Abstract
AIM
To investigate the expression of annexin II in gastric carcinoma and its role in the metastasis of gastric cancer.
METHODS
The expression of annexin II in 51 cases of gastric carcinoma and 24 cases of adjacent tissues was detected by immunohistochemistry. The relationship between annexin II and clinical features of gastric cancer was analyzed. Annexin II specific siRNA was used to inhibit the expression of annexin II in gastric cancer HGC-27 cells, and the effects of annexin II on the migration and secretion of matrix metalloproteinases (MMPs) were observed.
RESULTS
The positive rate of annexin II protein was 82.4% in gastric cancer tissues and 37.5% in adjacent tissues. There was significant difference between the two groups (P < 0.01); and the positive expression of annexin II was not related to the sex and age of the patients (P > 0.05). The expression of annexin II protein was correlated with tumor size, histological differentiation, TNM stage, Lymph node metastasis and other clinical features were significantly correlated, the difference was statistically significant (P < 0.05). Inhibition of annexin II expression, gastric cancer HGC-27 cells migration and secretion of MMPs were significantly decreased, the difference was statistically significant (P < 0.05).
CONCLUSION
Annexin II is highly expressed in gastric cancer tissues, annexin II protein expression is related to tumor size, histological differentiation, TNM staging, lymph node metastasis and other clinical features were significantly correlated. Annexin II high expression can promote the invasion and metastasis of gastric cancer.
Keywords: Gastric cancer, Annexin II, Metastasis, Real-time quantitative PCR, Human gastric cancer, HGC-27 cells, Matrix metalloproteinases
Core tip: In this study, we retrospectively analyzed the expression of annexin II in 51 cases of gastric carcinoma and 24 cases of adjacent tissue to investigate the relationship between the positive expression of annexin II and the invasion and metastasis of gastric cancer.
INTRODUCTION
Gastric cancer is one of the most common malignancies in my country, the first in the digestive tract malignant tumor, ranking the fourth in the world[1], Because of its symptoms hidden, the disease developed rapidly, when diagnosed in the late, the treatment effect is often poor, a serious threat to human health, but the early treatment of gastric cancer is good, 5 years after radical resection rate of up to 90% or more, So in-depth study of the occurrence and development of gastric cancer mechanism, For the prognosis of gastric cancer and the treatment of gastric cancer has a very important clinical significance.
Annexin II is a calcium-dependent phospholipid-binding protein, Mainly in the form of monomer, dimer and tetramer, the study shows[2-6]: Annexin II are highly expressed in liver cancer, breast cancer, colon cancer, lung cancer and other malignant tumors, And play an important role in the occurence, development and infiltration of tumor. In this study, the expression of annexin II in 51 cases of gastric carcinoma and 24 cases of adjacent tissues was detected by immunohistochemistry, to investigate the relationship between the positive expression of annexin II and the clinical characteristics of gastric cancer. And annexin II specific siRNA was used to inhibit the expression of annexin II in gastric cancer HGC-27 cells, Detection of cell adhesion, invasion, migration, matrix metalloproteinases (MMPs) secretion and other capacity changes.
MATERIALS AND METHODS
Materials
Fifty-one cases of surgical specimens of gastric cancer were collected during the period from January 2012 to June 2014, among them, 30 males and 21 females; aged 32-78 years, mean age 58.6 ± 3.2 years; the above cases were not treated before radiotherapy, chemotherapy and other anti-tumor therapy. From the above 51 cases of gastric cancer, twenty-four patients were randomly selected from the normal tissue of the stomach. All specimens were fixed with formaldehyde, Paraffin embedded, sliced. Gastric cancer HGC-27 cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology. Study has been reviewed by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Methods
Immunohistochemical staining: Annexin II antibody was purchased from Abcam Reagent Company; Immunohistochemistry kit, DAB kit was purchased from Pikiton Reagent Company. Sliced was kept in high temperature and high pressure tissue antigen repair. The dyeing step is carried out according to the kit instructions. Positive plates were used as positive control, PBS instead of primary antibody as a negative control. The results showed that annexin II positive expression of brown matter in the cytoplasm appears brown matter. Each slice 5 representative of the high power field, Each field count 100 cells, The percentage of positive cells and the intensity of staining were scored separately. Specific criteria are as follows: (1) according to the degree of staining score: no staining for 0 points, Dyeing strength is 1 point; Medium dyeing intensity of 2 points; Strong staining intensity of 3 points. (2) Score according to the percentage of colored cells: The percentage of cells in the stained cells was < 5% for 0 points, 5%-25% for 1 points, 26%-50% for 2 points, 51%-75% for 3 points, > 75% for 4 points. (1) + (2) for the total points, The total score of 0-7 points. 0 is negative (-), 1-2 is divided into weak positive (+), 3-5 are divided into positive (++), 6-7 is divided into strong positive (+++).
Cell culture: Gastric cancer HGC-27 cells were cultured in RPMI1640 cell culture medium containing 10% fetal bovine serum and cultured in 5% CO2, 37 °C, saturated cell culture incubator.
Cell transfection: Gastric cancer HGC-27 cells (1 × 105 cells/mL) were seeded in 24-well plates, Cultured overnight at 37 °C, 5% CO2 and saturated humidity. Serum-free medium wash cells, 1 μL (20 pmol) of Annexin II siRNA was dissolved in 49 μL serum-free medium, 1 μL of liposomes was dissolved in 59 μL of serum-free medium, After keeping at room temperature for 10 min, Mix the two type of liquids, room temperature for 20 min. The mixture was then added to the 24-well plate of the cells to be transfected, Add serum-free medium to 500 μL, Placed in 37 °C, 5% CO2 cell incubator culture, 6 h later replaced with 10% newborn bovine serum RPMI1640 medium.
Real-time quantitative PCR: Use Trizol method to extract total RNA from cells, The total RNA samples of the extracted osteoblasts were reverse transcribed into cDNA, Using the following reaction system: 2 μg template RNA,1 μL oligo dT primer, 2 μL dNTP mixture,1 μL Ace reverse transcriptase,1 μL RNase inhibitor,4 μL 5 × RT buffer, 10 μL RNase-free water. The reaction conditions were as follows: first step 37 °C 30 min, The second step is 84 °C for 30 s. The reaction product was stored at 4 °C. And then real -time quantitative PCR reaction. Using the following reaction system: 12.5 μL Real Master Mix (SYBR I), 2 μL template cDNA, 1 μL forward primer, 1 μL reverse primer, 8.5 μL RNase-free water. The reaction conditions are: first step 94 °C 5 min; second step 94 °C 60 s, 57 °C 30 s, 72 °C 30 s, a total of 30 cycles; the third step 72 °C 5 min; the last 4 °C end of the reaction. The experimental data were analyzed by Option Monitor V3.1 software of real-time PCR.
Cell invasion experiment: A 1:3 diluted matrigel (RMPI 1640 dilution) was added to the matrigel invasion chamber, 37 °C for 2 h, 2 × 104 gastric cancer cells were resuspended in 0.1% fetal bovine serum culture medium and inoculated in the chamber, RMPI 1640 medium containing 10% fetal bovine serum was added to the 24-well plate of the lower chamber, 37 °C for 24 h. Remove the invasion chamber, With a cotton swab gently wipe the room did not pass through the cells, formaldehyde fixed for 10 min, Hematoxylin-eosin staining, Cut the filter, the neutral gum seal, light microscopy count the number of cells through the membrane. The number of cells in the membrane was calculated by taking 10 fields under light microscope. Each independent experiment was repeated three times.
Cell migration experiments: First with a marker pen in the 6-well plate even behind a horizontal line, Cross through the hole. About 2 × 104 gastric cancer cells were seeded in 6-well plates when the cells are completely fused, the tip of the nose is as far as possible to the horizontal line behind the horizontal scratches. Wash the cells three times with PBS, wash off the cells under the cells, and then add serum-free medium. And incubated in a CO2 incubator at 37 °C. 0 and 24 h under inverted microscope to observe and take pictures. The scribe width was measured with Image J software and the cell mobility was calculated. Cell mobility = (0 h scratch width - 24 h scratch width)/0 h scratch width × 100%. Each independent experiment was repeated three times.
Cell gelatin zymography experiments: Cells were treated with annexin II siRNA Digest the cells and count, the corresponding conditioned medium was collected, 1500 g low-speed centrifugation 10 min after removal of cell debris, Take the supernatant 4 °C spare. And then take the protein quality samples separately with non-reduction Loading Buffer Homogeneously mixed, incubated at 55 °C for 5 min. Prepare 5% concentrated gum and 8% separated gel containing 0.1% gelatin. Constant pressure electrophoresis until the edge of bromophenol blue separation from the front of the separation of about 0.5 cm. The gel was removed and transferred to the elution solution, eluting twice every 30 min. Rinse with gelatin incubation once, and then placed in gelatin incubation buffer at 37 °C for 18 h. Dyeing solution 4 h After the rinsing of distilled water, And then take a photo by a gel image analyzer.
Statistical analysis
Each independent experiment was repeated more than three times. Student-t test was performed with SPSS 16.0 software, the results are expressed as mean ± SD, qualitative data were compared using χ2 test, P < 0.05 indicating that the difference was statistically significant.
RESULTS
Expression of annexin II Protein in gastric carcinoma and its relationship with clinical characteristics of gastric cancer
Expression of AnnexinII in gastric carcinoma and adjacent tissues: Annexin II protein positive expression of cytoplasm in the yellow granular material, Mainly located in the cytoplasm (Figure 1). In 51 cases of gastric cancer, 42 cases were positive, The positive rate was 82.4% of which strong positive in 3 cases, Positive in 12 cases, Weakly positive in 27 cases, Negative in 9 cases. In 24 cases of paracancerous tissue, there were 9 positive expression, of which strong positive 0 cases, positive in 3 cases, weak positive in 6 cases, The positive rate was 37.5%. The expression of AnnexinII protein in gastric cancer tissues was significantly higher than that in adjacent tissues, Differences were significant (Table 1, P < 0.01).
Figure 1.
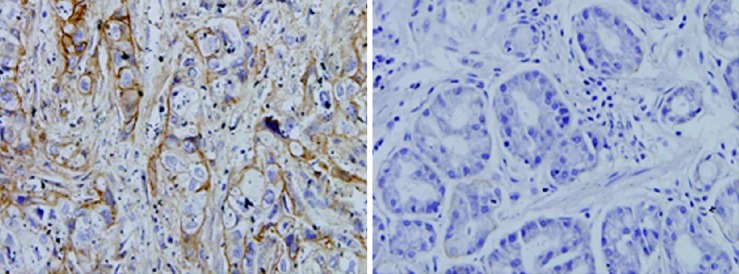
Immunohistochemistry to detect annexin II protein in gastric cancer tissues and adjacent tissues.
Table 1.
Expression of annexin II protein in gastric carcinoma and paracancerous tissues
| Group | n | Annexin II protein |
Positive rate, % | P value | |||
| - | + | ++ | +++ | ||||
| Gastric cancer | 51 | 9 | 27 | 12 | 3 | 82.4 | |
| Paracancerous | 24 | 15 | 6 | 3 | 0 | 37.5 | < 0.01 |
Expression of annexin ii protein in patients with clinical characteristics of gastric cancer: The study found, annexin II positive expression was not related to the sex of the patient (P > 0.05) while annexin II protein expression was associated with tumor size, histological differentiation, TNM staging, lymph node metastasis and other clinical features were significantly correlated, the difference was statistically significant (P < 0.05, Table 2).
Table 2.
Relationship between annexin II protein and clinical characteristics of gastric cancer patients
| Group | n | Annexin II positive | Positive rate, % | P value |
| Age in yr | ||||
| ≤ 60 | 27 | 23 | 85.2 | > 0.05 |
| > 60 | 24 | 19 | 79.2 | |
| Sex | ||||
| Male | 31 | 27 | 87.1 | > 0.05 |
| Female | 20 | 15 | 75 | |
| Tumor diameter in cm | ||||
| ≤ 5 | 19 | 12 | 63.2 | < 0.05 |
| > 5 | 32 | 30 | 93.8 | |
| Differentiation | ||||
| Well differentiated | 15 | 9 | 60 | < 0.05 |
| Poorly differentiated | 36 | 33 | 91.7 | |
| Clinical stage | ||||
| I+ II period | 17 | 10 | 58.8 | < 0.05 |
| III + IV period | 34 | 32 | 94.1 | |
| Lymph node metastasis | ||||
| Have | 33 | 31 | 93.9 | < 0.05 |
| Not have | 18 | 11 | 61.1 |
The role of annexin II in gastric cancer metastasis
SiRNA inhibits annexin II expression: The siRNA fragment targeting annexin II was transfected into human gastric cancer HGC-27 cells, the mRNA and protein expression of annexin II in human gastric cancer HGC-27 cells were detected by real time-PCR and Western blot after 24 h. And transfected unrelated interfering siRNA fragments (control siRNA) as a negative control. The results show, annexin II siRNA can significantly inhibit the transcription and protein expression of annexin II mRNA in human gastric cancer HGC-27 cells, the difference was statistically significant (P < 0.05; figure 2).
Effect of annexin II on the migration ability of gastric cancer cells: Scaling experiments were performed to detect changes in cell migration ability. Compared with the control group transfected with Control siRNA, the migration ability of human gastric cancer HGC-27 cells transfected with Annexin II siRNA was significantly decreased, differences were statistically significant (P < 0.01; Figure 3).
Figure 3.
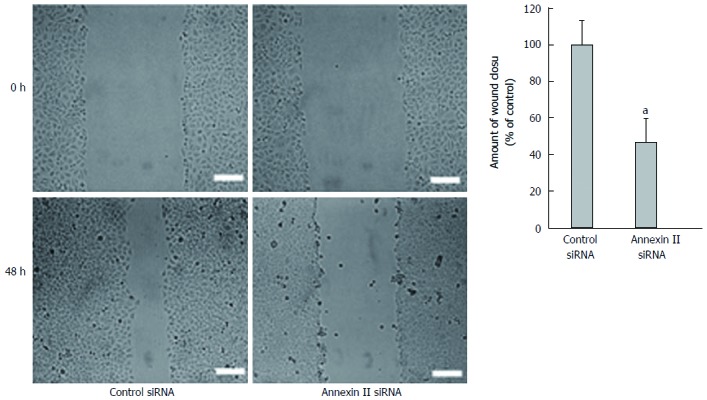
Effect of annexin II siRNA on the migration ability of human gastric cancer HGC-27 cells. aP < 0.05. siRNA: Small interfering RNA.
Effect of annexin ii on secretion of mmps in gastric cancer cells: The changes of MMPs secretion were detected by gelatin zymography. Compared with the control group transfected with Control siRNA, Human gastric cancer HGC-27 cells transfected with AnnexinII siRNAs after MMPs secretion was significantly reduced, Differences were statistically significant (P < 0.01; Figure 4).
Figure 4.
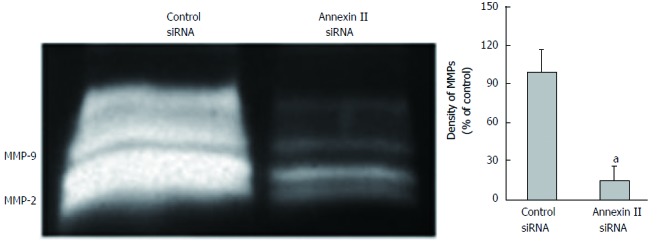
Effect of annexin II siRNA on secretion of matrix metalloproteinases in human gastric cancer HGC-27 cells. aP < 0.05. MMPs: Matrix metalloproteinases; siRNA: Small interfering RNA.
DISCUSSION
Annexins is a class of calcium-dependent phospholipid-binding proteins, which are widely present in the cell membrane, cytoplasmic or extracellular matrix. There are two basic functional domains on the structure: Highly conserved C-terminal core regions and highly variable N-terminal domains. The former has a binding site of Ca2+ and membrane, in the form of calcium-dependent reversible binding to cell membrane phospholipids, the latter determines annexins specific biological functions. Annexins participate in a series of activities of cells, such as endocytosis, exocytosis, cell proliferation, differentiation and apoptosis, and cytoskeleton activity, signal transduction and etc[7]. Annexin II as a member of the annexins family A subfamily, Also known as p36, AXA2, LIP2, etc. Its gene is located in 15q21-q22, containing 1.4 kb encoding gene[8,9].
In this study, the expression of annexin II in gastric cancer tissues and adjacent tissues was analyzed. The results showed that the expression of annexin II protein in gastric cancer tissues was significantly higher than that in adjacent tissues. To analyze the correlation between the expression of annexin II protein and the clinical characteristics of gastric cancer patients. The positive expression of annexin II was not related to the sex and age of the patients, while annexin II protein was expressed with tumor size, histological differentiation, TNM stage, lymph node metastasis and other clinical features were significantly correlated, the difference was statistically significant. The results suggest that annexin II protein is closely related to the malignant degree of gastric cancer.
Tumor metastasis is the most important malignant phenotype of the tumor and is the direct cause of this high mortality. Tumor metastasis is required in tumor cells themselves, host cells and extracellular matrix between the interaction can be completed, Involves a series of complex processes and steps, Including tumor cells from the primary tumor lesions, through the secretion of MMPs degradation of the extracellular matrix into the blood vessels or lymphatic vessels migrate to the distant, and then attack to the secondary organization or organ in the formation of metastases[10-14]. Therefore, the study of tumor metastasis for clinical diagnosis and treatment is important. To clarify whether annexin II in the process of gastric cancer metastasis play a role, In this study, annexin II-specific siRNA was used to inhibit annexin II expression in gastric cancer HGC-27 cells, after the expression of annexin II was reduced, gastric cancer HGC-27 cells migration, MMPs secretion and other capacity changes. The study found: In gastric cancer HGC-27 cells using annexin II- specific siRNA inhibited annexin II expression, gastric cancer cell migration, MMPs secretion and other capacity were reduced, suggesting that annexin II in the process of gastric cancer metastasis plays an important role.
In summary, annexin II in gastric cancer showed high expression, and the degree of differentiation of gastric cancer, TNM stage, lymph node metastasis and other clinical features are closely related, application of annexin II specific siRNA in gastric cancer HGC-27 cells inhibited annexin II expression, gastric cancer cell migration, MMPs secretion and other capacity are reduced, suggesting that annexin II plays an important role in the process of gastric cancer metastasis. The development and progression of gastric cancer is a complex multi-factor comprehensive results. At present, the etiology and pathogenesis of gastric cancer are more studied, but it is not clear. This study confirmed that annexin II was highly expressed in gastric cancer tissues and had the important role in gastric cancer metastasis. With the deepening of related research, annexin II gene expression, biological function and the role of progress in gastric cancer will be gradually clear, is expected to be used in the diagnosis and treatment of gastric cancer, predict the prognosis of markers and therapeutic targets.
COMMENTS
Background
Gastric cancer is one of the most common malignancies in my country, the first in the digestive tract malignant tumor, ranking the fourth in the world, Because of its symptoms hidden, the disease developed rapidly, when diagnosed in the late, the treatment effect is often poor, a serious threat to human health, but the early treatment of gastric cancer is good, 5 years after radical resection rate of up to 90% or more.
Research frontiers
Annexin II is a calcium-dependent phospholipid-binding protein, mainly in the form of monomer, dimer and tetramer, the study shows: Annexin II are highly expressed in liver cancer, breast cancer, colon cancer, lung cancer and other malignant tumors, and play an important role in the occurence, development and infiltration of tumor.
Innovations and breakthroughs
The authors restropectively analysed the expression of annexin II in 51 cases of gastric carcinoma and 24 cases of adjacent tissues, to investigate the relationship between the positive expression of annexin II and the invasion and metastasis of gastric cancer.
Applications
Annexin II in gastric cancer showed high expression, And the degree of differentiation of gastric cancer, TNM stage, lymph node metastasis and other clinical features are closely related, suggesting that annexin II plays an important role in the process of gastric cancer metastasis.
Terminology
Annexins is a class of calcium-dependent phospholipid-binding proteins, they are widely present in the cell membrane, in the form of calcium-dependent reversible binding to ceembrane phospholipids, the latter determines Annexins specific biological functions.
Peer-review
This is an interesting study with great promise. AnnexinII was highly expressed in gastric cancer tissues and had the important role in the effect of promoting gastric cancer metastasis.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The medical records and research data of the subjects are kept confidential. When the results are published, Information related to the subject will not be disclosed. The research program meets the medical ethics. The research topic is ethical.
Conflict-of-interest statement: All the authors have no conflicts of interests to declare.
Data sharing statement: No additional unpublished data are available.
Peer-review started: May 16, 2017
First decision: June 22, 2017
Article in press: August 25, 2017
P- Reviewer:Eleftheriadis NP, Garcia-Olmo D, Goral V, Mura G
S- Editor: Gou SX L- Editor: A E- Editor: Ma YJ
Contributor Information
Feng Han, Department of Gastroenterology, the PLA No. 59 Hospital, Kaiyuan 661600, Yunnan Province, China; Department of Gastroenterology, The Second Affiliated Hospital, Kunming Medical University, Kunming 650101, Yunnan Province, China.
Shikha Shrestha, Department of Gastroenterology, The Second Affiliated Hospital, Kunming Medical University, Kunming 650101, Yunnan Province, China.
Hua Huang, Department of Gastroenterology, The Second Affiliated Hospital, Kunming Medical University, Kunming 650101, Yunnan Province, China.
Huang-Yong Lv, Department of Gastroenterology, the PLA No. 59 Hospital, Kaiyuan 661600, Yunnan Province, China.
Chuan Nie, Department of Gastroenterology, the PLA No. 59 Hospital, Kaiyuan 661600, Yunnan Province, China.
Ling Lin, Department of Gastroenterology, the PLA No. 59 Hospital, Kaiyuan 661600, Yunnan Province, China. lml19910@163.com.
Ming-Liang Lu, Department of Gastroenterology, The Second Affiliated Hospital, Kunming Medical University, Kunming 650101, Yunnan Province, China.
References
- 1.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Abd N, Fawzy A, Elbaz T, Hamdy S. Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinoma. Tumour Biol. 2016;37:211–216. doi: 10.1007/s13277-015-3524-x. [DOI] [PubMed] [Google Scholar]
- 3.Jeon YR, Kim SY, Lee EJ, Kim YN, Noh DY, Park SY, Moon A. Identification of annexin II as a novel secretory biomarker for breast cancer. Proteomics. 2013;13:3145–3156. doi: 10.1002/pmic.201300127. [DOI] [PubMed] [Google Scholar]
- 4.Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC. Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol. 2006;81:146–156. doi: 10.1016/j.yexmp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Tristante E, Martínez CM, Jiménez S, Mora L, Carballo F, Martínez-Lacaci I, de Torre-Minguela C. Association of a characteristic membrane pattern of annexin A2 with high invasiveness and nodal status in colon adenocarcinoma. Transl Res. 2015;166:196–206. doi: 10.1016/j.trsl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Jia JW, Li KL, Wu JX, Guo SL. Clinical significance of annexin II expression in human non-small cell lung cancer. Tumour Biol. 2013;34:1767–1771. doi: 10.1007/s13277-013-0715-1. [DOI] [PubMed] [Google Scholar]
- 7.Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D. Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med. 2016;14:89. doi: 10.1186/s12967-016-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Mol Life Sci. 2009;66:2623–2642. doi: 10.1007/s00018-009-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laohavisit A, Davies JM. Annexins. New Phytol. 2011;189:40–53. doi: 10.1111/j.1469-8137.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 13.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 14.Porporato PE, Payen VL, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci. 2016;73:1349–1363. doi: 10.1007/s00018-015-2100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


