Abstract
AIM
To test whether a delayed and short course of rapamycin would induce immunosuppressive effects following allogeneic orthotopic liver transplantation (OLT) in rats.
METHODS
Allogeneic OLTs were performed using Dark Agouti livers transplanted into Lewis recipients, and syngeneic OLTs were performed using the Lewis rat strain. Rapamycin (1 mg/kg per day) was administered by gavage from day 4 to day 11 post-transplantation. Lymphocyte cellular compartments were analyzed by flow cytometry in draining lymph nodes, non-draining lymph nodes and the spleen at days 11 and 42 in rapamycin-treated rats, untreated control rats and syngeneic grafted rats. Skin grafts from Dark agouti or from F344 RT were performed at day 30 on liver grafted rats treated with rapamycin.
RESULTS
An 8-d course of rapamycin treatment initiated 4 d following transplantation resulted in the survival of grafted rats for more than 100 d. In contrast, untreated rats died of liver failure within 13 to 21 d. The analysis of the cellular compartment revealed an increase in two cellular subpopulations, specifically myeloid-derived suppressor cells (MDSCs) and CD8+CD45RClow T cells, without major modifications in the regulatory T cell (Treg) compartment in treated rats in the early stages after grafting. We evaluated the ability of treated rats to reject third-party allogeneic skin grafts to confirm their immune competence. In contrast, when skin was collected from rats syngeneic to the grafted liver, it was not rejected.
CONCLUSION
Our results demonstrate that short and delayed rapamycin treatment allows for tolerance in allogeneic OLT. The results also allowed for the identification of the mechanisms of tolerance induced by rapamycin by identifying MDSCs and CD8+CD45RClow T cells as associated with the state of tolerance.
Keywords: Myeloid-derived suppressor cells, Liver transplantation, Rapamycin, Regulatory T cells
Core tip: Rapamycin is an immunosuppressive drug that is rarely used for liver transplantation treatment due to its side effects. Here, we show that a delayed and short course of rapamycin initiated on day 4 following allogeneic orthotopic liver transplantation in rats resulted in the survival of grafted rats for more than 100 d. The analysis of the cellular compartment revealed an increase in two cellular subpopulations, specifically myeloid-derived suppressor cells and CD8+CD45RClow T cells. The treated liver grafted-rats rejected third-party allogeneic skin grafts, but tolerated skin from syngeneic liver donor rats. Our results identify one of the mechanisms by which the state of tolerance is established.
INTRODUCTION
Current immunosuppression treatments following solid organ transplantation are based on calcineurin inhibitors (CNIs). However, prolonged CNI exposure leads to nephrotoxicity[1], neurotoxicity[2], an increased risk of cancer[3], and hypertension[4], all of which are associated with long-term morbidity and mortality. Reducing the use of CNIs by combining or replacing them with immunosuppressive drugs that exert different mechanisms of action remains the primary strategy to lower the incidence of these adverse events. The drug rapamycin, a mammalian target of the rapamycin (mTOR) inhibitor, is a potential alternative due to its strong immunosuppressive properties in humans.
Rapamycin mediates immunosuppressive effects by preventing T cell cycle progression from the G1 to the S phase, thereby blocking T cell proliferation[5]. Rapamycin also acts on dendritic cells (DCs) by inhibiting their capacity to differentiate from immature into mature DCs, thus reducing their ability to activate T cells. In turn, immature DCs promote the expansion of CD4+CD25+Foxp3+ regulatory T cells (Tregs) while concomitantly inducing T cell anergy and apoptosis and promoting graft tolerance[6]. In a model of experimental graft-vs-host disease facilitating the detection of donor-derived luciferase-labeled conventional T cells in vivo, the combination of Tregs and CNIs, but not rapamycin, suppresses Treg function[7]. In an allogeneic transplantation model, myeloid-derived suppressor cells (MDSCs) can also be recruited during mTOR inhibitor treatment[8]. Furthermore, following the activation of nitric oxide synthase (iNOS) and arginase (Arg1), MDSCs inhibit T cell proliferation and induce their apoptosis[9].
Currently, rapamycin is widely used to prevent rejection following renal[10], liver[11], and heart[12] transplantation. However, its use is primarily restricted to renal transplantation and is not recommended as a first-line treatment for liver transplantation due to deleterious side effects such as delayed wound-healing processes[13], thrombosis of the hepatic artery inducing graft loss, and increased mortality[14,15]. In liver transplantation, previous clinical trials have successfully demonstrated the possibility to introduce mTor inhibitors at day 30 in combination with reduced CNI treatments[16]. Here, we evaluated the possibility of using the mTor inhibitor alone in order to avoid CNI side effects in patients. Therefore, we tested a novel delayed and shortened rapamycin administration protocol in a model of orthotopic liver transplantation rejection in rats.
MATERIALS AND METHODS
Animals
Inbred Lewis RT1l male (LEW) and Dark Agouti RT1av1 male (DA) rats weighing 220 to 280 g were purchased from Janvier Labs (Le Genest-Saint-Isle, France) and F344 RT1v male rats weighing 220 to 280 g were purchased from Charles River Labs (L’Arbresle, France). The rats were maintained in animal facilities for at least one week prior to surgery under standard conditions. All experimental protocols were performed according to European Union guidelines and with the approval of the Regional Ethics Committee in Animal Experimentation no. 16, Ile-de-France, France (authorization no.11/12/12-11B).
Orthotopic liver transplantation
Non-arterialized OLT was performed according to Kamada’s technique[17]. The allografts were transplanted orthotopically. Vena porta clamping ranged from 19-23 min. Allogeneic OLTs were performed by grafting a DA liver (donor) into a LEW rat (recipient). Syngeneic OLTs were performed with LEW rats (donor and recipient).
Treatment
Rapamycin[18] was administered by oral gavage (1 mg/kg body weight per day) every day starting on day 4 after transplantation until day 11. Non-draining lymph node (non-dLN; axillary), dLN (periportal), spleen, and liver samples were collected for analysis on day 11 and day 42 after sacrifice. The residual concentration of rapamycin was analyzed in naive and liver-grafted rats after the administration of rapamycin for 8 d. Whole blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes for chromatographic analysis on days 0, 4, 9 and 11.
Flow cytometry
Cell suspensions obtained by mechanical shredding (draining lymph nodes, not draining lymph nodes and the spleen) were used for phenotypic analysis. Anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-CTLA-4, anti-CD62L, anti-CD278 (ICOS), anti-CD11b/c, anti CD161, and anti-MHC-II, anti-Foxp3, anti-IFNγ, anti-TNFα labelled with phycoerythrin (PE), phycoerythrin cyanine 7 (PE-Cy7) fluorescein isothiocyanate (FITC), peridinin chlorophyll protein complex eFluor 710 (perCP eFluor 710), allophycocyanin (APC), and eFluor 450 were purchased from ebioscience (Paris, France). Anti-CD45RC-PE was purchased from BD Biosciences and Anti-CD3-Vioblue and Helios-APC were purchased from Miltenyi Biotec (Paris, France). Events were acquired on a FACS Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR, United States) software.
Skin grafting
Skin grafting was performed according to the method of Billingham and Medawar[19]. Full-thickness tail skin (1 cm2) from two donors (DA and F344) was grafted onto the right and left dorsal thorax, respectively, of liver-grafted recipients after 30 d of tolerance (DA skin and F344 skin onto LEW rats). Rejection was defined as more than 80% graft necrosis upon daily inspection.
Histologic analysis
Grafted livers were excised, fixed in 10% formaldehyde buffer, paraffin-embedded, sectioned (5 μm) and stained with hematoxylin and eosin (H and E) according to a standard protocol. The histological grading of rejection was performed based on the BANFF classification[20].
Serum biochemical testing
Serum samples were stored at -20 °C until the analysis was performed. Transaminase enzyme and total bilirubin levels were measured to evaluate hepatocellular injury.
Statistical analysis
Differences between the groups were evaluated using an unpaired-t-test performed with Prism software 5.0 (Graph Pad Software, Inc., La Jolla, CA, United States). The results were considered statistically significant when P < 0.05.
RESULTS
Short and delayed rapamycin treatment prevents the rejection of allogeneic orthotopic liver transplantation
In this study, we used a model of DA liver transplantation in LEW recipient rats. These two rat strains differ in their major histocompatibility antigens. Allogeneic transplantation resulted in the death of all untreated rats within 13 to 21 d due to allogeneic liver rejection (Figure 1A). Four grafted rats were sacrificed for histological examination of their transplanted livers on day 11 and exhibited the characteristic signs of acute rejection according to the BANFF classification (mean score = 8.75 ± 0.25, Figure 1B and C). Next, we evaluated whether delayed rapamycin treatment exerts immunosuppressive effects. Four rats received rapamycin at a dose of 1 mg/kg per day beginning on day 4 post-transplantation and continuing until day 30. Under these conditions, 3 out of 4 grafted animals survived over 100 d without any additional treatment, suggesting that delayed and long-term rapamycin treatment is effective (Figure 1A).
Figure 1.
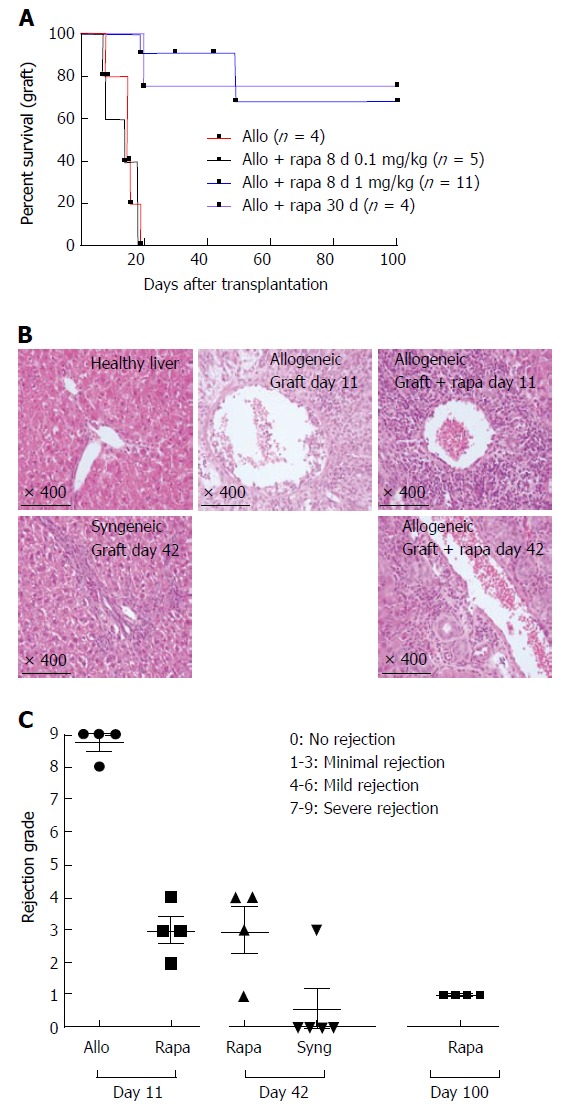
A short course of rapamycin prolongs liver transplantation in rats. A: Fully allogeneic liver transplantation (DA donor to LEW recipient) was performed, and the rats were given no treatment or were treated with rapamycin as follows: a short course at a dose of 1 mg/kg (oral gavage for 8 d from day 4 to day 11, n = 11), a short course at a dose of 0.1 mg/kg (8-d treatment from day 4 to day 11, n = 5) or a long course at a dose of 1 mg/kg (30-d treatment from day 4 to day 34, n = 4). Survival curves based on cumulative data are shown; B: H and E-stained histological sections from biopsy samples of a healthy liver and a grafted liver at the time of sacrifice on day 11 after liver transplantation (no treatment vs short course of rapamycin at 1 mg/kg) and on day 42 (short course of rapamycin at 1 mg/kg vs syngeneic); C: Histological grading of liver grafts using Banff scoring on day 11 and day 42 after transplantation, n = 4-5 rats/group.
We then tested whether a shorter course of rapamycin also leads to transplant liver maintenance. Eleven grafted rats received 1 mg/kg per day of rapamycin from day 4 to day 11 (8 d) and only 2 out of 11 rats died on days 19 and 49 (Figure 1A). To evaluate immunosuppression levels in transplanted livers, 4 rats were sacrificed on day 11 at the end of the treatment period. Compared to untreated control transplanted rats, the treated rats demonstrated a dramatic reduction in their histological rejection grade (Figure 1B and C). Four additional transplanted rapamycin-treated rats were sacrificed on day 42 (1 mo after the end of rapamycin treatment) to compare their histological scores to those observed in syngeneic liver-transplanted animals. Although significantly higher than the scores observed in rats that did not reject their syngeneic liver transplants, the histological scores of the rapamycin-protected animals remained constant between day 11 and day 42 and were dramatically reduced compared to those of the untreated, transplanted rats (Figure 1B and C). Importantly, 4 protected animals sacrificed at day 100 did not display any histological signs of liver rejection. In addition, the blood analysis of residual rapamycin levels after 48 h of treatment administered in non-grafted control rats was under the threshold of detection of our platform, but rapamycin persisted in livers on day 21 (10 d after the end of rapamycin treatment, 6.66 ± 0.44 ng/g liver). Notably, none of the treated rats demonstrated dehiscence of either the abdominal wall or the vascular anastomosis, therefore circumventing a major side effect of the treatment on wound-healing processes. We also tested a reduced dose of rapamycin, 0.1 mg/kg, administered from day 4 to day 11. Under these experimental conditions, all animals rejected their transplanted livers with the same kinetics observed in the non-treated animals (Figure 1A). Therefore, graft acceptance following delayed, short-term treatment is dose-dependent.
We next assessed hepatocellular injury by quantifying total bilirubin, ALAT and ASAT in the blood of transplanted animals administered a short course of rapamycin on days 11 and 42. On day 11, we observed a dramatic decrease in total bilirubin in the treated rats compared to the untreated control rats (15.50 ± 4.51 μmol/L vs 109.5 ± 11.43 μmol/L, respectively) and decreasing trends for ALT (147.66 ± 66.02 UI/L vs 263.25 ± 45.88 UI/L, respectively) and AST (1257 ± 240.62 UI/L vs 558.25 ± 364.01 UI/L, respectively). On day 42, transaminase levels continued to decrease in rapamycin-treated rats compared to day 11, but remained higher than in rats with syngeneic liver transplants (ALAT = 609.8 ± 133.7 UI/L vs 152 ± 61.50 UI/L, respectively; ALAT = 130 ± 22.84 UI/L vs 35.50 ± 4.36 UI/L, respectively) (Figure 2). Therefore, the relationship between these biological abnormalities and the time from treatment appears to correlate with BANFF scores.
Figure 2.
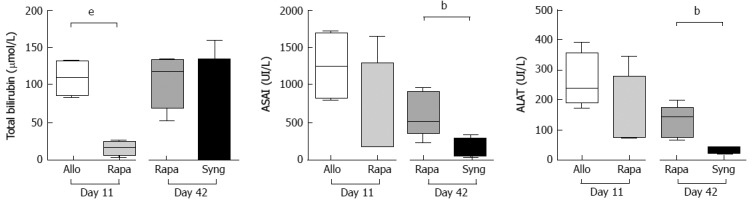
The protective effects of a short course of rapamycin on liver function. Fully allogeneic liver transplantation (DA donor to LEW recipient) was performed, and the rats were given no treatment or were treated with 1 mg/kg rapamycin (8-d treatment from day 4 to day 11). Total bilirubin, ALAT, and ASAT expression levels were measured in rat sera on postoperative days 11 (control vs rapamycin) and 42 (rapamycin vs syngeneic graft). The graph shows the cumulative data from 4-5 rats/group. P-values are indicated when the differences between the two groups of rats are significant (bP ≤ 0.01, eP ≤ 0.001). ALAT: Alanine aminotransferase; ASAT: Aspartate aminotransferase.
Based on our results, a delayed and short course of rapamycin effectively prevents allogeneic OLT rejection and correlates with low histological scores and long-term preserved liver function.
Allogeneic orthotopic liver tolerance under rapamycin treatment is associated with activated CD4+ and CD8+ regulatory T cell phenotypes and MDSC expansion
Given the in vivo effects of a delayed and short course of rapamycin in the allogeneic liver transplant model, we next sought to evaluate the involvement of different cell populations in these tolerance effects at the end of treatment and one month later.
We performed a flow cytometry analysis of the CD4+Foxp3- (CD4conv) and CD8+Foxp3- (CD8conv) T cell populations in non-dLNs and dLNs and in the spleens of grafted animals at two important time points: day 11 post-transplantation (the timing of liver rejection and the end of treatment) and day 42 (when tolerance appears to be strong in the rapamycin-treated group). We did not observe any differences in the percentage or the number of CD4conv and CD8conv T cells at day 11 or day 42 (data not shown). Although we did not observe any changes in the naïve compartment on day 11, on day 42, the percentage of naïve CD4conv (CD4+Foxp3-CD62L+CD45RC+) T cells in dLNs and non-dLNs increased in the rapamycin-treated group compared to day 11 and was statistically significantly higher compared to the rats that received syngeneic liver transplants (Figure 3A).
Figure 3.
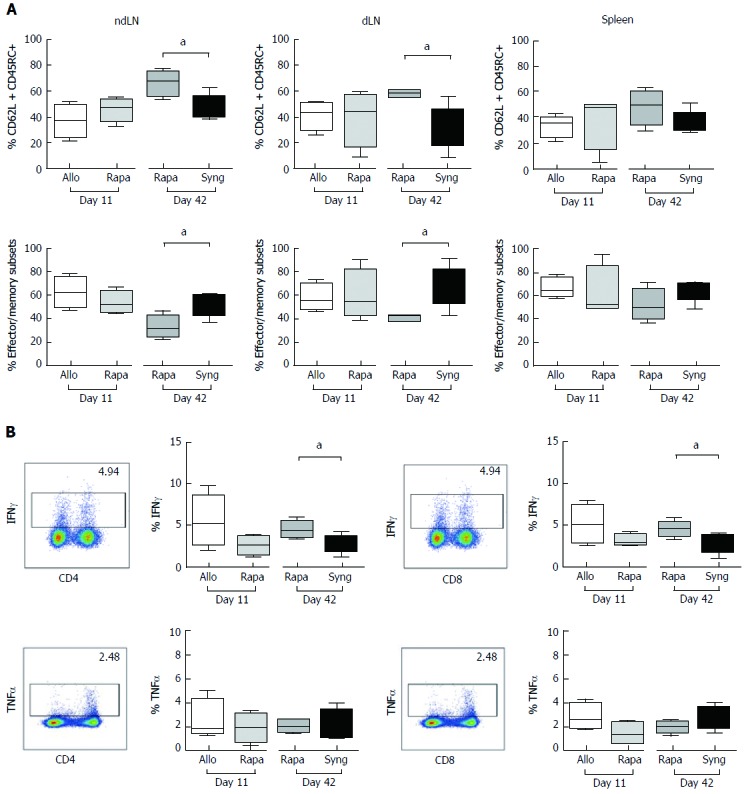
The effects of a short course of rapamycin treatment on naïve and effector memory cells (A) seems to transiently inhibit the T cell function (B). A: The ndLNs, dLNs and spleens of grafted animals were collected on day 11 and day 42 and conventional T cells were analyzed by flow cytometry. Naïve T cells, defined as CD62L+CD45RC+, and effector/memory cells, defined as (CD62L-CD45RC-) + (CD62L+CD45RC-) + (CD62L-CD45RC+), were analyzed in CD4+Foxp3- and CD8+Foxp3- T cell populations. The results are expressed as the mean +/- SEM of the percentages of effector/memory and naïve T cells, respectively. P-values are indicated when the differences between the two groups of rats are significant (aP ≤ 0.05). The graph shows the cumulative data from 4-5 rats/group; B: Quantification of IFNγ and TNFα expression by CD4+ and CD8+ T cells in the dLNs of grafted animals after PMA/ionomycin stimulation for 4 h. The graph shows the cumulative data from 4-5 rats/group. The results are expressed as the mean +/- SEM. P-values are indicated when the differences between the two groups of rats are significant (aP ≤ 0.05).
We then quantified IFNγ and TNFα production by CD4+ and CD8+ T cells in the dLNs of grafted animals. Compared to untreated rats, rapamycin reduced the expression of IFNγ in both CD4+ and CD8+ T cells on day 11 post-transplantation (CD4+ = 5.59% ± 1.15% vs 2.6% ± 0.54%, CD8+ = 5.07% ± 1.58% vs 3.13% ± 0.35%, Figure 3B), but with weak modification of the expression of TNFα. One month after the end of the treatment, IFNγ expression in CD4+ and CD8+ T cells was higher in the rapamycin-treated rats compared to the rats with syngeneic grafts, but the percentage of secreting cells remained weak. Therefore, a short course of rapamycin appears to transiently inhibit the T cell inflammatory cytokine production capacity.
To identify the cellular mechanisms associated with liver acceptance, we then analyzed both CD4+ and CD8+ Tregs in grafted animals on day 11 and day 42. No major changes in the frequency of CD4+CD25+Foxp3+ Tregs were observed in the treated rats (Figure 4A). However, rapamycin treatment induced CD25 overexpression based on the mean intensity of fluorescence (MFI) on day 11. Additionally, ICOS (MFI) overexpression in CD4+CD25+Foxp3+ Tregs observed on day 42 in rapamycin-treated rats compared to untreated rats grafted with syngeneic livers suggested the activation of CD4+ Tregs 1 mo after the end of rapamycin treatment (Figure 4A). According to previous studies, the transcription factor Helios is required to maintain regulatory T cell identity[21,22]. In our model, Helios frequency in CD4+Foxp3+ cells was higher in rapamycin-treated rats one month after the end of treatment on day 42 than in syngeneic-grafted rats, suggesting a stable regulatory profile for this transcription factor.
Figure 4.
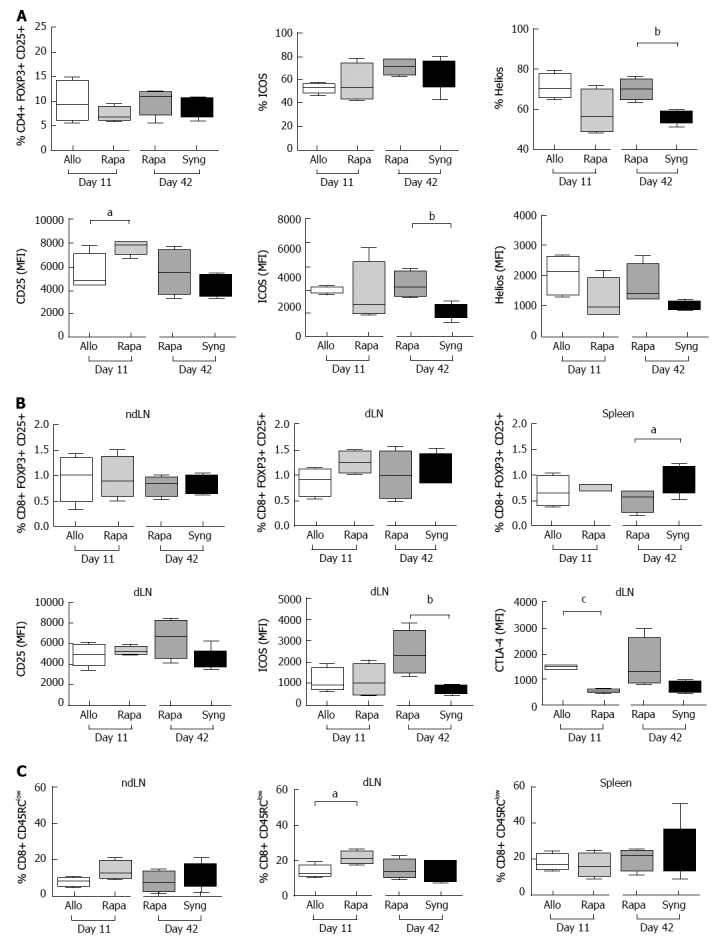
The effects of a short course of rapamycin treatment on CD4+ and CD8+ regulatory T cell phenotypes. A: The dLNs of grafted animals were collected on day 11 and day 42 and regulatory CD4+ T cells were analyzed by flow cytometry. Different activation markers were studied (CD25+, ICOS+, Helios+) after gating on CD25+FOXP3+ among CD4+ cells. The results are expressed as the mean +/- SEM of the percentages of CD25+, ICOS+, and Helios+ or the MFI values for CD25, ICOS, and Helios; n = 4-5 rats/group. P-values are indicated when the differences between the two groups of rats are significant (aP ≤ 0.05, bP ≤ 0.01). MFI, mean fluorescence intensity; B: The ndLNs, dLNs and spleens of grafted animals were collected on day 11 and day 42 and the percentages of CD25+Foxp3+ regulatory CD8+ T cells were analyzed by flow cytometry. The MFI values of the activation markers (CD25, ICOS, and CTLA-4) are shown for the dLNs. The results are expressed as the mean +/- SEM. P-values are indicated when the differences between the two groups of rats are significant (aP ≤ 0.05, bP ≤ 0.01, cP ≤ 0.0001); C: The percentages of CD45RClow cells among CD8+ cells in the dLNs, ndLNs and spleens of grafted rats are shown. The results are expressed as the mean +/- SEM. P-values are indicated when the differences between the two groups of rats are significant (aP ≤ 0.05).
CD8+Foxp3+CD25+ regulatory cells are central mediators of tolerance during allogeneic heart transplantation in rats[23]. In our model, compared to untreated rats, rapamycin-treated rats demonstrated a slight increase in CD8+Foxp3+CD25+ cells in the dLNs on day 11 and increased MFI CD25, ICOS and CTLA-4 expression in these cells on day 42, suggestive of their activated status compared to rats transplanted with syngeneic livers (Figure 4B). Additionally, CD8+CD45RClow Tregs in transplanted livers are associated with organ transplant tolerance[24]. We observed a significant increase in CD8+CD45RClow Tregs in the dLNs of rapamycin-treated rats on day 11.
Several recent studies have shown the beneficial effects of MDSCs during solid organ allograft rejection in humans and mice[25]. In our OLT model, we phenotypically characterized these myeloid cells in the spleens of grafted rats based on their expression of CD11b/c, their intermediate expression of CD161, and the lack of MCH class II protein expression. Compared to untreated control rats, rapamycin treatment increased the frequency of MDSCs in the spleen on day 11 (1.603 ± 0.302% vs 4.085 ± 0.4596%) (Figure 5). The MDSC proportion remained relatively stable on day 42 and comparable to that of MDSCs observed in the syngeneic group on day 42. Therefore, these two subpopulations (CD8+CD45RClow Tregs and MDSC) appear to be involved in tolerance during the early phase of rejection.
Figure 5.
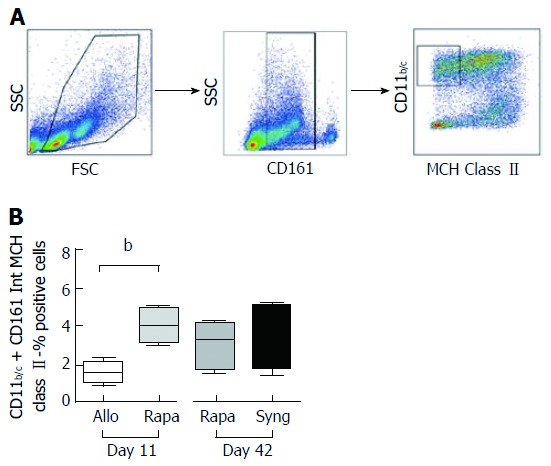
A short course of rapamycin increases myeloid-derived suppressor cells numbers on postoperative day 11 in allogeneic liver-grafted rats. The spleens of grafted animals were collected on day 11 and day 42, and myeloid-derived suppressor cells (MDSCs) were analyzed by flow cytometry. A: Gating strategy for MDSC analysis: CD11b/c+CD161intMCH class II-; B: Cumulative data indicating MDSC percentages. The results are expressed as the mean +/- SEM of the MDSC percentages. P-values are indicated when the differences between the two groups of rats are significant (bP ≤ 0.01).
A short course of rapamycin induces antigen-specific tolerance
To test whether a short course of rapamycin treatment induces donor-specific tolerance, skin allografts were performed one month after liver transplantation (20 d after the end of rapamycin treatment). We grafted donor skin collected from syngeneic liver donor rats (DA) or skin collected from third-party donor rats (neither donor nor recipient, F344) onto rapamycin-treated rats. In control LEW rats grafted with both DA and F334 skin, we observed no signs of rejection on day 7. On day 18, all skin transplants were rejected. In rapamycin-treated rats that did not reject their DA liver transplants, we observed the first signs of F344 skin rejection starting on day 7 and all skin grafts were fully rejected on day 10. Conversely, no signs of rejection were observed for DA-grafted skin on day 7 and black hairs from DA donor skin were detectable on protected animals until day 60 (Figure 6A and B).
Figure 6.
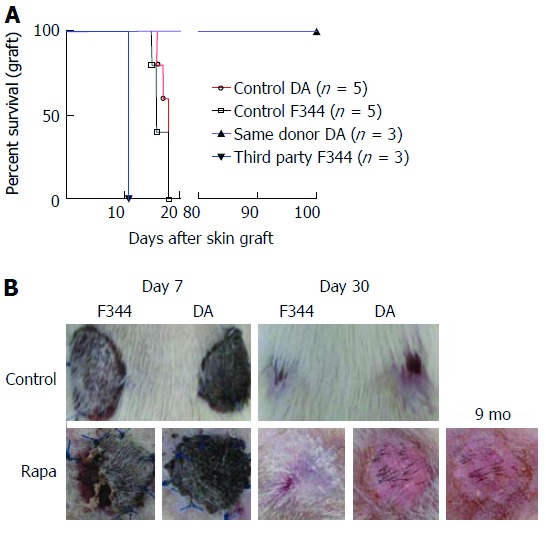
A short course of rapamycin induces antigen-specific tolerance. Allogeneic liver transplantations were performed (DA donor to LEW recipients), and the rats were treated with a short course of rapamycin (1 mg/kg, days 4 to 11). On day 30 after liver transplantation, skin grafts were performed with skin from the same DA donor or from third-party F344 rats. The control groups received allogeneic skin grafts from DA or F344 donors with rats (LEW) that did not undergo liver transplantation. A: Cumulative survival curves; days indicate the days post-skin graft, n = 3-5 rats/group; B: Representative images are shown on day 7 and day 30 after skin grafts.
DISCUSSION
Since the establishment of a small-animal liver transplant model[26], different preclinical studies have demonstrated the feasibility of reducing or even substituting immunosuppressive drugs in favor of alternative therapies[27-29] to overcome the side effects responsible for significant morbidity and mortality[15]. In this context, we were strongly invested in the development of new pharmacological strategies to induce tolerance in transplantation. Notably, we have studied different approaches based on low-dose IL-2 administration in different pathophysiological settings. In allogeneic stem cell transplantation, we observed that low dose IL-2 +/- rapamycin treatment had no effect on GVHD, particularly in a model of xeno-GVHD using human cells[30]. In contrast, a similar protocol significantly delayed allogeneic skin graft rejection in mice. At the beginning of this work, we tried to transfer this therapeutic protocol in a model of allogeneic liver transplantation to rats considered as a less immunogenic setting. Fortuitously, in a group of control rats, we were surprised to observe that a 30-d rapamycin administration initiated at day 4 was sufficient to allow for allogeneic liver acceptance. We initially decided to delay rapamycin administration to day 4 to avoid the known side effects of the molecule when used at the time of transplantation. Additionally, the elevated grade of histopathological rejection observed as soon as day 11 led us to treat the rats at day 4 to avoid the potentially irreversible rejection process if administered too late. Due to the impressive results obtained with 30 d of treatment, we tested the potential of a reduced treatment duration. In summary, we investigated a novel rapamycin administration protocol to prevent acute liver rejection. A short (8 d) and delayed (starting on day 4 after transplantation) course of rapamycin was sufficient to control acute OLT rejection in rats and induce a state of operational tolerance. Indeed, more than 75% of grafted animals treated with 1 mg/kg rapamycin via oral administration survived more than 100 d without any additional immunosuppressive treatment. This is in marked contrast to previous results and represents a dramatic improvement over a previously described protocol using the same rat strains. Indeed, the authors tested the administration of rapamycin between days 0 and 7, resulting in an MST of OLT of 24 d[31]. Our results are of significant importance because they demonstrate that delayed rapamycin treatment does not jeopardize the safety of the transplanted organ. Rapamycin can also be stopped without impairing the developed graft tolerance. These results are consistent with a previous study that tested a delayed and short-course treatment with an anti-CD3 mAb[32] to obtain strong and long-term tolerance of allogeneic cardiac allografts. Therefore, in the context of transplantation, establishing a strong but time-limited allogeneic immune response prior to the initiation of a therapeutic approach may be favorable in the establishment of sustainable and specific tolerance.
We also sought to identify the immunological mechanisms underlying this long-term tolerance obtained with rapamycin. CD4+ Tregs have been regarded as a potential therapeutic modality for the prevention and treatment of allogeneic immune responses for a long time[33]. They also demonstrate potential for the specific targeting of cells involved in the allogeneic immune response without inducing general immunosuppression. We observed the preservation of immune potential as indicated by the capacity of tolerant rats to reject allogeneic skin grafts and accept skin grafts that originated from rats with the same background as the accepted livers. We quantified Tregs contents in the dLNs of grafted animals on day 11 (at the end of the rapamycin treatment) and one month later. In contrast to the results of a previous report[34], we did not observe a major change in the percentage of Tregs. However, CD25 overexpression on day 11 and the overexpression of ICOS in CD4+ Tregs on day 42 suggested the activation of these cells in rapamycin-treated rats. We also observed an increase in CD8+Foxp3+CD25+ regulatory cells, but this increase was only present on day 11 and only in the spleen. At the same time point, CD8+Foxp3+CD25+ Tregs overexpressed CD25, ICOS and CTLA4 in the dLNs of rapamycin-treated animals compared to syngeneic grafted rats, again suggesting an activated CD8+Foxp3+CD25+ Tregs phenotype[35]. CD8+CD45RClow Tregs[36] have also been associated with organ transplant tolerance[24,37]. We observed a significant increase in CD8+CD45RClow Tregs in the dLNs of rapamycin-treated rats on day 11. In murine cardiac allograft models, a short course of rapamycin leads to the recruitment of MDSCs and increases their expression of iNOS. According to Nakamura et al[8], even if the T cell activity is suppressed in a dose-dependent manner in vitro, monocytic MDSCs treated with rapamycin suppress CD4+ T cell proliferation more efficiently than granulocytic MDSCs treated with rapamycin. Therefore, mTOR down-regulation promotes monocytic MDSC migration in vitro and in vivo[38]. Altogether, our results suggest that MDSCs and CD8+CD45RClow Tregs are involved in the early stages of tolerance initiation followed by sustained tolerance that involves both CD4+ and CD8+Foxp3+CD25+ Tregs.
In summary, our results provide new perspectives on research regarding the use of rapamycin in OLT. Notably, the precise mechanisms of tolerance remain to be identified by sequential depletion of the different regulatory cell subtypes to confirm their potential roles in graft acceptance. Additionally, if rapamycin should be reintroduced early in the therapeutic arsenal for the prevention of liver transplant rejection in humans, several steps must be taken. First, the main concern in using early rapamycin involves hepatic artery thrombosis. Consequently, the efficacy of our protocol remains to be tested in an additional model of OLT with consideration of this central parameter in a large animal model for example. Finally, the compatibility of early rapamycin administration should be evaluated with the gold standard of liver rejection prevention that relies on FK506 or CNI administration. Such a comparative study deserves specific experiments that would allow for a direct comparison of the clinical effects of these two type of molecules and also for the identification of the mechanisms of tolerance that take place depending on the molecule used. This information would even support therapeutic decisions consistent with the mechanisms of tolerance identified.
ACKNOWLEDGMENTS
We want to acknowledge the staff of our animal facility platform (EFPA) for their excellent animal care. We are also indebted to France Noizat-Pirenne and Etablissement Français du Sang for access to their cytometry platform.
COMMENTS
Background
Solid organ transplantation remains the last option for vital organ failure. Even though the control of the immune response in the recipient is now better controlled, the side effects of the immunosuppressive approach are still present and the therapeutic approach needs perpetual improvement. Current immunosuppression treatments following solid organ transplantation are based on calcineurin inhibitors (CNIs). However, reducing the use of CNIs by combining or replacing them with immunosuppressive drugs that exert different mechanisms of action remains the primary strategy to lower the incidence of adverse events.
Research frontiers
The main challenge in transplantation is to identify therapeutic protocols that allow for organ specific tolerance with reduced or absent side effects.
Innovations and breakthroughs
Our data revealed that (1) rapamycin as mono-therapy allows for long-term allogeneic liver protection in rats; (2) this treatment was slightly delayed as opposed to rats treated at day 0; and (3) an 8-d treatment was as efficient as a 3-wk treatment to induce long-term tolerance. We also identified two populations of immune cells (MDSC and CD8+CD45low) that were increased in tolerant rats. These results reveal notable new perspectives by identifying the precise mechanisms at the origin of this state of tolerance.
Applications
These results support the development of similar therapeutic approaches in large animal models.
Terminology
Allogeneic orthotopic liver transplantation (OLT) is performed with a liver from a donor with a different genetic background from the recipient. A syngeneic graft is performed within the same genetic background.
Peer-review
An interesting and readable manuscript to explore a delayed and short course of rapamycin initiated on day 4 following allogeneic OLT in rats resulted in the survival of grafted rats for more than 100 d. This study is very clear, and the manuscript is well written. The main flaws of the study are its limited translational components.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Regional Ethics Committee in Animal experimentation no16, Ile de France, France (authorization no.11/12/12-10B).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 3, 2017
First decision: March16, 2017
Article in press: June 1, 2017
P- Reviewer: Chiu KW, Demirag A, Rodriguez-Peralvarez ML
S- Editor: Gong ZM L- Editor: A E- Editor: Li D
Contributor Information
Salim Hamdani, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, F-94000 Créteil, France.
Allan Thiolat, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, F-94000 Créteil, France.
Sina Naserian, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, F-94000 Créteil, France.
Cynthia Grondin, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, F-94000 Créteil, France.
Stéphane Moutereau, AP-HP, Laboratoire de Biochimie, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
Anne Hulin, AP-HP, Laboratoire de Pharmacologie-Toxicologie Biologiques, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
Julien Calderaro, AP-HP, Anatomie et Cytologie Pathologique, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
Philippe Grimbert, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, APHP, Inserm, CIC Biothérapie, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
José Laurent Cohen, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, APHP, Inserm, CIC Biothérapie, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
Daniel Azoulay, APHP, Service de Chirurgie HPB et Transplantation Hépatique, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France; Department of Hepato-Pancreato-Biliary Surgery and Liver Transplantation, Henri Mondor Hospital, 94010 Créteil, France. daniel.azoulay@aphp.fr.
Caroline Pilon, Université Paris-Est, UMR_S955, UPEC, Inserm, U955, Equipe 21, APHP, Inserm, CIC Biothérapie, Groupe Hospitalier Henri-Mondor Albert-Chenevier, F-94000 Créteil, France.
References
- 1.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246:339–346. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 3.Tjon AS, Sint Nicolaas J, Kwekkeboom J, de Man RA, Kazemier G, Tilanus HW, Hansen BE, van der Laan LJ, Tha-In T, Metselaar HJ. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl. 2010;16:837–846. doi: 10.1002/lt.22064. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648–1654. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 5.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura T, Nakao T, Yoshimura N, Ashihara E. Rapamycin Prolongs Cardiac Allograft Survival in a Mouse Model by Inducing Myeloid-Derived Suppressor Cells. Am J Transplant. 2015;15:2364–2377. doi: 10.1111/ajt.13276. [DOI] [PubMed] [Google Scholar]
- 9.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–280. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 11.Glover TE, Watson CJ, Gibbs P, Bradley JA, Ntzani EE, Kosmoliaptsis V. Conversion From Calcineurin to Mammalian Target of Rapamycin Inhibitors in Liver Transplantation: A Meta-Analysis of Randomized Controlled Trials. Transplantation. 2016;100:621–629. doi: 10.1097/TP.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y, Wang X, Fan J, Rao Z, Lu Y, Lin T. Conversion From Calcineurin Inhibitors to Mammalian Target-of-Rapamycin Inhibitors in Heart Transplant Recipients: A Meta-Analysis of Randomized Controlled Trials. Transplant Proc. 2015;47:2952–2956. doi: 10.1016/j.transproceed.2015.09.059. [DOI] [PubMed] [Google Scholar]
- 13.Mehrabi A, Fonouni H, Wente M, Sadeghi M, Eisenbach C, Encke J, Schmied BM, Libicher M, Zeier M, Weitz J, et al. Wound complications following kidney and liver transplantation. Clin Transplant. 2006;20 Suppl 17:97–110. doi: 10.1111/j.1399-0012.2006.00608.x. [DOI] [PubMed] [Google Scholar]
- 14.Moreno R, Berenguer M. Post-liver transplantation medical complications. Ann Hepatol. 2006;5:77–85. [PubMed] [Google Scholar]
- 15.Romero FA, Razonable RR. Infections in liver transplant recipients. World J Hepatol. 2011;3:83–92. doi: 10.4254/wjh.v3.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 18.Stepkowski SM. Preclinical results of sirolimus treatment in transplant models. Transplant Proc. 2003;35:219S–226S. doi: 10.1016/s0041-1345(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 19.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385–402. [Google Scholar]
- 20.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci USA. 2016;113:6248–6253. doi: 10.1073/pnas.1604765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Liu Z, Witkowski P, Vlad G, Manavalan JS, Scotto L, Kim-Schulze S, Cortesini R, Hardy MA, Suciu-Foca N. Rat CD8+ FOXP3+ T suppressor cells mediate tolerance to allogeneic heart transplants, inducing PIR-B in APC and rendering the graft invulnerable to rejection. Transpl Immunol. 2004;13:239–247. doi: 10.1016/j.trim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Miyanari N, Ichiguchi O, Akizuki E, Matsumura F, Matsuda T, Okabe K, Liang J, Ohshiro H, Mori K, et al. Infiltrating CD45RC- T cells are associated with immunologic unresponsiveness induced by donor class I major histocompatibility complex antigens in rats. Hepatology. 1998;28:450–458. doi: 10.1002/hep.510280224. [DOI] [PubMed] [Google Scholar]
- 25.Thomson AW, Turnquist HR. Regulators with potential: substantiating myeloid-derived suppressor cells in human organ transplantation. Am J Transplant. 2013;13:3061–3062. doi: 10.1111/ajt.12464. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Charters AC, Chandler JG, Orloff MJ. A technique for orthotopic liver transplantation in the rat. Transplantation. 1973;16:664–669. doi: 10.1097/00007890-197312000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Wang GY, Zhang Q, Yang Y, Chen WJ, Liu W, Jiang N, Chen GH. Rapamycin combined with allogenic immature dendritic cells selectively expands CD4+CD25+Foxp3+ regulatory T cells in rats. Hepatobiliary Pancreat Dis Int. 2012;11:203–208. doi: 10.1016/s1499-3872(12)60149-0. [DOI] [PubMed] [Google Scholar]
- 28.Fujiki M, Esquivel CO, Martinez OM, Strober S, Uemoto S, Krams SM. Induced tolerance to rat liver allografts involves the apoptosis of intragraft T cells and the generation of CD4(+)CD25(+)FoxP3(+) T regulatory cells. Liver Transpl. 2010;16:147–154. doi: 10.1002/lt.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng JF, Chen F, Liu H, Liu J. Induction of immune tolerance by pre-infusion of apoptotic lymphocytes derived from peripheral blood of donor rats before liver transplantation. Minerva Chir. 2013;68:183–189. [PubMed] [Google Scholar]
- 30.Pérol L, Martin GH, Maury S, Cohen JL, Piaggio E. Potential limitations of IL-2 administration for the treatment of experimental acute graft-versus-host disease. Immunol Lett. 2014;162:173–184. doi: 10.1016/j.imlet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura N, Ohsaka Y, Hamashima T, Yasui H, Yura H, Kobayashi Y, Shiho O, Oka T. Rapamycin in experimental liver transplantation in the rat. Transplant Proc. 1996;28:1796–1797. [PubMed] [Google Scholar]
- 32.Goto R, You S, Zaitsu M, Chatenoud L, Wood KJ. Delayed anti-CD3 therapy results in depletion of alloreactive T cells and the dominance of Foxp3+ CD4+ graft infiltrating cells. Am J Transplant. 2013;13:1655–1664. doi: 10.1111/ajt.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballarin R, Spaggiari M, Di Benedetto F, De Ruvo N, Cautero N, Montalti R, Guerrini GP, Longo C, Mimmo A, D’Amico G, et al. [Is there an age limit for radical surgery in case of tumors infiltrating the duodenum?] Minerva Chir. 2010;65:1–9. [PubMed] [Google Scholar]
- 34.Tian L, Lu L, Yuan Z, Lamb JR, Tam PK. Acceleration of apoptosis in CD4+CD8+ thymocytes by rapamycin accompanied by increased CD4+CD25+ T cells in the periphery. Transplantation. 2004;77:183–189. doi: 10.1097/01.TP.0000101005.44661.3E. [DOI] [PubMed] [Google Scholar]
- 35.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 36.Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, Gonzalez-Dunia D, Saoudi A. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–3301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 37.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Hervé C, Li XL, Heslan M, Usal C, Tesson L, Ménoret S, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1096–1106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Bi Y, Yang H, Chen X, Liu H, Lu Y, Zhang Z, Liao J, Yang S, Chu Y, et al. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid-derived suppressor cells in protecting against murine immunological hepatic injury. J Leukoc Biol. 2014;95:961–970. doi: 10.1189/jlb.0913473. [DOI] [PubMed] [Google Scholar]


