Abstract
Background
Left atrial (LA) remodeling develops as a result of longstanding pressure overload. However, determinants and clinical outcome of excessive remodeling, so called giant left atrium (GLA), are not clear.
Methods
Clinical characteristics of patients with GLA (antero-posterior diameter higher than 65 mm), including echo-Doppler parameters, and follow-up clinical outcomes from a tertiary referral hospital were investigated.
Results
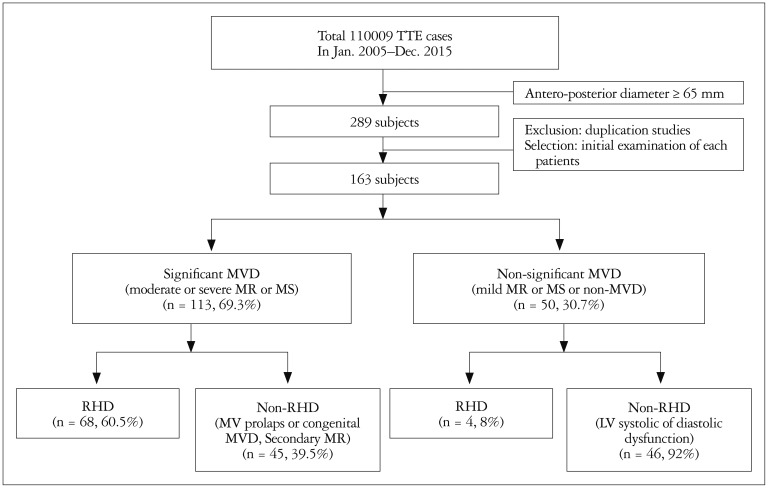
Among 68519 consecutive primary patients who underwent echocardiography over a period of 10 years, data from 163 GLA cases (0.24%) were analyzed. Main causes were significant rheumatic mitral stenosis (n = 58, 36%); other causes comprised significant rheumatic mitral regurgitation (MR; n = 10, 6%), mitral valve (MV) prolapse or congenital mitral valvular disease (MVD) (n = 20, 12%), and functional MR (n = 25, 15%). However, mild rheumatic MV disease (n = 4, 3%) or left ventricular (LV) systolic or diastolic dysfunction without significant MR (n = 46, 28%) were also causes of GLA. During median follow-up of 22 months, 42 cases (26%) underwent composite events. MV surgery was related to lower rate of composite events. In multivariate analysis, MV surgery, elevated pulmonary arterial systolic pressure, and increased LA volume index were independent predictors of future events (p < 0.05) regardless of underlying diseases or history of MV surgery.
Conclusion
Although rheumatic MVD with atrial fibrillation is the main contributor to GLA, longstanding atrial fibrillation with LV dysfunction but without MVD also could be related to GLA. Even in GLA state, accurate measurement of LA volume is crucial for risk stratification for future events, regardless of underlying disease.
Keywords: Left atrium, Remodeling, Cardiovascular events, Echocardiography
Introduction
Left atrial (LA) size increases in response to long-standing hemodynamic or electrical loads.1),2) The incidence of new onset atrial fibrillation, heart failure, ischemic stroke, acute coronary syndrome, and cardiovascular death increase with an increase in LA size.3),4),5) However, giant left atrium (GLA), which is due to excessive LA remodeling, is a rare condition with a prevalence of only 0.3–0.6%.6),7),8) Despite the fact that the terminology “GLA” is often used, the cut-off size to diagnose GLA has not always been clearly defined, though an LA antero-posterior direction (AP) dimension higher than or 65 mm was used in some previous reports.6),7) In addition to long-duration loading of the left atrium, other factors may also contribute to this rare condition. Despite uncertainty regarding the underlying pathophysiologic mechanism, rheumatic mitral valvular disease (MVD) is known to be the main cause of GLA, and few studies reported that mitral regurgitation (MR) from mitral valve (MV) prolapse or hypertrophic cardiomyopathy could be related to this rare condition.7) However, the actual prevalence, contributors, and clinical outcomes of GLA have not previously been investigated. In this study, we therefore investigated GLA clinical characteristics and clinical outcomes. We then evaluated predictors for clinical outcome and whether the concept of “the bigger the worse” is applicable to this rare condition.
Methods
Study population and clinical characteristics
This was a single-center, retrospective, observational study. The study proposal was approved by the Institutional Review Board of our hospital and informed consent was waived due to the retrospective nature of the study. From a total of 110009 consecutive echocardiography cases performed from Jan 2005 to Dec 2015, cases with an LA-AP diameter 65 mm or greater than 65 mm were selected. In cases with repeated examination, the initial examination was selected. Finally, 163 patients (0.24%) among 68519 primary patients were selected for final analysis (Fig. 1). The medical records of these 163 patients were reviewed and associated data were collected by a study coordinator. History of underlying cardio-cerebro vascular disease and previous cardiac surgery were reviewed. In addition, information about hypertension, diabetes, dyslipidemia, and current medications were collected. Blood hemoglobin, estimated glomerular filtration rate (eGFR), brain natriuretic peptide (BNP) (n = 84), and C-reactive protein (CRP) (n = 121) measured within 1 year were collected. Significant MVD was defined as a moderate or severe degree of stenosis or regurgitation. Patients with previous MV surgery were included in the significant MVD group.
Fig. 1. Schematic flow diagram of the study population. TTE: transthoracic echocardiography, MR: mitral regurgitation, MS: mitral stenosis, MV: mitral valve, MVD: mitral valvular disease, RHD: rheumatic heart disease.
Clinical outcome assesment
Primary composite end-point for follow-up comprised all-cause death, new onset cerebrovascular accident (CVA), heart failure admission, and acute myocardial infarction. To evaluate clinical outcomes, a coordinator reviewed medical records and contacted patients or relatives by telephone.
Transthoracic echocardiography
Left ventricular (LV) dimensions and septal and posterior wall thicknesses were measured at end-diastole and end-systole in the two-dimensional parasternal long axis or short axis views. LV ejection fraction was calculated using the Teichholz method. LV mass was measured by Devereux's methods as recommended by the American Society of Echocardiography.9) LA volume was measured using the prolate ellipsoidal method at the point of LV end-systole when LA size was maximal.10),11) LA volume index (LAVI) was calculated as divided by body surface area. Peak velocity of tricuspid regurgitation (TRV) was measured and pulmonary arterial systolic pressure (PASP) was calculated as 4 × TRV2 + right atrial (RA) pressure, where RA pressure was estimated according to inferior vena cava diameter and its respiratory variations.9) Mean pressure gradient through the MV were measured by continuous wave Doppler and quantification of MR was done by proximal isovelocity surface area methods.12) Severity of valvular regurgitation and stenosis were defined according to current guidelines but severity of functional MR followed uniformly to primary MR.13)
Statistical analysis
Clinical characteristics and echocardiographic parameters are presented as means ± standard deviations for continuous variables and numbers (percentages) for categorical variables. The significance of correlations between groups in continuous variables was assessed using the Pearson correlation coefficient. The significance of differences in clinical and echocardiographic findings between study groups was evaluated by independent t-test for continuous variable or the chi-square test for dichotomous variables. Cox proportional hazard regression analysis was performed to determine the probability distribution of time to each event and composite end-points. The variables not following normal distribution were expressed as median and interquartile range. Mann-Whitney U-test for independent two group comparison and log transformed values were used for the Cox regression analysis. All analyses were performed using SPSS (version 20.0, IBM Corp., Armonk, NY, USA), and p values less than 0.05 were considered significant.
Results
Baseline clinical characteristics
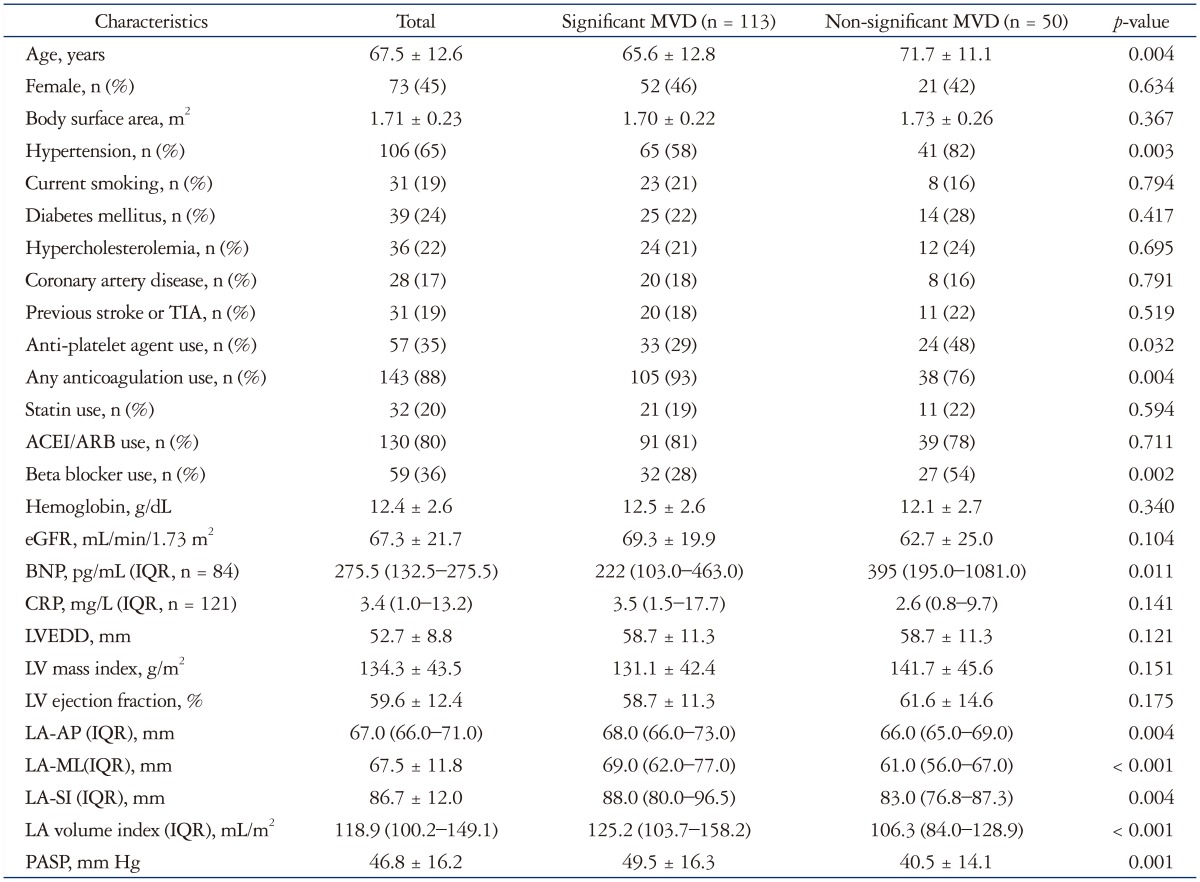
Mean age of the 163 subjects was 67.5 ± 12.6 years, and 73 (45%) were female. Main contributors to GLA were rheumatic mitral stenosis (n = 58, 36%), rheumatic MR (n = 10, 6%), MR due to MV prolapse or congenital MVD (n = 20, 12%), secondary MR of more than mild degree (n = 25, 15%), mild rheumatic mitral stenosis or regurgitation (n = 4, 3%), and significant LV dysfunction without significant MR (n = 46, 28%) (Fig. 2). Among those patients with significant LV systolic or diastolic dysfunction, three patients had hypertrophic cardiomyopathy and two patients had constrictive pericarditis. Twenty five patients were suspected as restrictive cardiomyopathy. Almost all patients had atrial fibrillation (n = 161, 99%), one patient had sinus rhythm and remaining patient had ventricular pacing rhythm. Of 163 patients, 139 patients (85.3%) were taking warfarin and 4 patients (2.4%) were taking novel oral anticoagulants. Remainder 20 patients were taking aspirin or other antiplatelet agents. Thirty three patients (20%) had previous history of MV surgery, of them 25 patients underwent MV replacement surgery due to rheumatic heart disease (RHD), one patient underwent MV repair and 7 patients underwent MV replacement (n = 7) due to degenerative MR. The median LA-AP diameter and LAVI were 67 (66–71) mm and 118.9 (100.2–149.1) mL/m2, respectively. The mean LV ejection fraction and PASP were 59.6 ± 12.4% and 46.8 ± 16.2 mm Hg, respectively. Mean blood hemoglobin concentration was 12.4 ± 2.6 g/dL and mean eGFR was 67.3 ± 21.7 mL/min/1.73 m2 (Table 1).
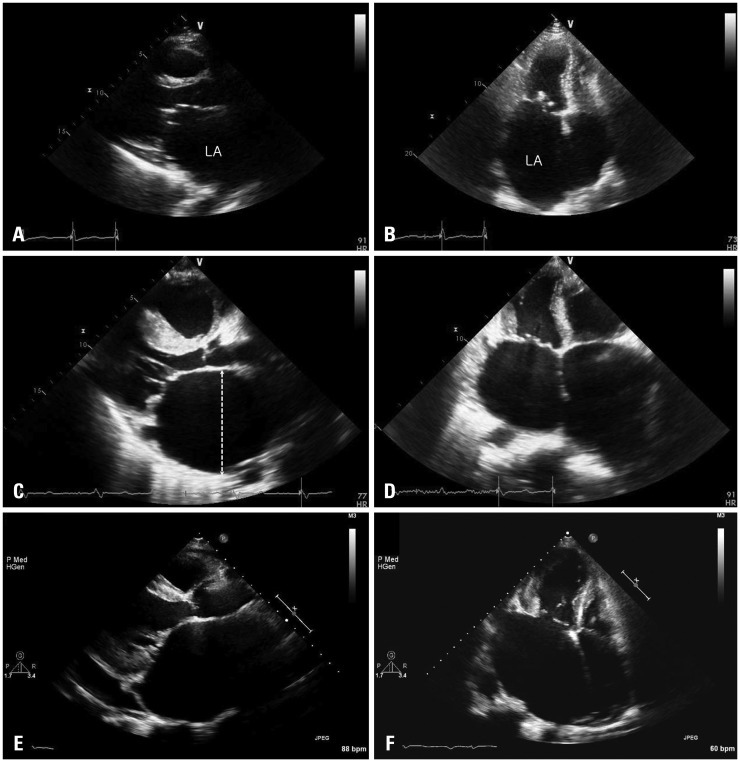
Fig. 2. Representative cases of a giant LA. Parasternal long axis view (A) and apical 4-chamber view (B) of rheumatic mitral stenosis. Parasternal long axis view (C) and apical 4-chamber view (D) of hypertrophic cardiomyopathy with moderate MR. Parasternal long axis view (E) and apical 4-chamber view (F) of severe rheumatic MR. Dotted line in C shows antero-posterior diameter of left atrium. LA: left atrium, MR: mitral regurgitation.
Table 1. Baseline clinical characteristics.
MVD: mitral valvular disease, TIA: transient ischemic attack, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, eGFR: estimated glomerular filtration rate, BNP: brain natriuretic peptide, IQR: inter-quartile range, LVEDD: left ventricular end-diastolic dimension, LA: left atrial, AP: antero-posterior direction, ML: medio-lateral direction, SI: supero-inferior direction, PASP: pulmonary arterial systolic pressure
Comparison among groups with different causes of GLA
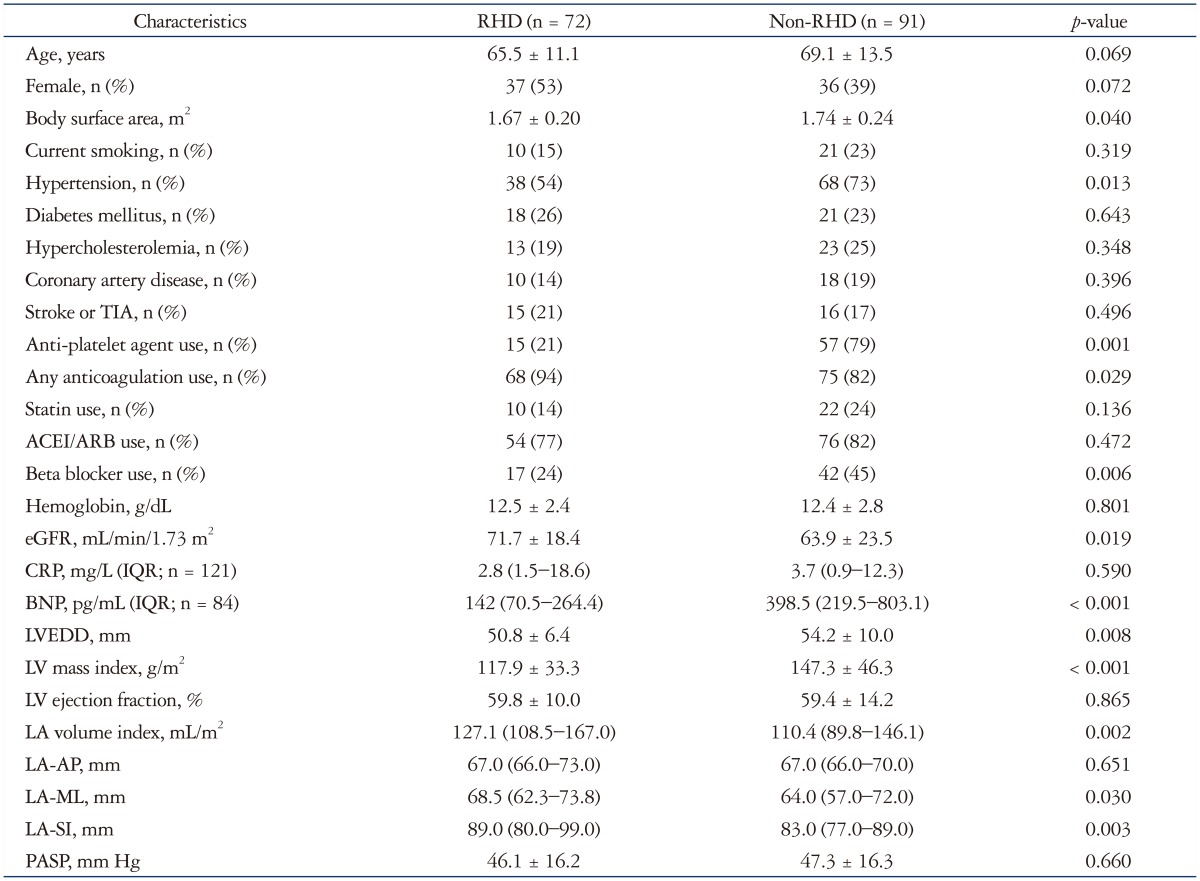
Patients with significant MVD were younger and had a higher PASP and LAVI than the other patients. They had a lower prevalence of hypertension, beta-blocker use, and lower serum BNP level than the other patients (Table 1). When patients were assigned to groups based on the presence or absence of RHD, we found that RHD patients had a lower LV mass index, BNP level, and beta-blocker use and higher LAVI than non-RHD patients (Table 2). LA-AP diameter (r = 0.773, p < 0.001), LA–medio-lateral direction (ML) diameter (r = 0.887, p < 0.001), and LA–supero-inferior direction (SI) diameter (r = 0.842, p < 0.001) were significantly correlated to LAVI, but the degree of correlation was better for LA-ML diameter than LA-AP diameter.
Table 2. Baseline clinical characteristics according to presence of rheumatic valve disease.
RHD: rheumatic heart disease, TIA: transient ischemic attack, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, eGFR: estimated glomerular filtration rate, CRP: c-reactive protein, IQR: inter-quartile range, BNP: brain natriuretic peptide, LVEDD: left ventricular end-diastolic dimension, LA: left atrial, AP: antero-posterior direction, ML: medio-lateral direction, SI: supero-inferior direction, PASP: pulmonary arterial systolic pressure
Clinical follow-up results
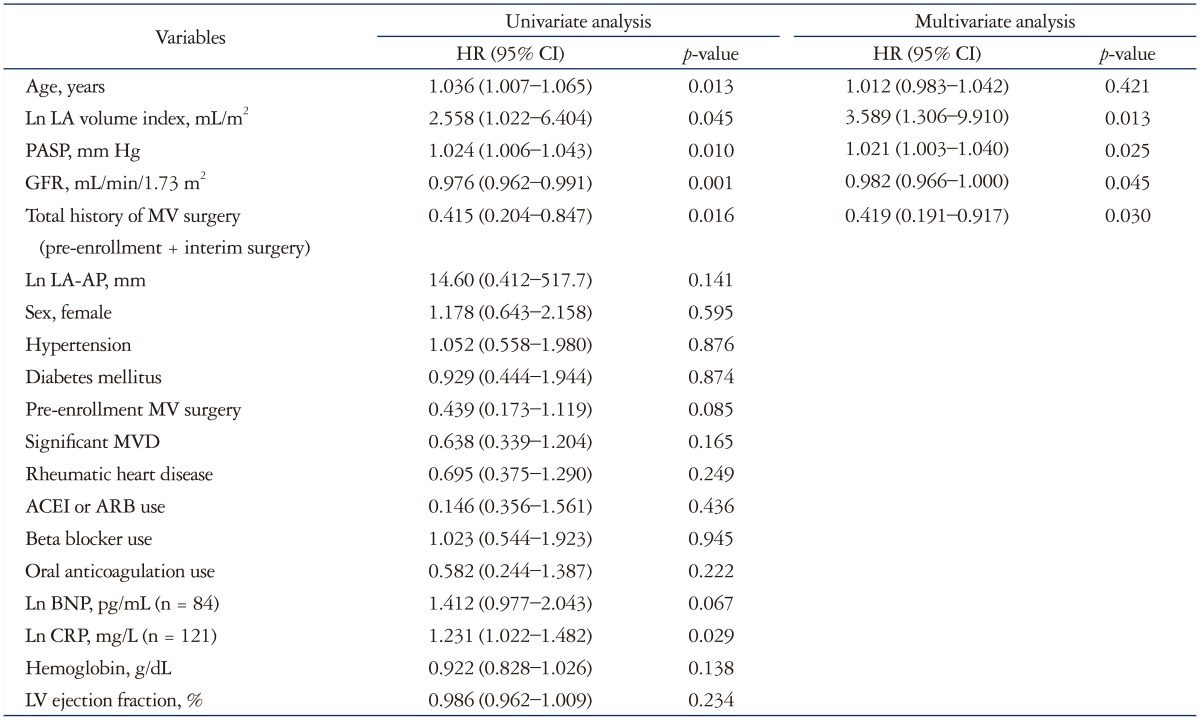
Median duration of follow-up was 22 months. During the follow-up period, 42 cases (26%) underwent composite events. Of these 42 cases, 18 patients were admitted due to heart failure (5 patients finally died), eight patients due to CVA (one patient died), one patient due to acute myocardial infarction (this patient died), while the death of the remaining 15 patients was classified as due to all-cause death. A total of 22 patients (13.5%) therefore died. During follow-up period 26 patients underwent interim MV surgery. Non-MVD patients, mainly those with LV systolic and diastolic dysfunction, had a tendency to have more composite events than MVD patients, but this difference was not statistically significant. The different components of all-cause death and heart failure readmission did not differ significantly between the MVD and non-MVD groups. Previous history of MV surgery or interim MV surgery was not significantly related to composite primary end-points, individually (all p > 0.05). However, any history of MV surgery, when combined both pre-enrollment and interim MV surgery, was related to lower incidence of composite primary end-points (hazard ratio 0.42, 95% CI 0.20–0.85, p = 0.016). In addition, there was no significant difference in clinical outcomes between RHD and non-RHD groups. In univariate analysis, age, LAVI, eGFR, PASP, and Ln CRP was related to composite end-points. In multivariate analysis, any history of MV surgery, eGFR, PASP, and LAVI were independently related to composite end-points (Table 3). LA diameters, however, were not significantly related to composite end-points (AP, p = 0.141; ML, p = 0.259; and SI, p = 0.142).
Table 3. Univariate and multivariate analyses for composite end-points.
HR: hazard ratio, LA: left atrial, PASP: pulmonary arterial systolic pressure, GFR: glomerular filtration rate, MVD: mitral valvular disease, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, Ln: natural logarithm, CRP: C-reactive protein, BNP: brain natriuretic peptide, LV: left ventricular
Discussion
Main finding of this study is that although rheumatic MVD with atrial fibrillation is a major contributor to GLA, other diseases like functional MR and long-standing diastolic dysfunction combined with atrial fibrillation can result in excessive LA enlargement. The event rate during follow-up of 5 years, reached 26%, which is not negligible percentage regardless of underlying causes. No history of MV surgery, elevated PASP and increased LA volume index are independent predictors of future events within this group of patients. Therefore, in patients with GLA, accurate measurement of LA volume rather than just diameter is crucial for risk stratification for future events, regardless of underlying disease.
Possible mechanisms of excessive LA remodeling
In addition to the classic concept of long-standing pressure or volume overload, direct rheumatic involvement14),15) in the left atrium would provide a structural substrate for excessive LA enlargement, sometimes called LA aneurysm. Previous studies showed that pathologic changes of the left atrium differed between RHD and non-RHD groups; there was a higher prevalence of fibrosis, post-inflammatory changes, Aschoff body, and higher expression of inflammatory markers in the RHD group than the non-RHD group.2),16) Rheumatic pancarditis not only causes MV regurgitation, but also damages the entire heart by weakening its tissues.7) A diseased LA wall is therefore primed to dilate, especially when significant valve regurgitation co-exists. In our study, patients with underlying RHD had a significantly larger left atrium than non-RHD patients, which supports role of rheumatic involvement in left atrium to make GLA. Another interesting finding in our study is that even in the absence of significant MR, GLA developed due to advanced diastolic dysfunction such as hypertrophic cardiomyopathy and constrictive pericarditis. This observation is contrary to classical concepts that rheumatic MVD is a unique determinant of excessive LA remodeling. Moreover, the duration of pressure overload to the LA was usually shorter in these diastolic dysfunctional patients than rheumatic MVD patients. As a recent study showed, some genetic mutations may be related to LA size, or some genetic or inherent background could predispose to excessive remodeling or aneurysmal change of the left atrium.17)
Prognostic importance of LA volume within GLA
GLA can directly compress adjacent bronchopulmonary structures,18) restrict LV movement,19) result in thrombus formation even in patients taking anticoagulation medicines, and/or elevate pulmonary venous pressure as a reflection of long-standing LV diastolic dysfunction. The mean 2- or 3-year mortality rate was as high as 14%, and the all-event rate was 26%. An interesting finding is that the dogma of “the bigger the worse”5) can be applied even at the stage of excessive remodeling or aneurysmal changes of the left atrium. Some study results also support favorable effects of LA volume reduction procedure for the prognosis.7) Although causality is not certain, history of MV surgery was related to better prognosis in our study, it might suggest MV surgery improve the prognosis even in the stage of giant LA. However, this refers to LA volume rather than directional LA dimensions. In our study, LA-AP diameter was modestly but significantly correlated with LA volume. This could be due to asymmetric expansion during the remodeling process, especially greater expansion in the ML direction than the AP direction due to limited mediastinal space. Considering this, we recommend actual 3D-based measurements in cases with excessive LA remodeling.20) LA volume measurements based on echo data have some limitations due to assumption by methods such as the prolate-ellipsoidal method,11) area-length method, and biplane Simpsons' method9) that the LA is oval-shaped. We therefore argue that cardiac computed tomography or cardiac magnetic resonance imaging would provide more reliable measurement than echo data in cases with GLA.21)
Limitations of this study include its retrospective nature, use of an echo-database from single tertiary referral hospital, and a heterogeneous patient group. Furthermore, it was not possible to control for all related prognostic factors.
Acknowledgements
This study was funded by a grant from The Korean Society of Echocardiography.
References
- 1.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 2.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–765. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 3.Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, Dahlöf B, Devereux RB. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for end-point reduction in hypertension trial. Hypertension. 2007;49:311–316. doi: 10.1161/01.HYP.0000254322.96189.85. [DOI] [PubMed] [Google Scholar]
- 4.Manning WJ, Gelfand EV. Left atrial size and postoperative atrial fibrillation: the volume of evidence suggests it is time to break an old habit. J Am Coll Cardiol. 2006;48:787–789. doi: 10.1016/j.jacc.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 6.El Maghraby A, Hajar R. Giant left atrium: a review. Heart Views. 2012;13:46–52. doi: 10.4103/1995-705X.99227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolakis E, Shuhaiber JH. The surgical management of giant left atrium. Eur J Cardiothorac Surg. 2008;33:182–190. doi: 10.1016/j.ejcts.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Oh JK. Echocardiographic evaluation of morphological and hemodynamic significance of giant left atrium. An important lesson. Circulation. 1992;86:328–330. doi: 10.1161/01.cir.86.1.328. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 10.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging. 2009;2:282–289. doi: 10.1161/CIRCIMAGING.108.826602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, Schneck SL, Tyler R, Khandheria BK, Lester SJ. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9:351–355. doi: 10.1016/j.euje.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation. 2012;126:2005–2017. doi: 10.1161/CIRCULATIONAHA.112.121590. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 14.Plaschkes J, Borman JB, Merin G, Milwidsky H. Giant left atrium in rheumatic heart disease: a report of 18 cases treated by mitral valve replacement. Ann Surg. 1971;174:194–201. doi: 10.1097/00000658-197108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson J, Danielson GK, MacVaugh H, 3rd, Joyner CR. Plication of the giant left atrium at operation for severe mitral regurgitation. Surgery. 1967;61:118–121. [PubMed] [Google Scholar]
- 16.Qian Y, Meng J, Tang H, Yang G, Deng Y, Wei D, Xiang B, Xiao X. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010;12:371–377. doi: 10.1093/europace/eup438. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Di Tullio MR, Beecham A, Slifer S, Rundek T, Homma S, Blanton SH, Sacco RL. A comprehensive genetic study on left atrium size in Caribbean Hispanics identifies potential candidate genes in 17p10. Circ Cardiovasc Genet. 2010;3:386–392. doi: 10.1161/CIRCGENETICS.110.938381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda H, Nakao M, Nohara H, Higami T, Mukohara N, Asada T, Ogawa K, Kawamura T. [The causes of prolonged postoperative respiratory care in mitral valve disease with a giant left atrium] Kyobu Geka. 1990;43:172–177. [PubMed] [Google Scholar]
- 19.Kawazoe K, Beppu S, Takahara Y, Nakajima N, Tanaka K, Ichihashi K, Fujita T, Manabe H. Surgical treatment of giant left atrium combined with mitral valvular disease. Plication procedure for reduction of compression to the left ventricle, bronchus, and pulmonary parenchyma. J Thorac Cardiovasc Surg. 1983;85:885–892. [PubMed] [Google Scholar]
- 20.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 21.Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, Truong QA. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3:80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]