Abstract
The ability to avoid and escape from predators are clearly relevant behaviors from the ecological perspective and directly interfere with the survival of organisms. Detected in the aquatic environment, risperidone can alter the behavior of exposed species. Considering the risk of exposure in the early stages of life, we exposed zebrafish embryos to risperidone during the first 5 days of life. Risperidone caused hyperactivity in exposed larvae, which in an environmental context, the animals may be more vulnerable to predation due to greater visibility or less perception of risk areas.
Introduction
Emotional states such as fear and anxiety are possible to be observed in zebrafish larvae. With a comprehensive behavioral range, these animals are already responsive to the environment at 24 hpf (hours post-fertilization) and within a week of life, they already respond to stimuli such as touch, sound, water movement or changes in light1,2. Unlike adults, zebrafish larvae prefer clear areas (dark areas are thought to simulate predator shade), avoid areas of light oscillation and recognize when placed in open areas2–4. Even during the first week of life, zebrafish larvae may already are sensitive to the same anxiolytics used for anxiety in humans3,5.
Behavioral changes have the potential to impact directly on the physical condition and the perpetuation of a species2,4. One of the most known behaviors in nature is the prey-predator relationship. To avoid predators and consequently potential life risks, the escape behavior is fundamental to the species maintenance. Emotions such as fear or anxiety can be observed in all vertebrates and are very important to maintainance and survival of the species, and the preservation of these escape patterns is observed in most fish species. Changes or alterations in this response may induce a direct risk to the individuals or even in severe populational consequences1,3.
In this context, aquatic contaminants are involved in many behavioral changes of exposed species. Several drugs consumed by the population promotes an increase in the amount of drug residues in the aquatic environment6–9. Even when detected in low amounts, the ability to cause changes in the physiology of non-target organisms has not yet been fully elucidated, but it is known that even at low concentrations (ng/L or μg/L) these drugs may have effects on exposed species6,8–10.
Risperidone (RISP), an atypical antipsychotic used mainly for the treatment of schizophrenia and bipolar mood disorder, has already been detected at different levels in aquatic environments11,12. RISP levels have already been recorded up to 0.0014 μg/L in seawater8, 0.0029 μg/L in effluent water7, and 0.0034 μg/L in drinking water13. In Belgium, the highest level of environmental contamination was recorded, presenting 0.364 μg/L in affluents and 0.154 μg/L in Dendre River effluent14. A few studies have been carried out to find out the consequences of species exposure and this type of contaminant at low concentrations15,16. Despite behavioral changes are common findings in mammals exposed to risperidone during embryonic development17, no reports about behavioral changes in the RISP-exposed fish were found in the current literature. Thus, in view of the great importance of preserving all the behavioral repertoire, we sought to identify the effects of RISP exposition on behavioral parameters in embryos and larvae zebrafish.
Results
Fish exposed to the concentration of 0.03 μg/L showed an increase in mortality when compared to the control group (Fig. 1) (p < 0.0001). No differences were found in hatching and heart rate.
Figure 1.
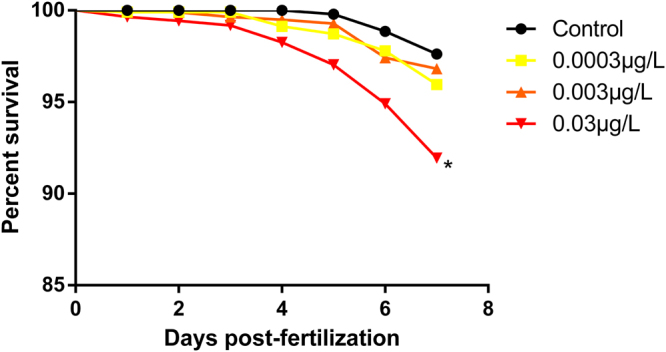
The larvae exposed to the highest concentration of risperidone tested showed increased mortality. Survival curve evaluated by Kaplan-Meier method. *p < 0.05. N = 160.
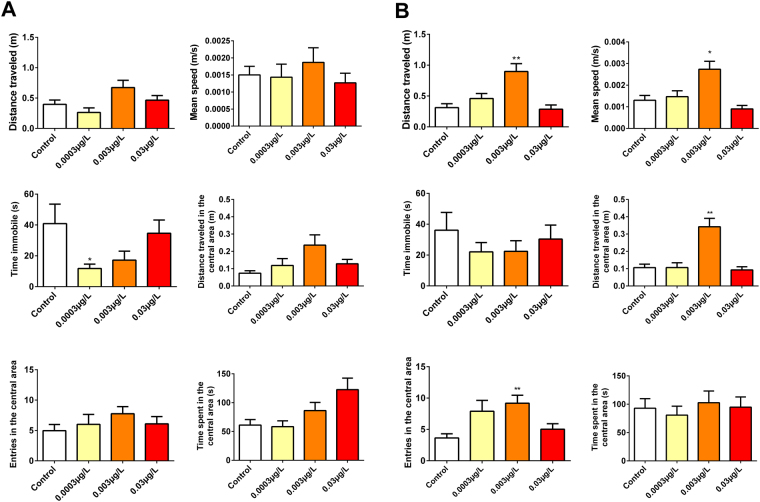
During the open field test, RISP at a concentration of 0.003 μg/L increased the total distance traveled (p = 0.0009), the number of entries in the central area (p = 0.0127) and the mean speed in the final phase of the test (6–12 min) (p = 0.0009). The concentration of 0.0003 μg/L increased the immobility time in the settling phase (p = 0.0542) (0–6 min), but this result did not last during the final test phase (6–12 min) (p = 0.6093) (Fig. 2).
Figure 2.
Open field test results. (A) The first phase (0–6 min). The concentration of 0.0003 µg/L RISP increased the immobility time of the exposed larvae. (B) The second phase (6–12 min). Hyperactivity can be observed by an increase in the distance, average speed and a number of entries in the central area. Means were compared by One-way ANOVA followed by Dunnett’s or Kruskal-Wallis test followed by Dunn’s were used depending on data normality. *p < 0.05. N = 30.
Larvae exposed to the concentration of 0.003 μg/L RISP decreased the response to the aversive stimulus and remained mostly in the stimulus area when compared to the control group (p = 0.0011). The other groups did not present alterations (Fig. 3).
Figure 3.
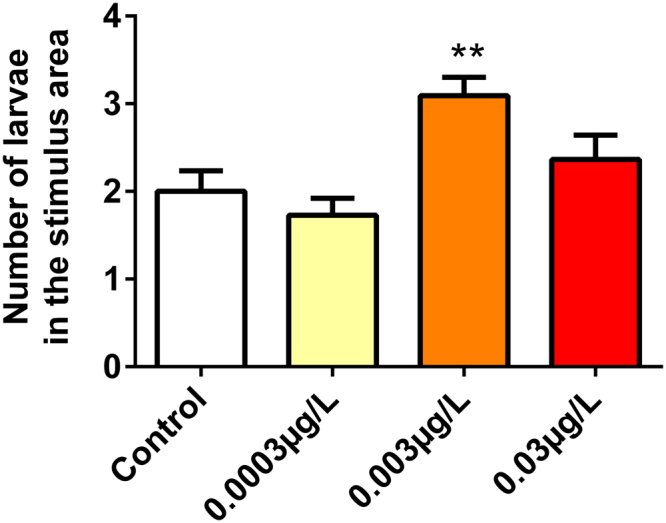
The concentration of 0.003 µg/L risperidone increased the number of animals that remained in the stimulus area. Means were compared by One-way ANOVA followed by Dunnett’s. *p < 0.05. N = 55.
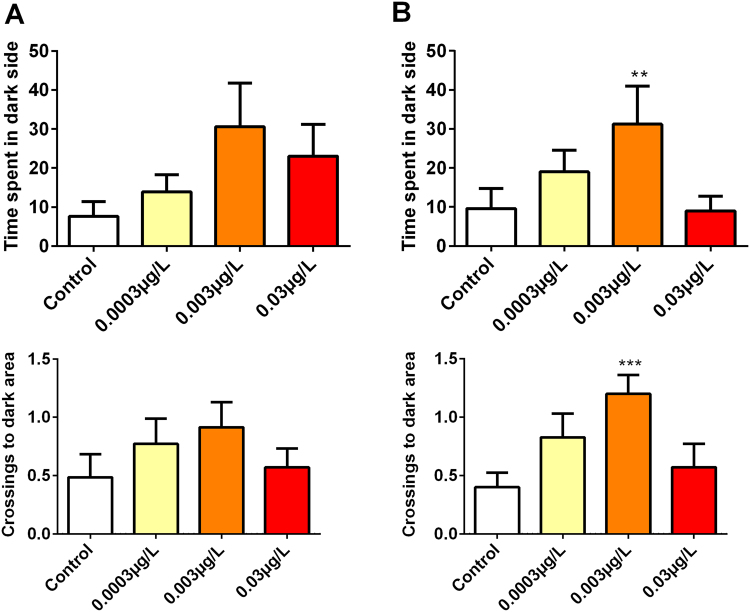
At the concentration of 0.003 μg/L larvae decreased the latency for entry into the dark side of the well (p = 0.0229), as well as increasing the number of entrances and residence time on this side during the second phase of the test (6–12 min) (p = 0.1532). In the initial phase of the test, there was no difference between groups (Fig. 4). There was no difference between the groups tested in relation to spontaneous movement.
Figure 4.
During the LDT, larvae exposed to the intermediate concentration of risperidone increased the time on the dark side and the number of crosses of the center line. Means were compared by One-way ANOVA followed by Dunnett’s or Kruskal-Wallis test followed by Dunn’s were used depending on data normality. *p < 0.05. N = 35.
Discussion
Here we show that the presence of RISP residues in water can alter the exploratory behavior of zebrafish embryos and larvae. In fact, during the first-time window (0–6 min) in the open field test, aversive stimuli and light/dark tests, larvae exposed to 0.0003 µg/L RISP increased the immobility time. The observed effects of extremely low RISP concentration were surprising since that this concentration was already detected in natural aquatic environments13, indicating the potential risk for populations exposed to this type of contaminant10. Moreover, larvae exposed to higher concentration of RISP displayed an increased mortality (5.68% in relation to the control group) and no significant alteration in hatching. These results are consistent with our previous study, where RISP affects parameters such as survival, hatching, heart rate and total larval length16.
In the 2nd time window (6–12 min) of the exploration tests, larvae presented increased exploratory activity and decreased response to the environmental stimuli, both indicating hyperactivity. The difference between 1st and 2nd-time window contradicts the actual knowledge that larvae do not present an adaptation period as seen in adults18. Further studies should be carried out to validate this hypothesis.
RISP is an atypical antipsychotic antagonist of serotonin and dopamine receptors19. Changes in the exploratory activity caused by RISP are common findings in adult rats17 and adult20 as well as larvae21 zebrafish. These changes in exploratory behavior are expected since that activity of dopaminergic and serotonergic neurons is related to motor coordination22,23.
The effects observed in our larvae appear to be associated with RISP exposure in early stages of development. In fact, the exposure to antipsychotics during the embryonic stage is associated with reduced levels of neurotransmitters leading to functional problems in exposed fish24 and mammals17,25,26. In addition, our larvae were exposed for 5 days and evaluated on the 6th day. This 24h-period without exposure may be related to these effects since the chronic administration of psychoactive drugs also appears to lead to an increase in the activity of dopaminergic and serotonergic receptors shortly after the drug withdrawal25–31.
In our study, the effects were mainly observed in the intermediary RISP concentration, but not in the higher. In fact, our tested concentrations were lower than plasma levels suitable to cause therapeutic effects32 or concentrations commonly tested in scientific experiments17,24,30,31 but exerted effects on zebrafish larvae, like those described with higher concentration. Thus, any comparison between our results and those reported in the literature is difficult, since no reports were found using RISP concentration as low as we used in the present study.
The last comment is about the possible implications of our results. In fish, dopamine and serotonin are involved in locomotion, attack/defense, learning/memory and eating behavior33. The ability to capture prey and escape from predators are clearly relevant behaviors from the ecological perspective, as they directly interfere with the growth and survival of organisms33,34. A prey may be more susceptible to predation as a result of non-detection of predators, poor escape performance, reduced resistance, inability to learn and greater visibility due to hyperactivity33,35. Our results highlight that RISP altered larvae activity patterns, which in an environmental context can directly influence the ability to avoid or evade predatory behavior which may result in significant repercussions on the maintenance of the species as well on the ecosystem.
Materials and Methods
Ethical Aspects
This study was approved by the Animal Use Ethics Committee (CEUA) of the University of Passo Fundo, UPF, Passo Fundo, RS, Brazil (Protocol #9/2015) and complied with the guidelines of the National Council for Animal Experimentation Control (CONCEA). Approximately 600 larvae were tested during this experiment.
Study strategy
We exposed embryos and larvae of zebrafish (Danio rerio, wild-type) to different concentrations of RISP already detected in the aquatic environment analyzing eventual changes in the larvae behavioral repertoire. This analysis was based on the different behavioral tests as follows: spontaneous movement, open field, light/dark as well as aversive stimulus. We opted for a 5-day chronic exposure to RISP since this period window correspond to the whole period of zebrafish organogenesis (2hpf to 120hpf)36.
Reproduction and maintenance of embryo
For breeding, healthy zebrafish wild-type, aged between 3 and 18 months were used. The animals were placed in barred bottom aquaria in ratios of 1:1 (males: females). After 12 hours of dark, during the morning the lights were on and after 1 hour the embryos were collected36,37. The methods of reproduction and maintenance of embryos are described in the previous work16.
After collection, the embryos were washed and classified as fertilized and unfertilized with the aid of light microscopy37,38. Embryos were maintained in E3 medium (reverse osmosis water + 60 mg/L Marine Ocean Instant Ocean) and distributed in 24 well cell culture plates (3 ml/well), 10 embryos per well and incubated in a water bath at 28 °C39. For the tests, embryos of up to 3hpf were accepted. The embryos were exposed to RISP from 3hpf to 120hpf.
Concentrations tested
The RISP concentrations were based on those already registered in the aquatic environment: 0.00034 µg/L13, 0.003 µg/L, and 0.03 µg/L. These concentrations were previously tested and changed survival, hatching, heart rate and total larval length16. The solutions were prepared and stored in amber glass bottles, where they remained heated in a water bath to be replenished in the wells when necessary.
Survival and hatching analysis
For analysis of survival and hatching, we have monitored all animals once a day in the morning for 7 days with the aid of a magnifying glass or optical microscopy. Embryos and larvae that do not show transparency, coagulated or without cell formation, cardiac movement or blood circulation were considered dead. Animals were considered “hatched” when partially or completely outside of the chorion. For this hatching and survival measurements, we analyzed 160 embryos by concentration (control and the three concentrations tested), totalizing 640 embryos
Spontaneous movement
In 24 hpf the embryos present spontaneous movements of the tail still inside the chorion. They are thus considered because they are induced by the development of the motoneurons without any control by the central nervous system37,40,41. These movements were recorded in 1 minute40 in 64 embryos by group (total of 256 embryos).
Heart rate
Heart rate was assessed at 48hpf in all groups during the morning. The heart rate was manually counted by light microscopy for 1 minute42 in 48 embryos by group (total of 192 embryos).
Open field test
To perform the open field test, the larvae at 6 dpf were placed in 10 mL wells containing only E3 medium and filmed (Canon EOS Rebel T5 Macro Lens EF 100mm) for 12 minutes. Similar to the behavior seen in mammals, zebrafish larvae also present thigmotaxis and recognize when placed in a new environment3,4,43.
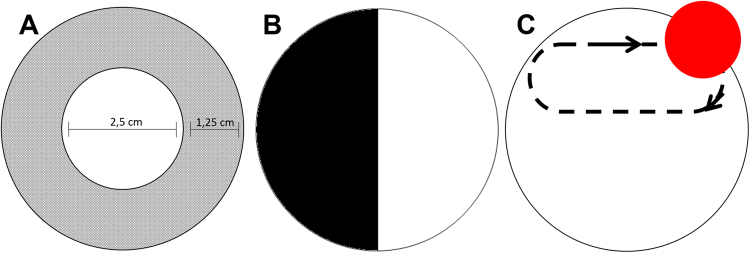
For thigmotaxic behavior analysis, we filmed 30 larvae by group (total of 120 larvae). In the videos, the well was virtually divided into a central and peripheral area (Fig. 5A)18 and the period were divided into two phases: adaptation period (0–6 min) and the exploratory period (6–12 min). ANY-maze software was used to analyze the following parameters: total distance travelled, time in the central area, distance travelled in the central area, entries in the central area, immobility time and mean speed.
Figure 5.
A schematic illustration of the experimental setup used to record zebrafish larvae behavior. (A) Open field test - Central and peripheral region established in the software ANY-Maze (B) Light/dark test (C) Aversive stimulus was produced with a red bouncing-ball.
Light/dark test (LDT)
For this test, a 6-well cell culture plate was used with one well (5 ml) divided into a dark (black) area and a clear (white) area. Unlike adults, zebrafish larvae prefer to stay in the white area of the well (Fig. 5B). It is believed that the dark area represents the shadow of a possible predator43,44.
Thirty-five larvae by group (total of 140 larvae) were placed in the test area and filmed for 6 minutes. The latency for entry into the dark side, the number of entries and dwell time on the dark side were evaluated using the ANY-maze software. As in the open field test, the LDT was divided between the initial phase (0–6 min) and final phase (6–12 min).
Aversive stimulus test (AST)
Aversive stimulus aims to test the cognitive ability of the larva to identify areas of danger. Tests with colorations have been used for their ecological relevance, since different species of fish, like zebrafish, use colors to differentiate possible foods, recognize specifics as well as avoid predators18.
For this test, the larvae were placed in 6-well cell culture plates (5 larvae per well, n = 55 by group totalizing 220 larvae) above an LCD monitor. After the adaptation period (2 min), using PowerPoint software (Microsoft Office Professional Plus 2013), we started the exposure to a visual stimulus area with a red sphere of 1.35 cm in diameter with a trajectory that traveled only half the well (Fig. 5C). The animals were stimulated for 5 min and at the end of the test were recorded the number of animals that remained in the stimulus area and those that remained in the non-stimulated area45.
Statistical analysis
For statistical analysis and graphing we used GraphPad Prism software version 6.01 for Windows. Survival and hatchability data were evaluated by Kaplan-Meier method. For the analysis of the heart rate data, spontaneous movement, open field test, LDT and AST, One-way ANOVA followed by Dunnet’s (a group of parametric data ) or Kruskal-Wallis test followed by Dunn’s were used (a group of non-parametric data). All groups were compared to the control group. Statistical significance was accepted when p ≤ 0.05.
Acknowledgements
This study was funded by the Universidade de Passo Fundo and CNPq. L.J.G.B. holds CNPq research fellowship (301992/2014–2).
Author Contributions
F.K. and L.J.G.B. conceptualize the experiments, wrote the manuscript and prepare the figures. F.K., R.I., J.G.S.R., H.H.A.B. and M.F. conducted experimental procedures. A.L.P. analyzed the results. All authors have read and approved the manuscript for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stewart W, Cardenas GS, McHenry MJ. Zebrafish larvae evade predators by sensing water flow. The Journal of Experimental Biology. 2013;216:388–398. doi: 10.1242/jeb.072751. [DOI] [PubMed] [Google Scholar]
- 2.Clift D, Richendrfer J, Thorn RJ, Colwill RM, Creton R. High-throughput analysis of behavior in zebrafish larvae: effects of feeding. Zebrafish. 2014;11:455–461. doi: 10.1089/zeb.2014.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Reviews in Neuroscience. 2011;22(1):63–73. doi: 10.1515/rns.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orger MB, Polavieja GG. Zebrafish behavior: opportunities and challenges. The Annual Rewiew of Neurosciencie. 2017;40:125–147. doi: 10.1146/annurev-neuro-071714-033857. [DOI] [PubMed] [Google Scholar]
- 5.Richendrfer H, Pelkowski SD, Colwill RM, Creton R. On the edge: pharmacological evidence for anxiety-related behavior in the zebrafish larvae. Behavior Brain Research. 2012;228:99–105. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.K’oreje KO, et al. Occurrence patterns of pharmaceutical residues in wastewater surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere. 2016;149:238–244. doi: 10.1016/j.chemosphere.2016.01.095. [DOI] [PubMed] [Google Scholar]
- 7.Bruce GM, Pleus RC, Snyder SA. Toxicological relevance of pharmaceuticals in drinking water. Environmental Science & Technology. 2010;44:5619–5626. doi: 10.1021/es1004895. [DOI] [PubMed] [Google Scholar]
- 8.Vidal-Dorsch DE, et al. Contaminants of emerginf concern in municipal watewater effluents and marine receiving water. Environmental Toxicology and Chemistry. 2012;31:2674–2683. doi: 10.1002/etc.2004. [DOI] [PubMed] [Google Scholar]
- 9.Fabbri E. Pharmaceuticals in the environment: expected and unexpected effects on aquatic fauna. Annals of the New York Academy of Sciences. 2015;1340:20–18. doi: 10.1111/nyas.12605. [DOI] [PubMed] [Google Scholar]
- 10.Calisto V, Esteves VI. Psychiatric pharmaceuticals in the environment. Chemosphere. 2009;77:1257–1274. doi: 10.1016/j.chemosphere.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Mannens G, Meuldermans W, Snoeck E, Heycants J. Plasma protein binding of risperidone and its distribution in blood. Psychopharmacology. 1994;114:566–572. doi: 10.1007/BF02244986. [DOI] [PubMed] [Google Scholar]
- 12.Prieto MJ, et al. Optimization and in vivo toxicity evaluation of G4.5 pamam dendrimer-risperidone complexes. PLOS One. 2014;9:1–10. doi: 10.1371/journal.pone.0090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder, S. A. Ocurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. Ozone: Science & Engineering. 20 (2008).
- 14.Vergeynst L, Haeck A, Wispelaere P, Langenhove HV, Demeestere K. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography-magnetic sector mass spectrometry: Method quality assessment and application in a Belgian case study. Chemosphere. 2015;119:S2–S8. doi: 10.1016/j.chemosphere.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 15.Idalencio R, et al. Waterborne risperidone decreases stress response in zebrafish. PLOS One. 2015;10:1–10. doi: 10.1371/journal.pone.0140800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalichak F, et al. Waterborne psychoactive drugs impair the initial development of zebrafish. Environmental Toxicology and Pharmacology. 2016;41:89–94. doi: 10.1016/j.etap.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Bardgett ME, et al. Adult rats treated with risperidone during development are hyperactive. Experimental and Clinical. Psychopharmacology. 2013;21(3):259–267. doi: 10.1037/a0031972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad F, Richardson M. Exploratory behaviour in the open fiels test adapted for larval zebrafish: impact of environmental complexity. Behavioural Processes. 2013;92:88–98. doi: 10.1016/j.beproc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Horacek J, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Igartua DE, et al. Development of nutraceutical emulsion as risperidone delivery systems characterization and toxicological studies. Journal of Pharmaceutical Sciences. 2015;104:4142–4152. doi: 10.1002/jps.24636. [DOI] [PubMed] [Google Scholar]
- 21.McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. The Journal of Comparative Neurology. 2004;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- 22.Brustein E, et al. Steps during the development of the zebrafish locomotor netword. Journal of Physiology Paris. 2003;97:77–86. doi: 10.1016/j.jphysparis.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.McPherson AD, et al. Motor behavior mediated by continuously generated dopaminergic neurons in the zebrafish hypothalamus recovers after cell ablation. Current Biology. 2016;25:263–269. doi: 10.1016/j.cub.2015.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prieto MJ, Guatierrez HC, Arévalo RA, Chiaramoni NS, Alonso SV. Effect of risperidone and fluoxetine on the movement and neurochemical changes of zebrafish. Open Journal of Medicinal Chemistry. 2012;2:129–138. doi: 10.4236/ojmc.2012.24016. [DOI] [Google Scholar]
- 25.Zuo J, et al. Distinct neurobehavioral consequences of prenatal exposure to supiride (SUL) and risperidone (RIS) in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:387–397. doi: 10.1016/j.pnpbp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Singh KP, Singh MK. In utero exposure to atypical antipychotic drug, risperidone: Effects on fetal neurotoxicoty in hippocampal region and cognitive impairment in rat offspring. Progress in neuro-psychopharmacology & Biological Psychiatry. 2017;75:35–44. doi: 10.1016/j.pnpbp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: Implications for antipsychotic drug treatment. The Journal of Pharmacology and Experimental Therapeutics. 2001;297:711–717. [PubMed] [Google Scholar]
- 28.Olsen CK, Kreilgaard M, Didriksen M. Positive modulation of glutamatergic receptors potentiates the supressive effects of antipsychotics on condiotioned avoidance responding in rats. Pharmacology, Biochemistry and Behavior. 2006;84:259–265. doi: 10.1016/j.pbb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Moran-Gates T, Grady C, Park YS, Baldessarini RJ, Tarazi FI. Effects of risperidone on dopamine receptor subtypes in developing rat brain. European Neuropsychopharmacology. 2007;17:448–455. doi: 10.1016/j.euroneuro.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y, Gardner MP, Tarazi FI. Effects of risperidone on glutamate receptor subtypes in developing rat brain. European Neuropsychopharmacology. 2009;19(2):77–84. doi: 10.1016/j.euroneuro.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y, Moran-Gates T, Gardner M, Tarazi FI. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. European Neuropsychopharmacology. 2010;20(3):1–14. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedel M, et al. Risperidone plasma levels, clinical response and side-effects. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:261–268. doi: 10.1007/s00406-004-0556-4. [DOI] [PubMed] [Google Scholar]
- 33.Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P. Effects of contaminants on behavior: Biochemical mechanisms and ecological consequences. BioScience. 2001;51:209–217. doi: 10.1641/0006-3568(2001)051[0209:EOCOBB]2.0.CO;2. [DOI] [Google Scholar]
- 34.Fuiman LA, Magurram AE. Development of predator defences in fishes. Reviews in Fish Biology and Fisheries. 1994;4:145–183. doi: 10.1007/BF00044127. [DOI] [Google Scholar]
- 35.Mesa MG, Poe TP, Gadomski DM, Petersen JH. Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. Journal of Fish Biology. 1994;45:81–96. doi: 10.1111/j.1095-8649.1994.tb01085.x. [DOI] [Google Scholar]
- 36.Avdesh, A. et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. Journal of Visualized Experiments. 69 (2012). [DOI] [PMC free article] [PubMed]
- 37.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 38.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of zebrafish, Danio rerio. Biological reviews. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 39.Buske C, Gerlai R. Maturation of shoaling behavior is accompanhied by chandes in the dopaminergic and serotoninergic systems in zebrafish. Developmental Psychobiology. 2012;54(1):28–35. doi: 10.1002/dev.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraysse B, Mons R, Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicology and Environmental Safety. 2006;63:253–267. doi: 10.1016/j.ecoenv.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Jin M, et al. Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquatic Toxicology. 2009;95:347–354. doi: 10.1016/j.aquatox.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Huang H, et al. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos folowing exposure to perfluorooctanesulphonicacid (PFOS) Aquatic Toxicology. 2010;98:139–147. doi: 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steenbergen PJ, Richardson MK, Champagne DL. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behavioural Brain Research. 2011;222:15–25. doi: 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn fro zebrafish? Genes, Brain and Behavior. 2004;3:63–74. doi: 10.1046/j.1601-183X.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 45.Nery LR, et al. Brain Intraventricular injection of amyloid-β in zebrafish embryos impairs cognition and increases tau phosphorylation, effects reversed bt lithium. PLOS One. 2014;9:1–7. doi: 10.1371/journal.pone.0105862. [DOI] [PMC free article] [PubMed] [Google Scholar]