasks within a controlled environment.[10] However, practical constraints (e.g., cost, time-consuming, labor intensive) make it difficult to integrate NPT into routine clinical care of cancer survivors.[11–13] To utilize NPT in a more efficient and effective manner, a user-friendly standardized tool that can facilitate timely referral by identifying children likely developing cognitive decrements is needed. Standardization allows for comparisons of scores over time in a psychometrically sound manner. A user-friendly instrument allows families to complete the tool routinely, particularly if it is brief and parents/patients can complete it outside of the clinic.
Research has suggested that perceived cognitive function (i.e., self- or proxy-reported cognition), PCF, can be related to structural imaging abnormalities,[14–19] though most previous studies have been limited to adults. For example, in a population-based study, de Groot et al[16] suggested that cognitive complaints (CC) might be an early warning sign related to progression of white matter lesions (WML) and imminent cognitive decline. In children, Mahone et al[14] found parent ratings of working memory in typically developing children to be correlated with frontal gray matter volumes. Several previous studies have considered parent-report questionnaires such as the Child Behavior Checklist (CBCL),[20] the Conners Parent Rating Scale (CPRS),[21] and the Behavior Rating Inventory of Executive Function (BRIEF)[22] as screening devices for surveillance of pediatric cancer survivors.[23] Yet these scales were developed using classical test theory (CTT), limiting their efficiency at discerning individual differences due to a fixed questionnaire format.
An ideal screening tool should be designed to sample neurocognitive deficits in pediatric cancer survivors, provide a brief-yet-precise score, be easy to administer, and be tailored to the pediatric patient’s current functional status. The latter feature is particularly important as it allows scores obtained at different times to be compared regardless of whether the same items are administered. The pediatric perceived cognitive function item bank (pedsPCF; as described in the METHODS section) was developed to fill this need.[24; 25] The pedsPCF was developed using qualitative data from patients, parents, clinicians and teachers, calibrated using item response theory models (IRT), normed on the US pediatric general population, and validated by analyses of its associations with clinical variables in pediatric cancer samples. The pedsPCF is available in both child- and parent-report forms. The pedsPCF can be administered using computerized adaptive testing (CAT) or in a short-form fixed questionnaire format.[26] Details of an item bank and its application can be found elsewhere, including several national efforts supported by the National Institutes of Health such as the Patient Reported Outcomes Measurement Information System (PROMIS; http://www.nihpromis.org), and will not be described here.
The parent-report pedsPCF has been found to be associated with computerized neuropsychological measures[25]. In this study, we took a further step to evaluate the association between the pedsPCF and leukoencephalopathy (i.e., degree of cerebral deep white matter injury), an important indicator of brain dysfunction in children with brain tumors. We used leukoencephalopathy to represent structural brain abnormalities in this study because it is an important adverse event in children with brain tumors, especially those who receive radiation and chemotherapy, and because it provides an objective validation criterion for parent-reported PCF. Leukoencephalopathy was graded with reference to the Common Terminology Criteria for Adverse Events (version 4.0).[27] PCF was reported using a brief version of the validated Perceived Cognitive Function Item Bank[24; 25] (pedsPCF; parent-report version).
METHODS
Sample
Patients and one of their parents were recruited from Lurie Children’s Hospital of Chicago, between November 2013 and January 2015. The inclusion criteria were 1) a diagnosis of a brain tumor (any type, stage), 2) chronological age between 5 and 21 years, 3) any type of treatment (e.g., chemotherapy, radiation, or surgery), and 4) any stage in the disease continuum (including pre- and post-treatment and long-term survival). Parents of all patients, and patients age 8 and older, were required to have sufficient English literacy to read and understand consent/assent forms and respond to age appropriate questions in the survey. All parents were asked to complete a 10-item short-form questionnaire derived from the pediatric perceived cognitive function item bank[25] and a symptom distress scale.[29] Patients age 8 and older completed the computerized adaptive testing version of the PROMIS Fatigue,[26] Mobility,[30] Upper Extremity,[30] Depressive Symptoms,[31] Anxiety,[31] and Peer Relationships item banks.[32] Self-report versions of those instruments are not available for patients 5–7 years of age. Clinical information was extracted from patients’ medical records. Data from patients who received magnetic resonance imaging (MRI) brain scans as a component of routine clinical care were analyzed for this project. The MRI scans were used to determine leukoencephalopathy status. Leukoencephalopathy status was graded by the study team (CB, SG) using an enhanced version of the Common Terminology Criteria for Adverse Events – CTCAE (v4.02)[27] (see ‘Leukoencephalopathy grading criteria’ below). The enhancement was made to increase objectivity of grading. Grading was done independently of all other assessments, with the grader blinded to patient information.
Pediatric Perceived Cognitive Function Item Bank (pedsPCF)
The 43-item pedsPCF was developed using a mixed methods approach. Items were generated via interviews with patients, parents, teachers and clinicians. Its psychometric properties were evaluated using both classical test theory and item response theory models (IRT). IRT was used to estimate final item parameters using data from 1,409 parents of children aged 7–17 drawn from the US general population[24] and validated on 515 children with cancer (53.3% were brain tumor patients).[25] For the general population sample, pedsPCF demonstrated stable measurement properties (i.e., no item bias) across child age, child gender, parent gender, and parent education. It also discriminated children with and without elevated levels of attention, social and thought problems as measured using the Child Behavior Checklist.[20] In a pediatric cancer sample, pedsPCF discriminated patients with presence vs. absence of a brain tumor, moderately correlated with years since diagnosis for children who received craniospinal or whole brain radiation, and moderately correlated with computerized neuropsychological testing. Its platform for CAT is available via the PROMIS Assessment Center™. Multiple short-forms of pedsPCF are available which provide scores predictive of the total score obtained when all 43 pedsPCF items are administered, as demonstrated by a high correlation between an example short-form and the full-length pedsPCF. The 10-item short-form was created by the study team based on the content and psychometric properties of pedsPCF items (e.g., high information function and high slope in IRT results). PCF scores are reported as T-scores relative to a general population (parents of the US pediatric general population) mean of 50, with a standard deviation of 10, with higher scores indicating better PCF.
Leukoencephalopathy grading procedures and criteria
For the purpose of grading in this study, all indications of white matter damage, including the subjective degree of deep white matter volume loss, were considered in determining the overall degree of leukoencephalopathy. This is a more broad and inclusive strategy than leukoencephalopathy grading secondary to post treatment radiation or chemotherapy alone, as used by the current CTCAE. For example, any process resulting in signal abnormality or volume loss such as infiltration of the white matter by tumor as well as anatomic injury to, or inclusion of, the deep white matter in a surgical bed or intervention path, was also considered during the grading process.
Grading was performed based on abnormal hyperintense signal on T2 FLAIR MRI sequences. MRI scanner manufacturer and technical sequence parameters were not held constant due to the retrospective nature of the study, as MRI scans were done as part of routine clinical care. Grading fell into 5 categories (0–4) as described in Table 1, from normal (grade=0), mild abnormality/minimal volume loss/limited to one lobe (grade=1), moderate abnormality/volume loss/limited to two lobes (grade=2), severe abnormality/volume loss/involving at least three lobes (grade=3), to near complete loss of white matter volume or complete infiltration of the white matter by signal abnormality throughout the entire hemisphere (grade=4). Patients’ MRI scans were graded for the degree of cerebral deep white matter injury as an analogue of leukoencephalopathy by a board certified neuro-radiologist (CB) from April to June 2015. A random subset of 20 patients was selected for verification of grading relative to a board certified pediatric neuro-oncologist (SG) in July 2015. Perfect (100%) agreement was found between the two raters. All reviewers were blinded to pedsPCF scores and all clinical information.
Table 1.
Leukoencephalopathy Grading
| Grade | Criteria |
|---|---|
| 0 | normal, injury not perceived |
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
Analysis
We used Spearman’s rho to evaluate the relationship between leukoencephalopathy grades and PCF scores. Analysis of variance (ANOVA) was used to evaluate whether PCF scores could differentiate patients with different grades of leukoencephalopathy at the level of the total score (using T-scores) and also for individual pedsPCF items (using ordinal rating scale responses). Effect sizes (ES) for comparisons between adjacent grades were calculated to determine the magnitude of difference between comparison groups; in which ES was considered small between 0.2 and 0.5 (exclusive), medium between 0.5 and 0.8 (exclusive), and large ≥ 0.8.[33]
RESULTS
Ninety-nine patient/parent dyads were recruited. Demographic and clinical characteristics of the sample are presented in Table 2. The average age of patients was about 12 years, roughly half were male, and about 3/4 were white. Most were attending school and about 2/3 received some form of special education services. The sample was composed primarily of children with medulloblastoma, astrocytic tumors, and gangliogliomas. Many had been treated with chemotherapy, about half with cranial radiation, about 2/3 had undergone brain tumor-related surgery, and about 1/5 had received all three types of treatment. Average years since diagnosis was 5.8 (SD=4.6) and years since last treatment was 4.3 (SD=3.7; 36.4% ≤ 1 year and 63.6% > 1 year). A majority of the parent respondents were married mothers with at least some college education. A majority of parents rated their children’s quality of life positively.
Table 2.
Sample demographic and clinical information
| Variable | Categories with the variable | Mean (SD) or percentage |
|---|---|---|
| Age (patient; in years) | Mean=12.7 (SD=4.4) | |
|
| ||
| Age (parent; in years) | Mean=43.1 (SD=6.9) | |
|
| ||
| Years since diagnosis | Mean=5.8 (SD=4.6) | |
|
| ||
| Years since last treatment | Mean=4.3 (SD=3.7) | |
|
| ||
| Years since last surgery | Mean=7.1 (SD=13.3) | |
|
| ||
| Years since last radiation | Mean=4.6 (SD=3.7) | |
|
| ||
| Years since last chemotherapy | Mean=3.9 (SD=3.9) | |
|
| ||
| Gender | Male | 47.4% |
| Female | 52.6% | |
|
| ||
| Histology | Medulloblastoma | 25.8% |
| Astrocytic (grade 1, 2 or 3) | 23.7% | |
| Ganglioglioma | 20.1% | |
| Ependymoma | 6.2% | |
|
| ||
| Ethnicity | Hispanic origin (yes) | 16.8% |
|
| ||
| Race | White | 73.7% |
| African American | 7.4% | |
|
| ||
| Attending School | Yes | 97.9% |
|
| ||
| Type of Classrooma | Regular classroom; no IEP | 38.3% |
| Regular classroom; with IEP | 41.5% | |
| Special education | 13.8% | |
| other | 6.4% | |
|
| ||
| Repeated grade | Yes | 11.3% |
|
| ||
| Treatment | Chemotherapy | 84.5% |
| Radiotherapy | 54.6% | |
| Surgery | 69.1% | |
| All of above | 21.7% | |
| None of above | 5.2% | |
|
| ||
| Radiation typeb | Craniospinal | 42.9% |
| Proton | 36.7% | |
| Limited field | 14.3% | |
|
| ||
| Karnofsky or Lansky performance statusc | 100 | 61.5% |
| 90 | 31.9% | |
| 80 | 6.6% | |
|
| ||
| Parent-rated child’s quality of life | Excellent | 24.2% |
| Very good | 36.8% | |
| Good | 28.4% | |
| Fair or Poor | 10.5% | |
Only those attending school were included. IEP: Individualized educational program
Based on patients who received radiation
Clinician rated
Leukoencephalopathy grade distributions were as follows: 36 grade 0; 27 grade 1; 22 grade 2; 13 grade 3, and 1 grade 4. Data from patients with grades 3 and 4 were combined for analyses since only one patient had a grade of 4. The mean pedsPCF T-score was 48.3 (SD=8.3; range: 30.5 to 63.7). PedsPCF was significantly correlated with leukoencephalopathy grades, r=−0.36, p<−0.001. ANOVA results showed that pedsPCF scores significantly differentiated patients with different leukoencephalopathy grades, F=4.14, p=0.0084. Post-hoc Tukey tests indicated that parents of patients with grades of 3 or 4 reported significantly (p<0.05) lower pedsPCF scores than parents of patients with a grade of 0 (see Figure 1). A negligible ES (0.09) was found between grade 1 versus 2, and a large ES (1.22) was found between grade 0 versus 3 or 4. ESs for other comparisons were either small (0.43) or moderate (0.50–0.62).
Fig 1.
Comparisons of pedsPCF means (95% confidence intervals) across leukoencephalopathy grades.
* F=−4.14, p=0.0084
** Post-hoc Tukey tests showed “grade=0” was significantly different from “grade=3 or 4”
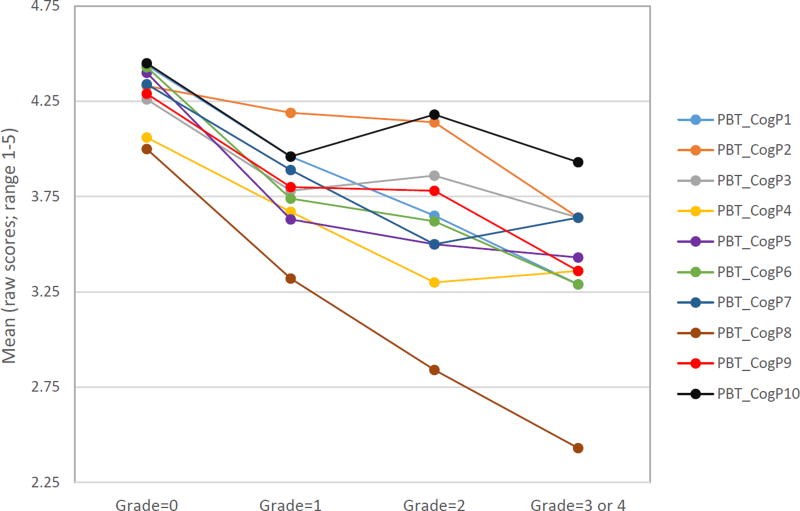
At the individual item level, 7 of 10 items significantly differentiated patients with different leukoencephalopathy grades (see Table 3 and Figure 2). Table 3 shows ESs for comparisons between adjacent grades (i.e., grade 0 vs. 1, 1 vs. 2 and 2 vs. [3 or 4]) for each item, ranging from 0.02 to 0.78, with all but 4 comparisons being in the expected direction. Of those, 5 comparisons had moderate ESs (all between grades 0 versus 1), 16 were small, and 8 were negligible. There was no systematic pattern to the magnitudes of differences between grades, although all but one item had either small or moderate ESs between grades 0 vs. 1. As shown in Figure 2, the item “It takes your child longer than other people to get his/her school work done” showed steadily decreased scores with increased leukoencephalopathy grades, with ESs ranging from 0.33 (grade 2 vs. [3 or 4]) to 1.27 (grade 0 vs. [3 or 4]). Another example was the item “Your child has to use written lists more often than other people his/her age so he/she will not forget things”, with ESs ranging from 0.23 (grade 1 vs. 2) to 1.08 (grade 0 vs. [3 or 4]). Either of these two items could potentially serve as a screening device if a single screening item were preferred. The three non-significant items were 1) It is hard for your child to pay attention to one thing for more than 5–10 minutes (F=1.55, p=0.2091), 2) Your child has trouble keeping track of what he/she is doing if he/she gets interrupted (F=1.73, p=0.1687), and 3) Your child has trouble paying attention to the teacher (F=1.49, p=0.2231). However, effect size comparisons among specific leukoencephalopathy grades for these items approached the upper boundary of a small ES (0.47 and 0.49) between grades 0 and 1 or between grades 2 and 3 or 4.
Table 3.
PedsPCF short-form items across leukoencephalopathy grades
| Item | F | P | Comparison (grade) |
Effect Size |
|
|---|---|---|---|---|---|
| PBT_CogP1 | Your child has to use written lists more often than other people his/her age so he/she will not forget things |
6.01 | 0.001** | 0 vs 1 | 0.43 |
| 1 vs 2 | 0.23 | ||||
| 2 vs 3 or 4 | 0.28 | ||||
|
| |||||
| PBT_CogP2 | It is hard for your child to pay attention to one thing for more than 5–10 minutes | 1.55 | 0.2091 | 0 vs 1 | 0.15 |
| 1 vs 2 | 0.05 | ||||
| 2 vs 3 or 4 | 0.49 | ||||
|
| |||||
| PBT_CogP3 | Your child has trouble keeping track of what he/she is doing if he/she gets interrupted | 1.73 | 0.1687 | 0 vs 1 | 0.49 |
| 1 vs 2 | −0.08 | ||||
| 2 vs 3 or 4 | 0.23 | ||||
|
| |||||
| PBT_CogP4 | Your child has to read things several times to understand them | 5.89 | 0.0011** | 0 vs 1 | 0.37 |
| 1 vs 2 | 0.30 | ||||
| 2 vs 3 or 4 | −0.05 | ||||
|
| |||||
| PBT_CogP5 | Your child forgets things easily | 7.12 | 0.0003** | 0 vs 1 | 0.70 |
| 1 vs 2 | 0.11 | ||||
| 2 vs 3 | 0.06 | ||||
|
| |||||
| PBT_CogP6 | Your child has to work really hard to pay attention or he/she makes mistakes | 7.94 | 0.0001** | 0 vs 1 | 0.78 |
| 1 vs 2 | 0.10 | ||||
| 2 vs 3 or 4 | 0.29 | ||||
|
| |||||
| PBT_CogP7 | Your child has trouble remembering to do things like school projects or chores | 6.29 | 0.0007** | 0 vs 1 | 0.51 |
| 1 vs 2 | 0.42 | ||||
| 2 vs 3 or 4 | −0.14 | ||||
|
| |||||
| PBT_CogP8 | It takes your child longer than other people to get his/her school work done | 6.02 | 0.001** | 0 vs 1 | 0.51 |
| 1 vs 2 | 0.36 | ||||
| 2 vs 3 or 4 | 0.33 | ||||
|
| |||||
| PBT_CogP9 | It is hard for your child to concentrate in school | 3.63 | 0.0167* | 0 vs 1 | 0.50 |
| 1 vs 2 | 0.02 | ||||
| 2 vs 3 or 4 | 0.41 | ||||
|
| |||||
| PBT_CogP10 | Your child has trouble paying attention to the teacher | 1.49 | 0.2231 | 0 vs 1 | 0.47 |
| 1 vs 2 | −0.20 | ||||
| 2 vs 3 or 4 | 0.25 | ||||
p<0.05
p<0.01
Fig 2.
Mean comparisons of individual pedsPCF items across leukoencephalopathy grades
Type of radiation received (“craniospinal or whole brain radiation” vs. others vs. no radiation) was not associated with leukoencephalopathy grade, X2 =4.72, p=0.58. Though patients who received craniospinal or whole brain radiation had worse pedsPCF scores (T=46.3) than the other two groups, T=49.4 and 49.2 for others and no radiation, respectively; these differences were not significant, F=1.37, p=0.33.
DISCUSSION
The prevalence of cognitive dysfunction in pediatric cancer survivors highlights the need for routine assessments to enable its early identification and timely intervention.[34] This is particularly important for childhood brain tumor survivors. Studies have shown the effectiveness of cognitive training strategies for pediatric cancer survivors who received neurotoxic therapy.[35–37] For example, Moore and colleagues[38] demonstrated the effectiveness of an early intervention program to improve mathematics abilities and visual working memory of childhood leukemia survivors compared to controls who received standard care. The impact of such interventions depends on effective identification of children who need them. The significant association found between the pedsPCF and Leukoencephalopathy grade in this study indicates that pedsPCF has the potential to identify patients for prompt formal cognitive testing, who can then benefit from such early interventions. Efforts have been made to develop brief neuropsychological testing batteries to facilitate identification.[39–42] Though those brief assessments are less time consuming than comprehensive neuropsychological assessment batteries, they still need to be administered by trained professionals and so many of the typical obstacles (e.g., cost) remain.
An alternative method of surveillance is via the use of self- or proxy-reported questionnaires as screening devices to identify children who require formal psychometric assessment in the form of either an abbreviated or comprehensive neuropsychological test battery. Bull et al[13] found that scores from the self- and parent-report Pediatric Quality of Life Inventory and from the teacher-report Behavior Rating Inventory of Executive Function discriminated children with a full-scale Intelligence Quotient below versus above 80. Hardy et al[23] found that parent-reported attention problems can serve as a valid indicator of cognitive deficits. The pedsPCF is a comprehensive item bank which taps the content areas of attention, concentration, executive function, language, spatial orientation, visuospatial ability, memory, personal orientation, and processing speed,[43] producing a global cognitive index via IRT scaling techniques. Hardy and colleagues[23] bemoan the lack of a validated rating scale measuring impairments related to mental processing speed, because such a scale would a particularly important predictor of neurocognitive late effects in pediatric cancer survivors. Our recommended single screening item “It takes your child longer than other people to get his/her school work done” taps this important domain.
The pedsPCF has been shown to be moderately correlated with neuropsychological test results at various magnitudes depending on years since diagnosis and the cognitive domain being measured.[25] The current investigation takes an additional step in evaluating the relationship between parent-reported PCF and cerebral compromise as indicated by degree of cerebral deep white matter injury. Results of this study provide initial empirical evidence that the pedsPCF is a tool with the potential to differentiate patients with undetectable/mild versus moderate/severe leukoencephalopathy, supporting the view that pedsPCF can be broadly, efficiently, and economically used to screen, for referral, children who can benefit from comprehensive neurocognitive testing.
The pedsPCF can be administered in a variety of formats (e.g., CAT or fixed short-form) as demonstrated in Lai et al,[24] and therefore it provides a range of administration and reporting options, including internet, personal computer, handheld and paper formats, making it accessible and affordable to all patients. Using a prototype symptom monitoring and reporting system, SyMon-SAYS,[44] as an example, patients and parents can complete the pedsPCF at home or in any location (such as a primary medical care office) using any device accessible to the internet. Clinicians can review pedsPCF scores during or prior to patient visits by accessing a data center on the internet or from a hardcopy printed at home or in clinic. The flexibility and accessibility of this system offers the promise of an innovative and practical screening tool that can identify patients who would benefit from comprehensive assessment with NPT or neuro-imaging.
The pedsPCF uses a T-score metric referenced to the pediatric general population, mean=50 and SD=10. A cut-off of 40 (i.e., 1 SD worse than norm) has been suggested for further exploration based on behavioral ratings and clinical variables.[24; 25] However, future studies should be conducted to evaluate how well this cut-off score discriminates children with (versus without) neurocognitive deficits and structural brain changes both cross-sectional and longitudinally. Although pedsPCF is available in both child- and parent-report forms, in this study we focused on parent-report rather than child-report cognition. We made this decision in consideration that immature meta-cognition (especially in children with a brain tumor whose cognitive function is likely compromised by tumors and/or neurotoxic treatments) may adversely impact a child’s ability to accurately report their own cognition.[28] As survivors need to eventually take the responsibility of managing their own late effects, future studies should be done to evaluate to what extent child-report pedsPCF is correlated with neuropsychological tests and brain imaging findings.
We modified the current grading system for leukoencephalopathy in order to make it more inclusive of white matter injury related to the entire course of patients’ underlying brain tumor, whether secondary to the primary lesion itself or the sequelae of treatment. Especially in patients further along their treatment course, structural MRI brain findings can be multifaceted and complex. MRI examinations may provide evidence of remaining tumor, one or more surgeries, and post-radiation or - chemotherapy treatment white matter effects. In many cases these etiological pathways are indistinguishable from an imaging standpoint, involving the same regions of anatomy. For this reason, although measuring leukoencephalopathy routinely in surveillance scans can provide objective information regarding changes of brain structure, challenges in correlating its results to patients’ functioning and daily lives make it unrealistic to use leukoencephalopathy grades as a screening tool. Instead, pedsPCF would be appropriate to serve this role, as described earlier. A strength of our grading method is its simplicity in assessing injury regardless of cause, especially in light of the complicated anatomic picture of many brain tumor patients. We acknowledge the possibility that different etiological pathways of brain injury may yield different clinical effects, so it is important to continue efforts to consider etiology in future studies of neurocognitive outcome.
A grading system such as that devised for the current study should be reproducible. We acknowledge that there remains room for improvement in the subjective elements of our criteria. The modifiers for our criteria included mild, moderate, and severe, and there is also subjectivity in the perception of T2 FLAIR abnormality and volume loss. We hope to improve the grading guidelines by better defining criteria for each grade, thereby increasing the objectivity of the leukoencephalopathy grading system. In our study we had good inter-observer agreement for a subset of cases between a neuro-radiologist and a pediatric neuro-oncologist. However, further investigation of inter-observer agreement should be undertaken, such as multiple neuroimaging specialists with pediatric brain tumor experience. Additionally, prospective investigation of our grading scheme, holding technical MRI parameters constant, would be highly desirable. It is acknowledged that subtle abnormalities, including the T2 FLAIR signal intensity present, can be influenced by MRI equipment and technique.
Limitations of this study are noted. We recruited a heterogeneous group of patients with different types of tumors, locations and treatments in order to maximize generalizability of the study results (i.e., relationship between leukoencephalopathy and PCF). We believe that the heterogeneity of our sample (with respect to patients’ ages and clinical histories) per se does not necessarily increase concerns about recall bias and response shift, though we recognize that these phenomena always exist in outcomes studies. We would expect limited impact of our sample’s clinical heterogeneity upon associations between parent-reported pedsPCF and leukoencephalopathy grades. These associations should be explored by future multi-center studies with a larger sample size. Additionally, this heterogeneity limited our ability to further evaluate potential contributing factors to leukoencephalopathy such as specific types and intensity of radiation, due to the small sample sizes within each category. Again, future multicenter studies with a larger sample should be conducted to determine whether our findings can be replicated across different pediatric brain tumor survivor subgroups. We did not collect information about parent health literacy, socioeconomic status, and other variables measuring family functioning. Past research indicates that poorer family functioning and greater social risk are associated with poorer executive function in children with focal brain insults.[45; 46] A majority of the current sample was from relatively educated families (87% of parents received at least some college), thus studies including patients with a broader range of socioeconomic status should be done to evaluate how these factors impact PCF scores and whether there are any differences in leukoencephalopathy between patients with similar treatment protocols but different socioeconomic status. This study focused on the association between PCF and leukoencephalopathy. Future longitudinal studies should be conducted to evaluate the predictive value of the PCF to imaging leukoencephalopathy analysis and neuropsychological testing results, particularly for formal face-to-face comprehensive testing, to develop a clearer understanding of the relationships among these three measurement modalities. Ultimately, a fuller picture of the causal relationships between cognition as measured via all three modalities and functional outcomes will be more clearly discerned with a larger, heterogeneous group.
In conclusion, this study provided empirical evidence that the efficient, inexpensive parent-report pedsPCF is significantly associated with cerebral compromise as indicated by structural brain changes in pediatric brain tumor survivors. The pedsPCF holds promise as a screening tool to efficiently refer patients for comprehensive neurocognitive testing in a timely manner to identify cognitive changes in childhood brain tumor survivors, making effective early intervention possible. Further studies should be done to examine the replicability of the current findings.
Acknowledgments
This work was supported by the National Institutes of Health/ National Cancer Institute (R01CA174452; PI: Jin-Shei Lai).
Footnotes
Compliance with Ethical Standards
Ethical approval
This study was approved by the Institutional Review Boards of both Northwestern University and Lurie Children’s Hospital of Chicago. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure of potential conflicts of interest
All authors (Lai, Bregman, Zelko, Nowinski, Cella and Goldman) report no disclosures and no conflicts of interest relevant to the manuscript.
Contributor Information
Jin-Shei Lai, Medical Social Sciences and Pediatrics, Feinberg School of Medicine at Northwestern University, Chicago, IL 60611, js-lai@northwestern.edu.
Corey Bregman, Medical Imaging (Radiology), Ann & Robert H Lurie Children’s Hospital, Chicago, Illinois 60611, CBregman@luriechildrens.org.
Frank Zelko, Pediatric Neuropsychology Service, Department of Child and Adolescent Psychiatry, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL 60611, fzelko@luriechildrens.org
Cindy Nowinski, Medical Social Sciences, Feinberg School of Medicine at Northwestern University, Chicago, IL 60611, c-nowinski@northwestern.edu
David Cella, Medical Social Sciences, Feinberg School of Medicine at Northwestern University, Chicago, IL 60611, d-cella@northwestern.edu
Jennifer J. Beaumont, Medical Social Sciences, Feinberg School of Medicine at Northwestern University, Chicago, IL 60611, j-beaumont@northwestern.edu.
Stewart Goldman, Hematology/Oncology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL 60611, SGoldman@luriechildrens.org.
References
- 1.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. The Journal of the American Medical Association. 2007;297(24):2762–2764. doi: 10.1001/jama.297.24.2762. [DOI] [PubMed] [Google Scholar]
- 2.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den BC, van der Pal HJ, Heinen RC, Jaspers MW, Koning CC, Oldenburger F, Langeveld NE, Hart AA, Bakker PJ, Caron HN, van Leeuwen FE. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. The Journal of the American Medical Association. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. Journal of Clinical Oncology. 2000;18(3):646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 4.Parisi MT, Fahmy JL, Kaminsky CK, Malogolowkin MH. Complications of cancer therapy in children: A radiologist's guide. Radiographics. 1999;19(2):283–297. doi: 10.1148/radiographics.19.2.g99mr05283. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. Journal of Clinical Oncology. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 6.Lavigne JV, Faier-Routman J. Psychological adjustment to pediatric physical disorders: A meta-analytic review. Journal of Pediatric Psychology. 1992;17(2):133–157. doi: 10.1093/jpepsy/17.2.133. [DOI] [PubMed] [Google Scholar]
- 7.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. Journal of Pediatric Psychology. 2005;30(1):9–27. doi: 10.1093/jpepsy/jsi012. [DOI] [PubMed] [Google Scholar]
- 8.Hays DM, Dolgin M, Steele LL, Patenaude AF, Hewett KD, Ruymann F, et al. Educational achievement, employment and workplace experience of adult survivors of childhood cancer. International Journal of Pediatric Hematology/Oncology. 1997;4:327–337. [Google Scholar]
- 9.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, Yasui Y, Mertens AC, Sklar CA, Nicholson HS, Zeltzer LK, Neglia JP, Robison LL. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood cancer survivor study. Journal of Clinical Oncology. 2003;21(17):3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 10.Walsh KS, Noll RB, Annett RD, Patel SK, Patenaude AF, Embry L. Standard of Care for Neuropsychological Monitoring in Pediatric Neuro-Oncology: Lessons From the Children's Oncology Group (COG) Pediatric blood & cancer. 2016;63(2):191–195. doi: 10.1002/pbc.25759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selove R, Kroll T, Coppes M, Cheng Y. Psychosocial services in the first 30 days after diagnosis: Results of a web-based survey of children's oncology group (COG) member institutions. Pediatric blood & cancer. 2012;58(3):435–440. doi: 10.1002/pbc.23235. [DOI] [PubMed] [Google Scholar]
- 12.Eisman EJ, Dies RR, Finn SE, Eyde LD, Kay GG, Kubiszyn TW, Meyer GJ, Moreland KL. Problems and limitations in using psychological assessment in the contemporary health care delivery system. Professional Psychology: Research and Practice. 2000;31(2):131. [Google Scholar]
- 13.Bull KS, Liossi C, Peacock JL, Yuen HM, Kennedy CR. Screening for cognitive deficits in 8 to 14-year old children with cerebellar tumors using self-report measures of executive and behavioral functioning and health-related quality of life. Neuro-oncology. 2015;17(12):1628–1636. doi: 10.1093/neuonc/nov129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahone EM, Martin R, Kates WR, Hay T, Horska A. Neuroimaging correlates of parent ratings of working memory in typically developing children. Journal of the International Neuropsychological Society. 2009;15(1):31–41. doi: 10.1017/S1355617708090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Annals of Neurology. 2000;47(2):145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: The Rotterdam Scan Study. Neurology. 2001;56(11):1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. British Journal of Psychiatry. 1996;168(4):477–485. doi: 10.1192/bjp.168.4.477. [DOI] [PubMed] [Google Scholar]
- 18.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. Journal of Clinical Oncology. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach TM. Integrative guide to the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: University of Vermont, Department of Psychology; 1991. [Google Scholar]
- 21.Conners CK. Conners' Rating Scales-Revised Technical Manual. North Tonawanda, New York: Multi Health Systems; 2000. [Google Scholar]
- 22.Gioia GA, Isquith P, Guy S, Kenworthy L. Behavior rating inventory of executive function (BRIEF): Professional Manual. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 23.Hardy KK, Willard VW, Wigdor AB, Allen TM, Bonner MJ. The potential utility of parent-reported attention screening in survivors of childhood cancer to identify those in need of comprehensive neuropsychological evaluation. Neuro-oncology practice. 2015;2(1):32–39. doi: 10.1093/nop/npu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai J-S, Butt Z, Zelko F, Cella D, Krull K, Kieran M, Goldman S. Development of a Parent-Report Cognitive Function Item Bank Using Item Response Theory and Exploration of its Clinical Utility in Computerized Adaptive Testing. Journal of Pediatric Psychology. 2011;36(7):766–779. doi: 10.1093/jpepsy/jsr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai J-S, Zelko F, Krull K, Cella D, Nowinski C, Manley P, Goldman S. Parent-reported cognition of children with cancer and its potential clinical usefulness. Quality of Life Research. 2014;23(4):1049–1058. doi: 10.1007/s11136-013-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai J-S, Stucky B, Thissen D, Varni J, DeWitt E, Irwin D, Yeatts K, DeWalt D. Development and psychometric properties of the PROMIS® pediatric fatigue item banks. Quality of Life Research. 2013;22(9):2417–2427. doi: 10.1007/s11136-013-0357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Common terminology criteria for adverse events: (CTCAE) Version 4.0. 2010 from http://evs.nci.nih.gov/ftp1/CTCAE/Documentation/CTCAE_Governance_2010-03-11.pdf.
- 28.Schneider W, Lockl K, Perfect T. Applied Metacognition. West Nyack, NY: Cambridge University Press; 2002. The development of metacognition knowledge in children and adolescents; pp. 224EP–259. [Google Scholar]
- 29.Hinds PS, Quargnenti A, Bush AJ, Pratt C, Fairclough D, Rissmiller G, Betcher D, Gilchrist GS. An evaluation of the impact of a self-care coping intervention on psychological and clinical outcomes in adolescents with newly diagnosed cancer. European Journal of Oncology Nursing. 2000;4(1):6–17. doi: 10.1054/ejon.1999.0051. discussion 18–19. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, Lai J-S, Yeatts KB, DeWalt DA. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. Journal of Clinical Epidemiology. 2011;64(7):794–804. doi: 10.1016/j.jclinepi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewalt DA, Thissen D, Stucky BD, Langer MM, Morgan Dewitt E, Irwin DE, Lai JS, Yeatts KB, Gross HE, Taylor O, Varni JW. PROMIS Pediatric Peer Relationships Scale: development of a peer relationships item bank as part of social health measurement. Health Psychol. 2013;32(10):1093–1103. doi: 10.1037/a0032670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 34.Iuvone L, Mariotti P, Colosimo C, Guzzetta F, Ruggiero A, Riccardi R. Long-term cognitive outcome, brain computed tomography scan, and magnetic resonance imaging in children cured for acute lymphoblastic leukemia. Cancer. 2002;95(12):2562–2570. doi: 10.1002/cncr.10999. [DOI] [PubMed] [Google Scholar]
- 35.Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN, Martin-Elbahesh K, Hardy KK, Merchant TE, Jeha S. Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial. Journal of Clinical Oncology. 2015;33(33):3894–3902. doi: 10.1200/JCO.2015.61.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler RW, Copeland DR, Fairclough DL, Mulhern RK, Katz ER, Kazak AE, Noll RB, Patel SK, Sahler OJZ. A Multicenter, Randomized Clinical Trial of a Cognitive Remediation Program for Childhood Survivors of a Pediatric Malignancy. Journal of Consulting and Clinical Psychology. 2008;76(3):367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van't Hooft I, Andersson K, Bergman B, Sejersen T, von Wendt L, Bartfai A. Sustained favorable effects of cognitive training in children with acquired brain injuries. NeuroRehabilitation. 2007;22(2):109–116. [PubMed] [Google Scholar]
- 38.Moore IM, Hockenberry MJ, Anhalt C, McCarthy K, Krull KR. Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatric Blood and Cancer. 2012;59(2):278–284. doi: 10.1002/pbc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krull KR, Okcu MF, Potter B, Jain N, Dreyer Z, Kamdar K, Brouwers P. Screening for Neurocognitive Impairment in Pediatric Cancer Long-Term Survivors. Journal of Clinical Oncology. 2008;26(25):4138. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 40.Lageman S, Cerhan J, Locke DC, Anderson SK, Wu W, Brown P. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. Journal of Neuro-Oncology. 2010;96(2):271–276. doi: 10.1007/s11060-009-9960-y. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong CL, Goldstein B, Shera D, Ledakis GE, Tallent EM. The predictive value of longitudinal neuropsychologic assessment in the early detection of brain tumor recurrence. Cancer. 2003;97(3):649–656. doi: 10.1002/cncr.11099. [DOI] [PubMed] [Google Scholar]
- 42.Ottensmeier H, Zimolong B, Wolff JE, Ehrich J, Galley N, von Hoff K, Kuehl J, Rutkowski S. Neuropsychological short assessment of disease-and treatment-related intelligence deficits in children with brain tumours. European Journal of Paediatric Neurology. 2015;19(3):298–307. doi: 10.1016/j.ejpn.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Lai JS, Zelko F, Butt Z, Cella D, Kieran MW, Krull KR, Magasi S, Goldman S. Parent-perceived child cognitive function: results from a sample drawn from the US general population. Child's Nervous System. 2011;27(2):285–293. doi: 10.1007/s00381-010-1230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai J-S, Yount S, Beaumont JL, Cella D, Toia J, Goldman S. A patient-centered symptom monitoring and reporting system for children and young adults with cancer (SyMon-SAYS) Pediatric Blood & Cancer. 2015;62(10):1813–1818. doi: 10.1002/pbc.25550. [DOI] [PubMed] [Google Scholar]
- 45.Anderson VA, Spencer-Smith MM, Coleman L, Anderson PJ, Greenham M, Jacobs R, Lee KJ, Leventer RJ. Predicting neurocognitive and behavioural outcome after early brain insult. Developmental Medicine & Child Neurology. 2014;56(4):329–336. doi: 10.1111/dmcn.12387. [DOI] [PubMed] [Google Scholar]
- 46.Anderson V, Catroppa C, Godfrey C, Rosenfeld JV. Intellectual Ability 10 Years after Traumatic Brain Injury in Infancy and Childhood: What Predicts Outcome? Journal of Neurotrauma. 2011;29(1):143–153. doi: 10.1089/neu.2011.2012. [DOI] [PubMed] [Google Scholar]