Abstract
A common feature of eukaryote genomes is large chromosomal regions where recombination is absent or strongly reduced, but the factors that cause this reduction are not well understood. Genomic rearrangements have often been implicated, but they may also be a consequence of recombination suppression rather than a cause. In this study, we generate eight high-quality genomic data sets of the filamentous ascomycete Neurospora tetrasperma, a fungus that lacks recombination over most of its largest chromosome. The genomes surprisingly reveal collinearity of the non-recombining regions and although large inversions are enriched in these regions, we conclude these inversions to be derived and not the cause of the suppression. To our knowledge, this is the first time that non-recombining, genic regions as large as 86% of a full chromosome (or 8 Mbp), are shown to be collinear. These findings are of significant interest for our understanding of the evolution of sex chromosomes and other supergene complexes.
Genomic rearrangements can be either a cause or a consequence of large-scale suppression of recombination in a genome. Here, Sun et al. show that recombination may be suppressed in collinear genomic regions, and that inversions may accumulate over time in a derived manner.
Introduction
Meiotic recombination plays an important role in maintaining and creating variation in sexual organisms, but can also break up advantageous gene complexes and thereby act as a barrier against establishing complex phenotypes. Suppression of recombination can in the latter case evolve to ensure that gene complexes are inherited together1. Complex phenotypes that segregate as Mendelian traits have often been mapped to multi-gene regions where recombination is reduced. Theory predicts that mutations that reduce recombination between genes in advantageous gene complexes will be favoured2, leading to the formation of so-called ‘supergenes’1,3. Numerous supergenes have recently been identified, for instance linking together genes determining phenotypically distinct morphs of mimetic butterflies4 and ruffs5,6, social structures in fire ants7 and locally adapted ecotypes of monkeyflower8. In the most well-studied supergene systems, those of animal and plant sex chromosomes, sexually antagonistic selection is thought to have driven an expansion of a region of suppressed recombination along almost the entire chromosome carrying the sex determination locus9.
In spite of the importance of recombination suppression for the evolution of adaptive linkage, it has been difficult to determine the underlying mechanisms for reducing recombination. Besides heterochromatin, which is generally restricted to repetitive regions of the chromosomes, all known examples of large-scale suppression of recombination are associated with structural rearrangements. Chromosomal inversions will, as single mutational events, immediately affect recombination patterns within the inverted region of the chromosome and are often proposed as causative mechanisms for recombination suppression10,11. In many supergene systems, including several young sex chromosomes12,13, inversions have been found in the regions of suppressed recombination, but on older sex chromosomes the degeneration of the heterogametic sex chromosome has precluded any direct identification of inversions. In humans, so-called evolutionary strata (regions with distinct levels of sequence divergence) have instead been identified, and these have been interpreted as being caused by a succession of structural rearrangements such as inversions and chromosomal fusions14. However, the strata hypothesis is not supported by all empirical data. Specifically, in sex chromosomes of humans15, Silene latifolia 16 and threespine stickleback17 there are regions where a gradual change in divergence levels has been interpreted as the result of a gradual expansion of suppressed recombination, but these regions are still associated with putatively rearranged regions or could be affected by mechanisms that scrambles the signal of divergence, such as gene conversion18. The ancient and highly diverged nature of many sex chromosome systems and the technical issues of studying structural variation in large genomes have so far made it difficult to conclusively untangle the relationship between suppression of recombination and structural rearrangements.
A further complicating factor is that while inversions can be advantageous by reducing recombination, they may also be associated with fitness costs. Crossover events within an inversion often cause unbalanced, inviable, gametes and can also have negative effects of fertility that increase with the size of the inversion19, 20. If another, non-structural mechanism suppresses recombination, selection against inversions would be relaxed and potentially increase their frequency in a population. Thus, an inversion found in a region of low recombination could instead be a consequence of suppressed recombination, rather than the cause.
Analogous to sex chromosomes, suppression of recombination has been identified along mating-type (mat) chromosomes of certain fungi21–23, including the filamentous ascomycete Neurospora tetrasperma. This species is a close relative to the model fungus N. crassa and maintains a self-fertile, pseudohomothallic, life cycle by producing ascospores that contain both necessary mating types in two different haploid nuclei. The ascospores can then germinate to form a heterokaryotic mycelium which can produce new sexual spores by selfing24. At meiosis, recombination suppression between the mat locus and the centromere ensures the proper packaging of the nuclei of opposite mating types into an ascospore25. However, recombination is suppressed far beyond this region and is lacking in most of the chromosome. Recombination suppression in N. tetrasperma is evidenced from analysis of marker segregation in crosses26 and cytological examinations have shown a lack of synapsis of most of the mat chromosome at pachytene26. Population genetic analyses have also shown that sequence divergence is elevated between the mat chromosomes, and that linkage disequilibrium is extensive27–29.
Ellison et al.30 generated high-quality assemblies from the two haploid genomes of one heterokaryotic strain of N. tetrasperma. These assemblies showed that one of the mat chromosomes carries several overlapping inversions covering much of the region where recombination is suppressed, whereas the mat chromosome of the other nuclei is collinear with N. crassa 31. The interpretation from this analysis was that the inversions were central in the evolution of the pseudohomothallic mating system of N. tetrasperma, and the cause of the recombination suppression. However, the influence of both structural rearrangements and unlinked recombination modifiers of recombination suppression has also been demonstrated experimentally in the same strain32.
Neurospora tetrasperma shows strong population structure and has been divided into at least eight phylogenetically distinct lineages27,33. All lineages show elevated divergences on the mat chromosome, but the size of the region and level of divergence varies between them27. If the inversions identified by Ellison et al.30 were fixed in the lineages, this would support the model that they represent an ancient and central event in the evolution of N. tetrasperma. Here, we investigate the structural variation on the mat chromosome to determine whether the previously identified inversions are shared between lineages and thus, can be interpreted as an ancestral trait of the species. We sequence eight haploid N. tetrasperma genomes from four heterokaryons, originating from four different lineages, and use these data to investigate their chromosome structure. Taken together, our results show that the majority of N. tetrasperma strains are collinear. All newly discovered inversions show limited phylogenetic distribution, indicating that they have taken place after the establishment of the non-recombining region. Collinearity is therefore the ancestral state of N. tetrasperma and all inversions that have been identified by Ellison et al.30 and herein are derived. These results establish the existence of large-scale recombination suppression in the absence of structural rearrangements, and caution against interpreting the presence of such rearrangements as being causative.
Results
A high degree of collinearity among Neurospora genomes
For three of the eight investigated N. tetrasperma genomes (Table 1), we created high-quality assemblies using optical mapping data together with Illumina paired-end and mate-pair reads, and for the other five we generated PacBio long-read data. Using these data sets, we were in all eight cases able to assemble the seven expected chromosomes into complete, or near-complete scaffolds and contigs (Table 1, Supplementary Tables 1 and 2, Supplementary Fig. 1). Whole-genome alignments against the N. crassa and N. tetrasperma reference genomes showed that all of these eight assemblies were mostly collinear with N. crassa and N. tetrasperma mat a, and none carried the inversions identified in N. tetrasperma mat A by Ellison et al.30 (Fig. 1, Supplementary Fig. 1). Furthermore, in order to expand our knowledge on the conservation of collinearity among the terminal, conidiating group of Neurospora, we sequenced two closely related species34 using PacBio: N. sitophila and N. intermedia (Table 1, Supplementary Table 2). Both genomes show a high degree of collinearity with both N. crassa and N. tetrasperma, indicating strong conservation of gene order in this clade (Supplementary Fig. 1).
Table 1.
Strains sequenced for this study
| Species | Homokaryon ID | Mating type | Heterokaryon ID | Lineage | Sequencing technology |
|---|---|---|---|---|---|
| N. tetrasperma | 9033 | mat A | P4492 | L1 | Optical mapping, Illumina |
| 9034 | mat a | Optical mapping, Illumina | |||
| CJ73 | mat A | J2 | L7 | PacBio | |
| CJ74 | mat a | PacBio | |||
| CJ85 | mat A | P505 | L8 | PacBio | |
| CJ86 | mat a | PacBio | |||
| 965A | mat A | 965 | L9 | Optical mapping, Illumina | |
| 965a | mat a | PacBio | |||
| N. intermedia | 8807 | mat a | PacBio | ||
| N. sitophila | W1434 | mat A | PacBio |
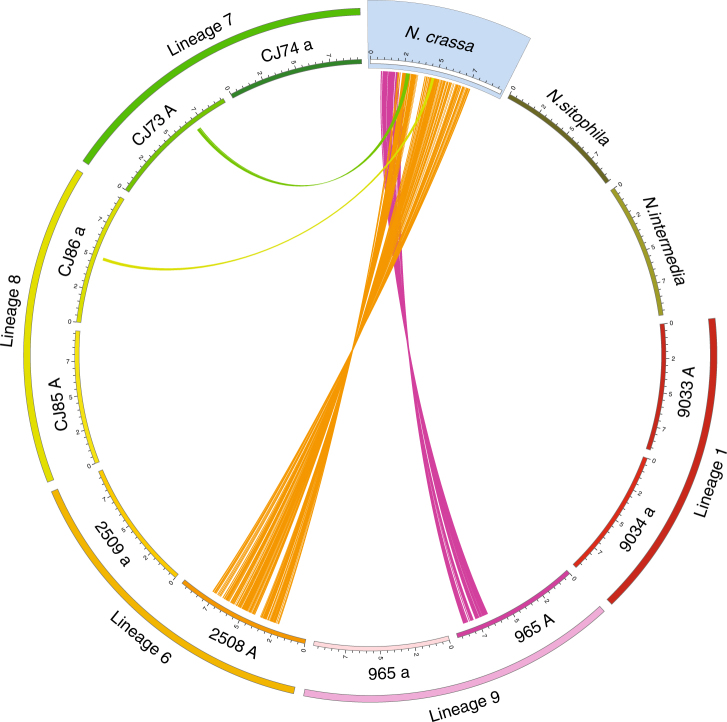
Fig. 1.
Whole chromosome pairwise alignments of the mat chromosome of N. tetrasperma against N. crassa. The mat chromosome (chromosome 1) of the 10 high-quality de novo assemblies (eight N. tetrasperma, one N. sitophila and one N. intermedia) generated for this study (Table 1), together with two mat chromosomes from Lineage 630, were aligned to the N. crassa OR74A assembly31. Chromosomes are scaled in Mbp and only inverted regions larger than 100 kbp are shown. For N. tetrasperma, genomes of each of the two mating types from a single heterokaryon were sequenced separately. Each heterokaryon represent a phylogenetically distinct lineage (Lineages 1, 6, 7, 8 and 9)27,33,34. The previously identified set of inversions in 2508 mat A (Lineage 6)30 is the biggest inverted region identified in N. tetrasperma. Strain 965 mat A (Lineage 9) carries a 1.9 Mbp inversion surrounding the mat locus, CJ86 mat a (Lineage 8) carries a 260 kbp inversion and CJ73 mat A (Lineage 7) a 320 kbp inversion. Both mating type strains of Lineage 1 completely lack large inversions on this chromosome, as do the N. sitophila and N. intermedia genomes
Lineage-specific inversions on the mat chromosome
In spite of general collinearity among the Neurospora genomes, several large inversions were found in N. tetrasperma. Using whole chromosome alignments, we detected a previously unknown 1.9 Mbp inversion surrounding the mat locus and two other large inversions (260 and 320 kbp) on the mat chromosome of N. tetrasperma (Fig. 1). In addition to our high-quality genomic data sets, we also analysed a published set of 92 N. tetrasperma strains sequenced with Illumina paired end reads27 (Supplementary Table 3). By aligning fragmented de novo assemblies of these 92 strains against both the N. tetrasperma mat a and mat A assemblies, we verified both breakpoints of one of the three inversions detected by Optical mapping or PacBio in Lineages 7, 8 and 9 (Fig. 1), while for two inversions we were only able to verify one breakpoint due to highly repetitive regions surrounding the second (Supplementary Table 4). Using the Illumina-data, we also identified three new unique inverted regions larger than100 kbp on the mat chromosome of Lineages 4 and 10, and show that the inversions identified by Ellison et al.30 on mat A in Lineage 6 are fixed on all mat A chromosomes in the sister clade Lineage 5 (Supplementary Table 4). All inverted regions over 100 kbp could further be shown to be fixed within one mating type in each lineage, except for a 3.6 Mbp inversion found in a single strain of Lineage 10 (CJ01) and a cluster of overlapping inversions (400 kbp in total) found on chromosome 5 in four genomes of Lineage 10 (Supplementary Table 4). For all inversions larger than 100 kbp that were identified in the Illumina data but not confirmed with other sequencing data, we used PCR, and in most cases also Sanger sequencing, to verify the breakpoints (Supplementary Table 4, Supplementary Fig. 2).
Enrichment of large inversions
A mechanism causing suppression of recombination is expected reduce the negative fitness effects of inversions in these regions. Large inversions that would have large fitness effects in recombining regions would then be expected to accumulate in regions where recombination is suppressed19. For all inversions (>1 kbp, identified in at least two different strains, Supplementary Table 5), we found an even distribution between the region of suppressed recombination and the rest of the genome (1.08×10−6 inversions per base pair for the region of suppressed recombination vs. 1.39×10−6 inversions per base pair for the rest of the genome). However, the mean size of inverted regions in the region of suppressed recombination is 45 times larger than in the rest of the genome (1.5 vs. 0.033 Mbp) and when including only the inverted regions larger than 100 kbp (Supplementary Table 4) in the analysis, we found a 22-fold enrichment per base pair in the region of suppressed recombination compared to the rest of the genome (p = 0.00061, one-sided binomial test). These results suggest that large inversions are significantly enriched in the suppressed recombination region in N. tetrasperma. When expanding the analysis by performing interspecific comparison between N. intermedia, N. sitophila and N. crassa only one inversion larger than 100 kbp is found in N. sitophila (Supplementary Fig. 1), which shows that inversions in Neurospora genomes are rare and further indicates a selection against large inversions in Neurospora species, but that the selective strength is reduced in the region of suppressed recombination on the mat chromosome in N. tetrasperma.
Discussion
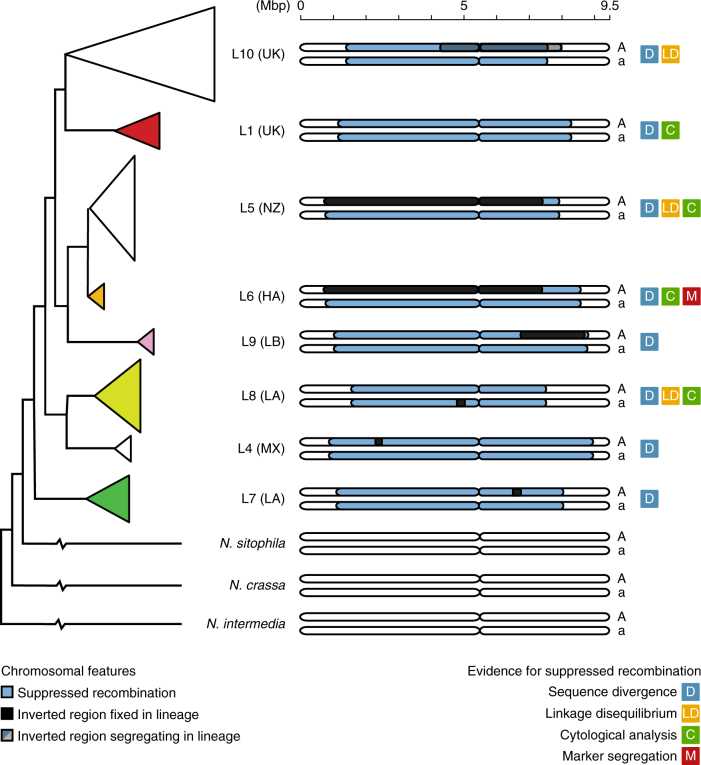
Previous studies have, through a number of different strategies26–29, established that the mat chromosome of N. tetrasperma contains a region where recombination is suppressed between the two mating types (Fig. 2), and based on an earlier genomic study30 inversions were hypothesized to be the causative factor. Here we show that the majority of N. tetrasperma lineages do not carry inversions capable of causing the large-scale suppression of recombination on the mat chromosome (Fig. 2). The inversions identified by Ellison et al.30 are limited to the mat A chromosome in Lineages 5 and 6, and while several lineages carry smaller inversions (2 Mbp or less) on one mating type chromosome, both mat chromosomes of Lineage 1 are completely collinear. It is therefore clear that something else than structural heterozygosity must maintain the suppression of recombination, covering a region as large as 8 Mbp or ~86 % of the entire chromosome27.
Fig. 2.
Structure of the mat chromosome across N. tetrasperma lineages. All inversions above 100 kbp on mat chromosomes identified in this study, plotted per lineage. The phylogenetic tree is adapted from Corcoran et al.27 and the colours of the filled triangles correspond to the colours used in Fig. 1. Several studies have provided different types of evidence for suppression of recombination along the mat chromosome in N. tetrasperma. Gallegos et al.26 investigated strains from Lineages 1, 5, 6 and 8 and determined both that they lack chromosomal synapsis at pachytene in most of the mat chromosome, and that no crossovers occurred between a set of genetic markers for a Lineage 6 heterokaryon. Corcoran et al.27 identified a region of elevated sequence divergence between the mating types within a heterokaryon for all N. tetrasperma lineages included in this study, as well as a region of elevated linkage disequlibirium for three of them. The combination of evidence indicates that all N. tetrasperma lineages carry a region of suppressed recombination on the mat chromosome (blue bars), but the size of this region differs between lineages27. Black bars show inverted regions that are fixed on a mating type within a lineage. All identified inverted regions are fixed in all investigated strains of the same mating type within its lineage, except a 3.6 Mbp inversion only identified in CJ01 mat A in Lineage 10. The inverted region in Lineages 5 and 6 is the only one that is shared between lineages. Neurospora sitophila, N. crassa and N. intermedia share the gene order with the majority of N. tetrasperma strains, but the mat chromosome recombines freely. This distribution of inversions shows that all identified inversions are derived and that only the suppression of recombination is shared between all analyzed strains of N. tetrasperma
The phylogenetic distribution of inversions (Fig. 2) also supports that the ancestral state of the non-recombining region must have been collinear and that the detected inversions are derived. We have previously reported on introgression of mat chromosomes in several lineages of N. tetrasperma from other species of Neurospora for which recombination is not suppressed27. This phenomenon complicates the interpretation of our data, in that introgression could have potentially reestablished collinearity from an ancestrally inverted stage. However, several lines of evidence speak against this hypothesis. Firstly, Lineages 1 and 8, which are generally collinear, do not show any signs of introgression in either mating type27. Secondly, the inverted region of Lineages 5 and 6 mat A is located on a chromosome that displays a strong signal of introgression from an unknown Neurospora species, which makes it unlikely that it represents the ancestral gene order. Thirdly, while mat A of Lineage 9 shows signs of introgression, it is mat a that carries the 2 Mbp inversion detected in this lineage.
It is worth pointing out that it is difficult to rule out that the inversions we see in this study took place in the genomic background of other species of Neurospora and were subsequently brought into N. tetrasperma by introgression. This scenario does not question that recombination suppression evolved in collinear chromosomal regions, but would lead to an overestimation of inversions that have taken place in the region of suppressed recombination in N. tetrasperma. This concern is only relevant for the inversions in Lineages 5 and 6, since for the other lineages it is the non-introgressed chromosome27 that carries the inversion. The fact that the gene order is highly conserved between all species of Neurospora included in this study, and the mat chromosome of N. tetrasperma sticks out by breaking this pattern, suggests that the conserved gene order is the ancestral, and that changes have taken place in N. tetrasperma.
We do not yet know what causes the suppression of recombination in N. tetrasperma, but there are several possible mechanisms. Sequence divergence has been shown to reduce recombination35, but since recombination is restored when introgressed32 into the more diverged N. crassa 36, this is an unlikely mechanism for suppression in this system. Heterochromatin reduces recombination37, but there is no evidence that the mat chromosome would be heterochromatinized in N. tetrasperma. Constitutive heterochromatin is limited to low-GC, repetitive regions38 and facultative heterochromatin is not accumulated in the non-recombining region during vegetative growth39. Recombination hotspots could be depleted in the non-recombining region, but since this region shows signs of large-scale introgression in several lineages of N. tetrasperma 27,36, a stable depletion of hotspots is also unlikely. Jacobson showed that the genetic background affects recombination on the mat chromosome32 and genes that affect recombination rates locally have been found in Neurospora 40, but there are no known genes that can affect recombination over as large regions as detected in this study. In yeast41 and mammals42 recombination hotspots are enriched for H3K4me2 histone methylation43, and in Arabidopsis44 and Ascobolus45 DNA methylation is also known to affect recombination rates. The presence or absence of similar modifications throughout the non-recombining region could potentially affect double-strand break formation and recombination initiation in N. tetrasperma. Little is known about the fine-scale recombination landscape and what shapes it in Neurospora species, and other ascomycetes show variation in both large-scale and small-scale distribution of crossovers, as well as the regulation of this distribution46–49, which makes definite predictions difficult. Further data on, for example, the distribution of crossovers, double-strand breaks and chromatin modifications would be necessary to understand recombination in Neurospora and for identifying the underlying causes of recombination suppression.
Our results show that large-scale suppression of recombination in genic regions can remain over evolutionary time, even in absence of structural rearrangements. We also show that the region of suppressed recombination has an accumulation of large derived inversions, supporting the hypothesis of relaxation of selection against inversions in regions of suppressed recombination. These results stress the point that the association of inversions with low levels of recombination is not enough to infer that the inversion is the cause of the suppression. Furthermore, a similar mechanism for suppressing recombination could potentially also explain the strata seen on the human Y chromosome. To what extent recombination suppression by non-structural means exist in other species is still unknown, but regulation of recombination rates show both conservation and divergence between as distantly related organisms as plants, fungi and animals50. The advent of improved methods for whole-genome sequencing should make it easier to determine how widespread structural rearrangements are in regions of suppressed recombination across species, and if large-scale recombination suppression in collinear regions is a common phenomenon.
Methods
Strains investigated in the study
We used three publicly available, well-annotated Neurospora genomes in this study, including N. crassa (N. crassa OR74A version 1231, sequenced by Broad Institute, corrected for the assembly error detected by Galazka et al.51), N. tetrasperma FGSC 2508 mat A and FGSC 2509 mat a (both sequenced by JGI30). In addition, 92 previously published N. tetrasperma genomes assembled from Illumina paired-end data27 were analysed.
For this study, we gathered genomic data from eight haploid strains of N. tetrasperma, originating from four heterokaryotic strains. These heterokaryons represent phylogenetically and reproductively isolated lineages of the monophyletic morphospecies27,33,34, and were obtained in previous studies by isolating mycelia grown from asexual spores (conidia) of single mating-type that the fungus occasionally produces52. Finally, we gathered genomic data from one N. sitophila and one N. intermedia strain (Table 1). All strains sequenced for this project were obtained from the Fungal Genetics Stock Center53 (http://www.fgsc.net/) except N. sitophila W1434, which was provided by Jacobson et al.54.
Illumina short-read data and optical mapping
We assembled high-quality genomes of three N. tetrasperma strains, using a combination of Illumina short-read data and optical mapping (Supplementary Table 1). We generated paired-end Illumina libraries with 500 bp insert size of these strains for an earlier study27 and these data were supplemented in this study with reads from 5 kbp mate-pair libraries (Supplementary Table 1). Strains were inoculated on a Vogel medium plate with 1% sucrose at 25 °C and DNA was extracted using the Easy-DNA Kit (Invitrogen) and sent to the Beijing Genomic Institute (BGI, Shenzheng, China) for sequencing. The DNA was fragmented, circularized and sequenced using Illumina HiSeq, generating 90 bp mate-pair reads. A series of filtration steps were set to remove the low-quality reads, including (1) 10 bp of the read for 500 bp library and 5 bp of the read from both ends for 5 kbp library, (2) reads with >10% of Ns’, (3) reads with >40 low-quality base calls (<Q20), (4) adapter contamination, and duplications. We used SOAPdenovo55 (http://soap.genomics.org.cn/soapdenovo.html; version 1.05) for the de novo assembly of the sequenced genomes. By testing multiple kmers, we chose kmer 21 as the final optimal value for de novo assembly. Single base correction and gap filling were performed by SOAPaligner (http://soap.genomics.org.cn/soapaligner.html; version 2.21).
To generate optical maps for the three strains, the fungal mycelium was digested by the lysing enzyme at 37° for 3 h. The collected DNA and protoplasts were centrifuged, and washed twice by phosphase buffer, and then suspended in STC buffer (0.125 M EDTA, pH 7.5, 0.9 M sorbitol). Equal volume of 2% low melting point agarose (LMPA) was poured into the medium to make the DNA plug. ArgusTM Optical Mapping System (OpGen) was used to evaluate the quality of DNA, and samples with a DNA molecule length of >150 kbp were considered as adequate quality for the optimal restriction enzyme digestion. The restriction enzymes were chosen by the OpGen Manager software, DNA samples were digested by MapCard (OpGen) and OpGen enzyme kits and the ArgusTM Optical Mapping System was used to collect the data. The restriction map was assembled into an optical map using OpGen optical map assembly, and the contigs generated by SOAPdenovo were placed and scaffolded using the OpGen alignment tool. Sequencing information, assembly statistics and data availability is shown in Supplementary Table 1.
Genome sequencing and assembly using PacBio
Whole genomes of five N. tetrasperma, one N. intermedia and one N. sitophila strain (Supplementary Table 2) were sequenced using the PacBio RSII platform56 (Pacific Biosciences). The strains were cultured by adding conidia to 200 ml of 3% liquid malt extract medium in 500 ml Erlenmeyer flasks, which were placed on rotary shaker at 30 °C for 3–4 days. The cultures were harvested by removing the mycelium from the liquid, placing it on a filter paper, which was folded between several layers of tissue paper and pressed to remove excess liquid. The mycelium was cut into small pieces and ~1 g was allotted into 2 ml tubes with screw-on caps, after which the tubes were stored at −20 °C.
To extract the DNA, two tubes of each strain were freeze-dried overnight and macerated using a TissueLyzer II bead-beater (Qiagen). Two 2 mm metal beads were placed in each tube, which were then shaken for 20 s at 25 Hz. If a tube contained large pieces of freeze-dried mycelium, it was shaken for another 10 s. This procedure was repeated until no large pieces remained, but no tube was shaken for more than 40 s in total. DNA was extracted from the macerated samples using Genomic Tip G-500 columns (Qiagen) and cleaned using the PowerClean DNA Clean-Up kit (MoBio Labs).
The extracted DNA was sent to the Uppsala Genome Center (Science for Life Laboratory, Uppsala, Sweden), where libraries were prepared and sequenced on a PacBio RSII system, using four SMRT cells per sample and the C4 chemistry and P6 polymerase (Pacific Biosciences). The raw sequence data was then filtered and assembled using the SMRT Analysis package and HGAP 3.0 assembler57 (Pacific Biosciences, https://github.com/PacificBiosciences/). Sequencing information, assembly statistics and data availability is shown in Supplementary Table 2.
Genomic comparison for the high-quality Neurospora genomes
The genomic comparison for all complete Neurospora genomes (three publicly available, three optical mapping and seven PacBio) were generated by LASTZ58 (http://www.bx.psu.edu/miller_lab/dist/README.lastz-1.02.00/README.lastz-1.02.00a.html; version 1.02) with low sensitive setting parameters, including no transition, step = 20 for seeding and no gap extension during alignment. The comparative genomics results were visualized by Circos59 (http://circos.ca/; version 0.64) in Fig. 1. Only inverted regions that were larger than 100 kbp, or located within a larger inverted region, were shown in the figure. The alignments for the inverted region were also manually inspected to control for alignment errors.
Identifying inversions from fragmented de novo assemblies
The de novo assemblies of 92 N. tetrasperma strains that had previously been generated from short paired-end Illumina reads27 were examined for the presence of inversions. These de novo assemblies were highly fragmented, with scaffold numbers ranging from 242 to 34,302 (mean 2845) and N50 from 23,658 to 1,140,656 bp (mean 172,026 bp) (Supplementary Table 3). In order to identify inversions, the MUMmer60 whole-genome aligner was used to align the 92 assemblies to the high-quality assemblies of the N. tetrasperma 2508A and 2509a strains (parameters: nucmer –c 200 –b 2000). Candidate inversions were called using custom python scripts (available at https://github.com/johannessonlab/InvDeNovo), which scanned MUMmer coordinate files to identify pairs of contigs in the de novo assemblies that aligned next to each other in the same chromosome of a reference assembly at two different locations and in different directions. If an inversion is present in the de novo assembly, this is the alignment pattern that is expected around the two inversion breakpoints (Supplementary Fig. 3). For each strain where candidate inversions larger than 100 kbp were identified, Illumina reads were then mapped to the de novo assembly using BWA61 in order to ensure that the signal of inversion was not caused by errors in the assembly procedure. Each breakpoint was also manually inspected to ensure that the signal was also not caused by a misalignment to repetitive sequences.
Confirmation of inversion breakpoints
Inversions detected in this study from de novo Illumina assemblies, and not verified by other sequencing data, were verified by using PCR and Sanger sequencing. DNA from strains CJ01, CJ02, 7585, 7586 and CJ24 was obtained by incubating conidia or mycelial tissue in 10% Chelex® 100 (Bio-Rad Laboratories) for 20 min at 95 °C. Primer pairs were designed around the putative inversion breakpoints (Supplementary Fig. 2, Supplementary Table 6). For the inversions in CJ01 and 7585, primers were designed to also match the nuclei of the alternative mating-type homokaryon from the same heterokaryons (i.e., CJ02 and 7586, respectively) enabling different primer combinations to confirm inverted or collinear gene order. In some cases, where breakpoints were found in non-aligning regions (7585 breakpoint 2, CJ24 breakpoint 4), it was impossible to determine the exact location of the breakpoint. Two primer pairs, one at each end of the region that did not align, were used to confirm presence of these breakpoints. PCRs were performed for each breakpoint and homokaryon pair simultaneously, for both inversion and collinear primer combinations. The Phusion® High Fidelity PCR kit (Thermo Scientific) was used for 10 µl reactions with the following thermal cycler conditions: 98 °C (30 s) [98 °C (10 s). *°C (30 s), 72 °C (1 min)]×30, 72 °C (10 min), where * depended on primer combination. PCR products were sequenced with the BigDye Terminator v3.1 cycle sequencing kit (Thermo Fisher) and read on an ABI3730xl DNA Analyzer (Applied Biosystems).
Filtering and enrichment analysis of inversions
Only inversions where both breakpoints were identified were included in this study. For inversions larger than 100 kbp, we verified breakpoints using PCR and Sanger sequencing and included only those that could be identified from at least two different types of data (i.e., both Illumina and PacBio, or both Illumina and PCR/Sanger). Inversions smaller than 100 kbp were included in the analysis only if they were found in at least two different strains. Supplementary Table 5 lists all inversions remaining after the final filtering, and Supplementary Table 4 lists all inversions larger than 100 kbp, where extra supporting evidence is available.
Enrichment of inversions in the non-recombining region on the mating-type chromosome were calculated by comparing the number of inversions per base-pair in the region of suppressed recombination on the mating-type chromosome (8,320,000 bp in strain 2509a, based on its maximum possible size as given by Corcoran et al.27) to the rest of the genome (30,097,195 bp in strain 2509a) using a one-sided binomial test. If the same inversion was found in several strains it was only counted once and the clusters of overlapping inversions found on the mat chromosome of Lineages 5 and 6 and on chromosome 5 on four Lineage 10 strains were also only counted as single inversion events.
Data availability
Raw PacBio reads generated in this study have been deposited in the Sequence Read Archive as BioProject PRJNA398702, and raw Illumina mate-pair reads as BioProject PRJNA239947. Genome assemblies have been deposited at Figshare62,63. Scripts for identifying inversions from fragmented assemblies is available through GitHub: https://github.com/johannessonlab/InvDeNovo.
Electronic supplementary material
Acknowledgements
We acknowledge funding to H.J. from the European Research Council and the Swedish Research Council. We thank Li Li and Zhiqin Lao from BGI for the sequencing service, and acknowledge support of the National Genomics Infrastructure (NGI)/Uppsala Genome Center and UPPMAX for providing assistance in massive parallel sequencing and computational infrastructure. Work performed at NGI/Uppsala Genome Center has been funded by RFI/VR and SciLifeLab, Sweden. We thank David Jacobson and Jennifer Anderson for valuable discussions.
Author contributions
Y.S., P.C. and H.J. planned and initiated the study. Y.S., J.S. and M.H. performed laboratory analyses. Y.S., J.S. and P.C. performed the computational analyses. All authors contributed to interpreting the data and completing the manuscript, but Y.S, J.S. and H.J. were mainly responsible for these tasks.
Competing interests
The authors declare no competing financial interests.
Footnotes
Yu Sun and Jesper Svedberg contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01317-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwander T, Libbrecht R, Keller L. Supergenes and complex phenotypes. Curr. Biol. 2014;24:R288–R294. doi: 10.1016/j.cub.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz-Barrientos D, Engelstädter J, Rieseberg LH. Recombination rate evolution and the origin of species. Trends Ecol. Evol. 2016;31:226–236. doi: 10.1016/j.tree.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth D. The status of supergenes in the 21st century: recombination suppression in Batesian mimicry and sex chromosomes and other complex adaptations. Evol. Appl. 2015;9:74–90. doi: 10.1111/eva.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joron M, et al. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature. 2011;477:203–206. doi: 10.1038/nature10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Küpper C, et al. A supergene determines highly divergent male reproductive morphs in the ruff. Nat. Genet. 2016;48:79–83. doi: 10.1038/ng.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamichhaney S, et al. Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax) Nat. Genet. 2016;48:84–88. doi: 10.1038/ng.3430. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, et al. A Y-like social chromosome causes alternative colony organization in fire ants. Nature. 2013;493:664–668. doi: 10.1038/nature11832. [DOI] [PubMed] [Google Scholar]
- 8.Lowry DB, Willis JH. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol. 2010;8:e1000500. doi: 10.1371/journal.pbio.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright AE, Dean R, Zimmer F, Mank JE. How to make a sex chromosome. Nat. Commun. 2016;7:12087. doi: 10.1038/ncomms12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkpatrick M. How and why chromosome inversions evolve. PLoS Biol. 2010;8:e1000501. doi: 10.1371/journal.pbio.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu. Rev. Ecol. Evol. Syst. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergero R, Charlesworth D, Filatov DA, Moore RC. Defining regions and rearrangements of the Silene latifolia Y chromosome. Genetics. 2008;178:2045–2053. doi: 10.1534/genetics.107.084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JA, Peichel CL. Molecular cytogenetic evidence of rearrangements on the Y chromosome of the threespine stickleback fish. Genetics. 2008;179:2173–2182. doi: 10.1534/genetics.108.088559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- 15.Iwase M, et al. The amelogenin loci span an ancient pseudoautosomal boundary in diverse mammalian species. Proc. Natl Acad. Sci. USA. 2003;100:5258–5263. doi: 10.1073/pnas.0635848100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergero R, Qiu S, Forrest A, Borthwick H, Charlesworth D. Expansion of the pseudo-autosomal region and ongoing recombination suppression in the Silene latifolia sex chromosomes. Genetics. 2013;194:673–686. doi: 10.1534/genetics.113.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natri HM, Shikano T, Merilä J. Progressive recombination suppression and differentiation in recently evolved neo-sex chromosomes. Mol. Biol. Evol. 2013;30:1131–1144. doi: 10.1093/molbev/mst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marais G, Galtier N. Sex chromosomes: how X-Y recombination stops. Curr. Biol. 2003;13:R641–R643. doi: 10.1016/S0960-9822(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 19.Navarro A, Ruiz A. On the fertility effects of pericentric inversions. Genetics. 1997;147:931–933. doi: 10.1093/genetics/147.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knief U, et al. Fitness consequences of polymorphic inversions in the zebra finch genome. Genome Biol. 2016;17:199. doi: 10.1186/s13059-016-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser JA, Heitman J. Evolution of fungal sex chromosomes. Mol. Microbiol. 2004;51:299–306. doi: 10.1046/j.1365-2958.2003.03874.x. [DOI] [PubMed] [Google Scholar]
- 22.Badouin H, et al. Chaos of rearrangements in the mating-type chromosomes of the anther-smut fungus Microbotryum lychnidis-dioicae. Genetics. 2015;200:1275–1284. doi: 10.1534/genetics.115.177709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menkis A, Jacobson DJ, Gustafsson T, Johannesson H. The mating-type chromosome in the filamentous ascomycete Neurospora tetrasperma represents a model for early evolution of sex chromosomes. PLoS Genet. 2008;4:e1000030. doi: 10.1371/journal.pgen.1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodge O, et al. Nuclear Phenomena Associated with Heterothallism and Homothallism in the Ascomycete Neurospora. Journal of Agricultural Research. 1927;35:289–305. [Google Scholar]
- 25.Raju NB, Perkins DD. Diverse programs of ascus development in pseudohomothallic species of Neurospora, Gelasinospora, and Podospora. Dev. Genet. 1994;15:104–118. doi: 10.1002/dvg.1020150111. [DOI] [PubMed] [Google Scholar]
- 26.Gallegos A, Jacobson DJ, Raju NB, Skupski MP, Natvig DO. Suppressed recombination and a pairing anomaly on the mating-type chromosome of Neurospora tetrasperma. Genetics. 2000;154:623–633. doi: 10.1093/genetics/154.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corcoran P, et al. Introgression maintains the genetic integrity of the mating-type determining chromosome of the fungus Neurospora tetrasperma. Genome Res. 2016;26:486–498. doi: 10.1101/gr.197244.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menkis A, Whittle CA, Johannesson H. Gene genealogies indicates abundant gene conversions and independent evolutionary histories of the mating-type chromosomes in the evolutionary history of Neurospora tetrasperma. BMC Evol. Biol. 2010;10:234. doi: 10.1186/1471-2148-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino ST, Nelson MA, Jacobson DJ, Natvig DO. Pseudohomothallism and evolution of the mating-type chromosome in Neurospora tetrasperma. Genetics. 1996;143:789–799. doi: 10.1093/genetics/143.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellison CE, et al. Massive changes in genome architecture accompany the transition to self-fertility in the filamentous fungus Neurospora tetrasperma. Genetics. 2011;189:55–69. doi: 10.1534/genetics.111.130690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galagan JE, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson DJ. Blocked recombination along the mating-type chromosomes of Neurospora tetrasperma involves both structural heterozygosity and autosomal genes. Genetics. 2005;171:839–843. doi: 10.1534/genetics.105.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menkis A, Bastiaans E, Jacobson DJ, Johannesson H. Phylogenetic and biological species diversity within the Neurospora tetrasperma complex. J. Evol. Biol. 2009;22:1923–1936. doi: 10.1111/j.1420-9101.2009.01801.x. [DOI] [PubMed] [Google Scholar]
- 34.Corcoran P, et al. A global multilocus analysis of the model fungus Neurospora reveals a single recent origin of a novel genetic system. Mol. Phylogenet. Evol. 2014;78:136–147. doi: 10.1016/j.ympev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Hunter N, Chambers SR, Louis EJ, Borts RH. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 1996;15:1726–1733. [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, et al. Large-scale introgression shapes the evolution of the mating-type chromosomes of the filamentous ascomycete Neurospora tetrasperma. PLoS Genet. 2012;8:e1002820. doi: 10.1371/journal.pgen.1002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Termolino P, Cremona G, Consiglio MF, Conicella C. Insights into epigenetic landscape of recombination-free regions. Chromosoma. 2016;125:301–308. doi: 10.1007/s00412-016-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis ZA, et al. Relics of repeat-induced point mutation direct heterochromatin formation in Neurospora crassa. Genome Res. 2009;19:427–437. doi: 10.1101/gr.086231.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson K, Rountree MR, Lewis ZA, Stajich JE, Selker EU. Regional control of histone H3 lysine 27 methylation in Neurospora. Proc. Natl. Acad. Sci. 2013;110:6027–6032. doi: 10.1073/pnas.1303750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catcheside DG, Corcoran D. Control of non-allelic recombination in Neurospora crassa. Aust. J. Biol. Sci. 1973;26:1337–1353. doi: 10.1071/BI9731337. [DOI] [PubMed] [Google Scholar]
- 41.Borde V, et al. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 2009;28:99–111. doi: 10.1038/emboj.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baudat F, et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010;327:836–840. doi: 10.1126/science.1183439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi K, Henderson IR. Meiotic recombination hotspots–a comparative view. Plant J. 2015;83:52–61. doi: 10.1111/tpj.12870. [DOI] [PubMed] [Google Scholar]
- 44.Yelina N, Diaz P, Lambing C, Henderson IR. Epigenetic control of meiotic recombination in plants. Sci. China Life Sci. 2015;58:223–231. doi: 10.1007/s11427-015-4811-x. [DOI] [PubMed] [Google Scholar]
- 45.Maloisel L, Rossignol J-L. Suppression of crossing-over by DNA methylation in Ascobolus. Genes Dev. 1998;12:1381–1389. doi: 10.1101/gad.12.9.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petes TD. Meiotic recombination hot spots and cold spots. Nat. Rev. Genet. 2001;2:360–369. doi: 10.1038/35072078. [DOI] [PubMed] [Google Scholar]
- 47.Fowler KR, Sasaki M, Milman N, Keeney S, Smith GR. Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 2014;24:1650–1664. doi: 10.1101/gr.172122.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam I, Keeney S. Nonparadoxical evolutionary stability of the recombination initiation landscape in yeast. Science. 2015;350:932–937. doi: 10.1126/science.aad0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croll D, Lendenmann MH, Stewart E, McDonald BA. The impact of recombination hotspots on genome evolution of a fungal plant pathogen. Genetics. 2015;201:1213–1228. doi: 10.1534/genetics.115.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenormand T, Engelstädter J, Johnston SE, Wijnker E, Haag CR. Evolutionary mysteries in meiosis. Phil. Trans. R. Soc. B. 2016;371:20160001. doi: 10.1098/rstb.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galazka JM, et al. Neurospora chromosomes are organized by blocks of importin alpha-dependent heterochromatin that are largely independent of H3K9me3. Genome Res. 2016;26:1069–1080. doi: 10.1101/gr.203182.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raju NB. Functional heterothallism resulting from homokaryotic conidia and ascospores in Neurospora tetrasperma. Mycol. Res. 1992;96:103–116. doi: 10.1016/S0953-7562(09)80923-4. [DOI] [Google Scholar]
- 53.McCluskey K, Wiest A, Plamann M. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J. Biosci. 2010;35:119–126. doi: 10.1007/s12038-010-0014-6. [DOI] [PubMed] [Google Scholar]
- 54.Jacobson DJ, et al. New findings of Neurospora in Europe and comparisons of diversity in temperate climates on continental scales. Mycologia. 2006;98:550–559. doi: 10.1080/15572536.2006.11832658. [DOI] [PubMed] [Google Scholar]
- 55.Luo R, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eid J, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 57.Chin C-S, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 58.Harris, R. S. Improved Pairwise Alignment of Genomic DNA, Ph.D. Thesis, The Pennsylvania State University (2007).
- 59.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun, Y., Svedberg, J., Hiltunen, M., Corcoran, P. & Johannesson, H. High-quality Neurospora tetrasperma genome assemblies. Figshare https://dx.doi.org/10.6084/m9.figshare.5327515.v1 (2017) [DOI] [PMC free article] [PubMed]
- 63.Sun, Y., Corcoran, P., Svedberg, J. & Johannesson, H. De novo genome assemblies of Neurospora tetrasperma based on Illumina HiSeq reads. Figshare https://dx.doi.org/10.6084/m9.figshare.5327809.v1 (2017)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw PacBio reads generated in this study have been deposited in the Sequence Read Archive as BioProject PRJNA398702, and raw Illumina mate-pair reads as BioProject PRJNA239947. Genome assemblies have been deposited at Figshare62,63. Scripts for identifying inversions from fragmented assemblies is available through GitHub: https://github.com/johannessonlab/InvDeNovo.