Abstract
Objective
We investigated the efficacy, safety, and patient satisfaction of once-weekly DPP-4 inhibitors (DPP-4Is).
Methods
Either of two once-weekly DPP-4Is, trelagliptin or omarigliptin, was administered alone or in combination with other antidiabetic drugs in 80 outpatients with type 2 diabetes mellitus for 3 months. The HbA1c, glycoalbumin (GA), body weight, and the Diabetes Treatment Satisfaction Questionnaire (DTSQ) scores were evaluated.
Results
Patients switching from other daily DPP-4Is (n=29) showed no significant changes in the HbA1c or GA levels. However, the HbA1c and GA levels of patients who had been naïve to DPP-4Is (n=37) significantly improved from 9.31±2.53% to 7.02±1.20% (p<0.001) and 26.7±11.8% to 17.3±5.7% (p<0.001), respectively. Several non-serious adverse events were reported, including nausea (n=1), abdominal distension (n=1), and constipation (n=1). In the DTSQs, the total score for six questions on the primary factors representing patient treatment satisfaction was not markedly changed in patients switching from daily to weekly DPP-4Is but was significantly improved from 21.0 to 28.0 (p<0.001) in patients naïve to DPP-4Is.
Conclusion
These findings suggest that the use of a once-weekly DPP-4I is effective and well-tolerated in diabetes treatment and improves treatment satisfaction.
Keywords: DPP-4 inhibitor, treatment satisfaction
Introduction
It has been reported that the risk of diabetic vascular complications is increased in patients with a history of intermittent treatments (1). Therefore, reducing the rate of treatment discontinuation is a pressing concern for preventing diabetic complications, including cardiovascular events. One suspected reason why diabetic patients discontinue treatment is that various kinds of medication are prescribed, resulting in a low adherence. Indeed, recent studies have shown that the rates of patient adherence to oral hypoglycemic agents is lower than those to drugs for other diseases, i.e. anti-hypertensive agents and agents for dyslipidemia (2,3). It has also been proven that the adherence to medications in diabetic patients increased with a decrease in the frequency of administration (4), and this increased adherence ameliorated their glycemic control (5). These reports underscore the necessity of further studies to examine whether or not a reduction in the frequency of administration improves patient satisfaction and the rates of treatment discontinuation.
In Japan, DPP-4 inhibitors (DPP-4Is) are prescribed to many patients with type 2 diabetes (6) and play an important role in the pharmacotherapy of diabetes. DPP-4Is not only improve glycemic control by inhibiting DPP-4, which degrades incretin secreted from the intestinal tract in a glucose concentration-dependent manner, but are also expected to exert cytoprotective effects on renal (7) and pancreatic β-cells (8). The once-weekly DPP-4Is trelagliptin and omarigliptin have recently been launched in Japan. The long-term efficacy of trelagliptin is derived from the inhibition of DPP-4 activities at low plasma concentrations (9). Omarigliptin has unique pharmacokinetics in that the chemical is passively reabsorbed in the renal tubules (10). As once-weekly DDP-4Is are commercially available only in Japan, their efficacy and safety in the general practices have not been established. From the perspective of adherence to medication described above, once-weekly DPP-4Is are also expected to help improve patient satisfaction.
We administered DPP-4I, trelagliptin or omarigliptin, alone or in combination with other antidiabetic drugs to patients with type 2 diabetes mellitus for three months and investigated the efficacy and safety as well as the patient satisfaction with the treatment.
Materials and Methods
The subjects were 80 outpatients with type 2 diabetes mellitus visiting TOSAKI Clinic for Diabetes and Endocrinology or Meieki East Clinic who had HbA1c levels ≥6.0% and <15.0% at baseline and who had had no changes in their diabetic treatment (e.g. diet therapy, exercise cure, medication) within the past 12 weeks (48 men, 32 women; mean age 57.1±14.9 years; mean duration of diabetes 6.0±6.8 years). The study excluded patients who had renal dysfunction [estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2], pregnant women, and patients who were judged as being inappropriate for the study by their physicians. Sixty-six patients completed the three-month administration. Fourteen cases discontinued treatment: patients who did not visit as scheduled (n=6); those with nausea (n=1), abdominal distention (n=1), and constipation (n=1); a patient who wished to switch to daily medication (n=1); a patient with hepatic metastasis of a malignant tumor (n=1); a patient who switched to a GLP-1 receptor agonist (n=1); and personal (n=1) or unknown reasons (n=1).
This study was conducted in accordance with the Ethical Guidelines for Clinical Research of the Ministry of Health, Labour and Welfare after providing explanation to the patients and obtaining their written informed consent. We obtained the approval of the institutional ethics review board of TDE Healthcare Corporation TOSAKI Clinic for Diabetes and Endocrinology (Approval No. 720902, 720903).
The subjects received trelagliptin 100 mg or omarigliptin 25 mg alone or in combination with other oral hypoglycemic agents, insulin, or GLP-1 receptor agonists. The switching group comprised patients who switched from daily DPP-4Is to once-weekly DPP-4Is (36 patients in total; receiving trelagliptin in 18 and omarigliptin in 18), and the naïve group comprised patients who had never used DPP-4Is before this study and received a weekly DPP-4I in addition to their existing treatment (44 patients in total; receiving trelagliptin in 27 and omarigliptin in 17).
This trial started on May 28, 2015. Patients who participated by November 25, 2015, were assigned to the trelagliptin group, and those who participated from November 26, 2015, were assigned to the omarigliptin group. Other anti-diabetic drugs were not changed during follow-up. In the switching group, 13 patients received a DPP-4I alone, and 23 received a DPP-4I in combination with other antidiabetic drugs. The daily DPP-IV inhibitors at baseline in the switching group were Sitagliptin 50 mg in 11, Sitagliptin 100 mg in 2, Alogliptin 25 mg in 6, Linagliptin 5 mg in 10, Teneligliptin 20 mg in 3, Vildagliotin 200 mg in 2, Anagliptin 200 mg in 1, and Saxagliptin 5 mg in 1. Concomitant antidiabetic drugs were sulfonylureas in 2, metformin in 13, sodium glucose co-transporter 2 (SGLT2) inhibitors in 2, glinides in 3, α-glucosidase inhibitors in 6, and pioglitazone in 1. Neither insulin nor GLP-1 receptor agonists was used. The mean number of concomitant drugs was 0.8 per patient. In the naïve group, 24 patients received a DDP-4I alone, and 20 received a DPP-4I in combination with other antidiabetic drugs. The concomitant antidiabetic drugs were sulfonylureas in 3, metformin in 10, SGLT2 inhibitor in 1, glinide in 1, α-glucosidase inhibitor in 1, insulin in 7, and GLP-1 receptor agonist in 1, and the mean number of concomitant drugs was 0.5.
Before the administration of trelagliptin or omarigliptin and at the end of the three-month administration, we evaluated the HbA1c, glycoalbumin (GA), body weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and serum biochemical laboratory data, including AST, ALT, γGTP, BUN, Cr, eGFR, LDL-C, HDL-C, and triglyceride, and the urinary albumin/creatinine ratio.
In addition, at baseline and the 1- or 2-month administration points, the Diabetes Treatment Satisfaction Questionnaire (DTSQ) as translated by Ishii et al. (11) was administered. The questionnaire consists of 8 questions quantified on a 7-point scale of 0 to 6 and is widely used to evaluate treatment satisfaction in diabetic patients.
Every four weeks, we asked the patients whether or not they were taking their medication as prescribed. At the same time, we confirmed episodes of hypoglycemia accompanied by a cognitive decline or hypoglycemic symptoms, such as cold sweats and tremors. For statistical analyses, a t-test was conducted using Microsoft Excel 2013, and p<0.05 was determined to be statistically significant.
Results
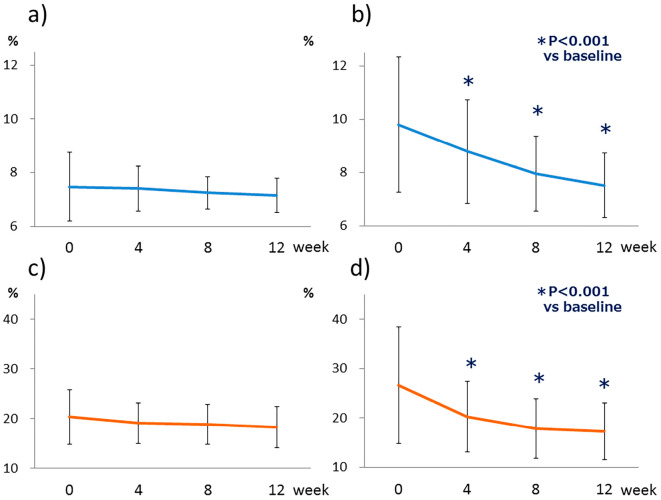
A total of 66 patients (29 in the switching group and 37 in the naïve group) were able to continuously receive DPP-4Is for 3 months. Although there were two instances of delayed doses of once-weekly DPP-4I, none of the patients omitted a dose. Patients in the switching group had no significant changes in their HbA1c or GA levels (Fig. 1a and c). However, the HbA1c and GA levels in the naïve group improved significantly from 9.31±2.53% to 7.02±1.20% (p<0.001) and 26.7±11.8% to 17.3±5.7% (p<0.001), respectively (Fig. 1b and d). The systolic and diastolic blood pressure were significantly decreased only in the naïve group (Table 1). The urinary albumin/creatinine ratio showed a decreasing trend without a significant difference(Tables 1-, -3), and eGFR levels showed no significant changes (Table 1).
Figure 1.
Continuous changes in the HbA1c and glycoalbumin levels during the three-month administration of weekly DPP-4 inhibitors. (a) HbA1c level in the switching group (n=29), (b) HbA1c level in the naïve group (n=37). (c) Glycoalbumin level in the switching group (n=16), (d) Glycoalbumin level in the naïve group (n=30).
Table 1.
Clinical Parameters of Patients Receiving Weekly DPP-4 Inhibitors (Trelagliptin or Omarigliptin) (Mean±Standard Deviation).
| Switching group | Naïve group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 3 months | p | n | Before | 3 months | p | n | ||
| Age (years) | 63.9±13.7 | 29 | 52.0±15.1 | 37 | |||||
| Sex (male/female) | 16/13 | 26/11 | |||||||
| HbA1c (%) | 6.98±1.27 | 6.66±0.64 | 0.170 | 29 | 9.31±2.53 | 7.02±1.20 | <0.001 | 37 | |
| Glycoalbumin | 20.3±5.5 | 18.3±4.1 | 0.110 | 16 | 26.7±11.8 | 17.3±5.7 | <0.001 | 30 | |
| Body weight (kg) | 62.1±12.2 | 62.4±12.0 | 0.227 | 29 | 69.6±13.8 | 69.5±13.7 | 0.855 | 37 | |
| BMI (kg/m2) | 24.2±4.0 | 24.4±4.0 | 0.159 | 29 | 25.6±4.4 | 25.6±4.3 | 0.846 | 37 | |
| Systolic BP (mmHg) | 129.3±18.8 | 128.4±16.6 | 0.753 | 29 | 133.8±27.4 | 124.6±17.9 | 0.022 | 37 | |
| Diastolic BP (mmHg) | 77.1±12.1 | 76.0±10.9 | 0.492 | 29 | 82.0±17.5 | 74.8±12.7 | <0.001 | 37 | |
| AST (IU/L) | 25.4±13.9 | 22.8±9.9 | 0.203 | 28 | 27.1±17.2 | 25.0±11.9 | 0.267 | 33 | |
| ALT (IU/L) | 25.4±13.9 | 22.5±11.2 | 0.162 | 28 | 36.8±30.2 | 33.5±24.3 | 0.386 | 33 | |
| γGTP (IU/L) | 46.4±44.7 | 36.4±27.6 | 0.024 | 28 | 52.6±51.6 | 40.2±27.6 | 0.021 | 33 | |
| BUN (mg/dL) | 15.8±5.8 | 16.1±5.1 | 0.625 | 29 | 15.2±3.7 | 14.6±3.2 | 0.137 | 33 | |
| Cr (mg/dL) | 0.73±0.28 | 0.71±0.29 | 0.227 | 29 | 0.72±0.17 | 0.72±0.18 | 0.769 | 33 | |
| eGFR (mL/min/1.73 m2) | 81.3±20.9 | 83.4±19.9 | 0.161 | 29 | 89.5±25.3 | 89.8±23.8 | 0.872 | 33 | |
| Urinary albumin/ creatinine ratio (mg/gCr) | 40.8±129.9 | 18.2±30.0 | 0.256 | 27 | 147.8±435.5 | 84.0±213.6 | 0.216 | 24 | |
| LDL-C (mg/dL) | 112.3±29.3 | 99.9±32.0 | 0.028 | 28 | 125.9±50.3 | 113.1±36.5 | 0.035 | 33 | |
| HDL-C (mg/dL) | 55.6±12.4 | 55.7±14.0 | 0.934 | 28 | 49.5±17.7 | 49.4±13.2 | 0.981 | 33 | |
| Triglyceride (mg/dL) | 155.8±89.9 | 163.7±124.4 | 0.714 | 28 | 192.6±127.0 | 166.5±92.0 | 0.161 | 33 | |
Table 2.
Clinical Parameters of Patients Receiving Trelagliptin (Mean±Standard Deviation).
| Switching group | Naïve group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 3 months | p | n | Before | 3 months | p | n | ||
| Age (years) | 65.3±13.7 | 17 | 50.1±15.3 | 24 | |||||
| Sex (male/female) | 11/6 | 18/6 | |||||||
| HbA1c (%) | 7.22±1.52 | 6.63±0.69 | 0.129 | 17 | 10.00±2.65 | 7.15±1.38 | <0.001 | 24 | |
| Glycoalbumin | 22.0±6.3 | 18.8±5.0 | 0.121 | 10 | 29.6±12.2 | 18.0±6.3 | <0.001 | 19 | |
| Body weight (kg) | 63.1±14.2 | 63.4±13.8 | 0.424 | 17 | 71.1±11.7 | 70.9±11.9 | 0.754 | 24 | |
| BMI (kg/m2) | 23.8±3.8 | 23.9±3.7 | 0.328 | 17 | 26.0±4.4 | 25.9±4.4 | 0.720 | 24 | |
| Systolic BP (mmHg) | 129.4±18.5 | 126.1±17.2 | 0.394 | 17 | 134.8±27.1 | 126.7±15.7 | 0.109 | 24 | |
| Diastolic BP (mmHg) | 79.3±12.8 | 75.5±11.9 | 0.106 | 17 | 84.5±17.8 | 76.8±11.9 | 0.006 | 24 | |
| AST (IU/L) | 27.9±17.4 | 22.5±11.2 | 0.066 | 17 | 27.1±17.5 | 25.3±12.6 | 0.377 | 22 | |
| ALT (IU/L) | 26.9±16.9 | 22.8±12.9 | 0.212 | 17 | 36.4±27.6 | 34.9±25.1 | 0.741 | 22 | |
| γGTP (IU/L) | 45.5±46.2 | 35.0±25.2 | 0.084 | 17 | 63.5±59.4 | 45.5±30.3 | 0.023 | 22 | |
| BUN (mg/dL) | 15.4±3.3 | 16.2±5.4 | 0.415 | 17 | 15.2±3.6 | 14.8±3.0 | 0.384 | 22 | |
| Cr (mg/dL) | 0.74±0.22 | 0.70±0.20 | 0.043 | 17 | 0.74±0.18 | 0.74±0.20 | 1.000 | 22 | |
| eGFR (mL/min/1.73 m2) | 78.7±19.8 | 83.3±20.1 | 0.028 | 17 | 90.1±25.7 | 90.6±26.2 | 0.824 | 22 | |
| Urinary albumin/ creatinine ratio (mg/gCr) | 20.1±26.9 | 15.3±14.0 | 0.300 | 15 | 175.2±474.1 | 99.6±231.7 | 0.200 | 20 | |
| LDL-C (mg/dL) | 106.8±30.5 | 88.1±29.2 | 0.033 | 16 | 129.4±55.0 | 115.9±38.4 | 0.105 | 22 | |
| HDL-C (mg/dL) | 55.5±13.0 | 54.9±15.4 | 0.747 | 16 | 50.8±14.5 | 50.1±12.4 | 0.717 | 22 | |
| Triglyceride (mg/dL) | 137.0±73.5 | 165.6±154.0 | 0.413 | 16 | 217.5±144.9 | 168.2±96.2 | 0.061 | 22 | |
Table 3.
Clinical Parameters of Patients Receiving Omarigliptin (Mean±Standard Deviation).
| Switching group | Naïve group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 3 months | p | n | Before | 3 months | p | n | ||
| Age (years) | 62.0±14.0 | 12 | 55.6±14.8 | 13 | |||||
| Sex (male/female) | 5/7 | 8/5 | |||||||
| HbA1c (%) | 6.63±0.74 | 6.69±0.59 | 0.548 | 12 | 8.03±1.76 | 6.78±0.78 | 0.006 | 13 | |
| Glycoalbumin | 17.6±2.3 | 17.5±1.8 | 0.741 | 6 | 21.7±9.8 | 16.2±4.6 | 0.013 | 11 | |
| Body weight (kg) | 60.6±9.2 | 61.0±9.3 | 0.381 | 12 | 66.8±17.2 | 67.0±16.7 | 0.648 | 13 | |
| BMI (kg/m2) | 24.9±4.5 | 25.0±4.5 | 0.336 | 12 | 24.9±4.5 | 25.0±4.3 | 0.586 | 13 | |
| Systolic BP (mmHg) | 129.1±19.9 | 131.6±16.0 | 0.569 | 12 | 131.9±29.0 | 120.8±21.5 | 0.111 | 13 | |
| Diastolic BP (mmHg) | 74.1±10.8 | 76.8±9.8 | 0.134 | 12 | 77.5±16.6 | 71.2±13.7 | 0.112 | 13 | |
| AST (IU/L) | 21.4±3.4 | 23.2±8.0 | 0.447 | 11 | 27.1±17.3 | 24.4±11.0 | 0.520 | 11 | |
| ALT (IU/L) | 23.1±7.6 | 22.0±8.7 | 0.559 | 11 | 30.7±36.4 | 37.0±23.4 | 0.354 | 11 | |
| γGTP (IU/L) | 45.2±43.4 | 38.3±31.6 | 0.124 | 12 | 30.9±18.0 | 29.5±18.2 | 0.630 | 11 | |
| BUN (mg/dL) | 16.3±8.3 | 16.1±4.8 | 0.855 | 12 | 15.1±4.0 | 14.3±3.8 | 0.123 | 11 | |
| Cr (mg/dL) | 0.71±0.35 | 0.72±0.38 | 0.470 | 12 | 0.69±0.15 | 0.68±0.13 | 0.559 | 11 | |
| eGFR (mL/min/1.73 m2) | 85.0±22.9 | 83.6±20.5 | 0.492 | 12 | 88.2±25.4 | 88.1±19.4 | 0.960 | 11 | |
| Urinary albumin/ creatinine ratio (mg/gCr) | 66.6±194.0 | 21.8±42.9 | 0.300 | 12 | 11.0±8.2 | 6.2±3.0 | 0.300 | 4 | |
| LDL-C (mg/dL) | 119.6±27.3 | 115.5±29.5 | 0.518 | 12 | 118.9±40.7 | 107.4±33.3 | 0.161 | 11 | |
| HDL-C (mg/dL) | 55.7±12.2 | 56.7±12.4 | 0.621 | 12 | 46.8±23.3 | 48.1±15.3 | 0.734 | 11 | |
| Triglyceride (mg/dL) | 180.8±106.2 | 161.2±74.9 | 0.351 | 12 | 142.8±58.7 | 163.0±87.4 | 0.252 | 11 | |
In biochemistry tests, the γ-GTP levels were decreased in both groups, and there were no significant differences in the AST or ALT level in either group (Table 1). The LDL-C levels were decreased in both groups, but there were no significant changes in the HDL-C levels or triglycerides in either group (Table 1). Three adverse events occurred during the three-month period: 1 case each of nausea, abdominal distension, and constipation. There were no cases of serious hypoglycemia.
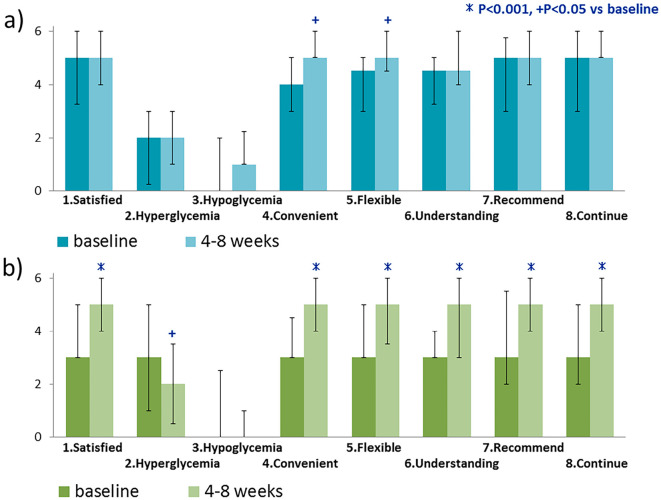
The DTSQ score significantly increased for two questions in the switching group: Q4 (Convenient) and Q5 (Flexible) (Fig. 2a). In the naïve group, there were significant increases in the scores of six questions: Q1 (Satisfied), Q4 (Convenient), Q5 (Flexible), Q6 (Understanding), Q7 (Recommend), and Q8 (Continue) (Fig. 2b). The total scores of 6 questions representing satisfaction (Q1, Q4, Q5, Q6, Q7, and Q8) showed an increasing trend from 27.5 to 29.5 (p=0.061) in the switching group and significantly increased from 21.0 to 28.0 (p<0.001) in the naïve group (Fig. 3).
Figure 2.
(a) Patient treatment satisfaction in the switching group (n=26); blue, before administration; light blue, after administration, (b) Patient treatment satisfaction in the naïve group (n=33); green, before administration; light green, after administration.
Figure 3.
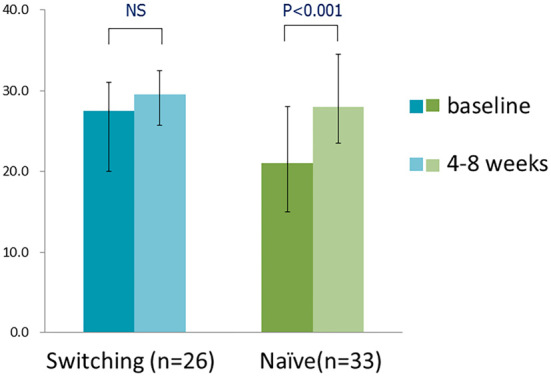
The total score of 6 questions of the primary factors representing satisfaction (Q1 [Satisfied], Q4 [Convenient], Q5 [Flexible], Q6 [Understanding], Q7 [Recommend], and Q8 [Continue] (switching group, n=26; naïve group, n=33); blue or green, before administration; light blue or light green, after administration).
Discussion
In the current study, a weekly DPP-4I (either trelagliptin or omarigliptin) was administered to patients who had previously received either daily DPP-4I or who were naïve to DPP-4Is. These novel weekly DPP-4Is induced no severe adverse events during the three-month treatment period and provided comparable results in glycemic control to daily DPP-4Is. The scores of satisfaction for convenience and flexibility in patients receiving weekly DPP-4Is were significantly better than those for daily DPP-4Is.
In this study, the HbA1c and GA levels showed a slight decreasing trend without significant changes by switching from daily to weekly DPP-4Is. A clinical study showed that once-weekly trelagliptin 100 mg was not inferior to once-daily alogliptin 25 mg in its HbA1c-lowering effect (12), and once-weekly omarigliptin 25 mg was comparable to once-daily sitagliptin 50 mg in its HbA1c-lowering effect (13). Therefore, the results obtained in this study do not imply the superior efficacy of weekly DPP-4Is to daily DPP-4Is. Daily DPP-4Is may not have exerted drastically superior effects to weekly administered agents due to the low adherence to daily DPP-4Is before switching to the weekly dosage in some patients. Among patients who completed the three-month administration, some took their weekly DPP-4I a day late, but none of them forgot to take it. If switching to weekly DPP-4s leads to improvement in the adherence to medication, it may be considered a good treatment option in terms of glycemic control.
The γ-GTP and LDL-C levels showed significant decreases in both groups. Although the mechanisms of these effects are not known, the improved patient satisfaction with treatments of diabetes induced by participation in this study and weekly DPP-4Is might motivate patients to improve their lifestyle.
In the treatment satisfaction survey, the score significantly increased for six questions in the naïve group but only for two questions in the switching group. The satisfaction level in the switching group was already high with prior administration of daily DPP-4Is, which may have resulted in a smaller increase in the satisfaction level after switching to weekly DPP-4Is. Since this study did not compare daily and weekly DPP-4Is in the naïve group, we are limited in our ability to determine which regimen results in greater satisfaction. In addition, this study is a single-arm study without a control group; therefore, the placebo effect must be considered when interpreting the results of DTSQ. An additional limitation of this study is that only those who gave their informed consent participated in the study. Therefore, the study may have included many patients who were already keen to receive the weekly formulation. Furthermore, due to the governmentally imposed restriction on the administration of new drugs in Japan, the study was conducted only in patients who were able to visit the clinic every two weeks, which may have led to the inclusion of many patients who were very willing to receive treatments. These limitations might have resulted in an inflated percentage of satisfied patients.
The total score for six questions as the primary factors representing treatment satisfaction was significantly higher in the naïve group than in the naïve group before administration, suggesting that weekly DPP-4Is might result in high treatment satisfaction in patients who show interest in proposed once-weekly oral drug administration. In a previous questionnaire survey in patients with type 2 diabetes, 67% and 33% of patients wished to receive weekly and daily formulations, respectively (14). According to this survey, the number of patients who wished to receive weekly formulation was higher among relatively young patients (18-44 years of age) and non-medicated patients than in others. In the current report, there were some patients who were not positive about taking antidiabetic drugs but started treatment regardless. Most of them were young and/or drug-naïve and agreed to take the medication because of the once-weekly administration regimen. Considering adverse reactions, it would be ideal to start with daily DPP4-Is. However, in patients who do not consent to the administration of daily formulation, weekly DPP-4Is are an option for achieving favorable glycemic control through early intervention.
Saisho et al. reported that the total score of 6 questions in the DTSQ was a significant predictor of patient dropout, and the optimal cut-off value was 22.5 (sensitivity 63.2%, specificity 70.8%) (15). In our current study, the total score of 6 questions significantly increased from 20.7±8.3 to 28.5±6.2 (p<0.001). The mean score that was less than the cut-off value at baseline exceeded the cut-off value after the introduction of weekly DPP-4Is, suggesting that treatment with weekly DPP-4Is may reduce the risk of dropout from treatments.
In the analysis of patients receiving other drugs daily (e.g. antidiabetic drugs, antihypertensive drugs, and antihyperlipidemic drugs), the total scores of the 6 questions significantly increased from 24.1±8.5 to 29.1±5.9 (p=0.021, n=19) in the switching group and from 21.9±8.7 to 28.9±6.1 (p<0.001, n=27) in the naïve group. These results were different from those of our pre-survey expectation. We presumed that the satisfaction levels in the patients who took many daily oral drugs would not increase even if the dosing frequency of only one drug decreased from daily to weekly. However, comments from patients included, “My blood glucose level improved even though the number of drugs decreased by one,” and, “Reducing the number of drugs, even by one, is good.” There may be differences in the viewpoints of patients and healthcare providers, and the convenience of the weekly formulation for patients may be greater than that healthcare providers expect. However, comments in the switching group included, “[The number of drugs I take] is irrelevant because I entrust my treatment to my physician,” and, “I had high expectations for the new drug but could not feel any particular change,” suggesting that preferences for weekly or daily formulations differ among patients, so patients should first be asked about their preferences before prescriptions are made. Other comments recorded on the treatment satisfaction survey included, “I don't need to carry it on bus trips. I will not forget to take it,” and, “I feel easier taking fewer drugs.” In the second Diabetes Attitudes, Wishes and Needs (DAWN2) study which is a survey using the Problem Areas in Diabetes Questionnaire (PAID) in 17 countries, 43% of Japanese patients with diabetes responded that pharmacotherapy affected most or all of their daily life, but only 11% responded that healthcare providers asked them how diabetes affected their daily life (Japan ranked 15th out of 17 countries) (16). Healthcare providers should therefore suggest alternative treatment options to their patients in order to identify patients' preferences and to establish a better physician-patient relationship.
Family members whose elderly diabetic patients needed nursing care because of dementia commented that reduced the dosing frequency reduced their burden. If the number of drugs taken daily can be reduced through the selection of a weekly formulation, it can be expected to reduce the burden on caregivers and may therefore be a good indication of these drugs for these patients.
Conclusion
We administered the novel once-weekly regimen of DPP-4Is trelagliptin or omarigliptin to patients with type 2 diabetes mellitus and investigated the efficacy and safety as well as the patient satisfaction. Despite the relatively short administration period of three months, the present study suggested that the use of weekly DPP-4Is was effective and safe and affected the patient satisfaction. However, the treatment satisfaction varied among patients and often differed from the presumptions of healthcare providers. Therefore, it is important to present options to patients before writing prescriptions. Longer-term studies evaluating the efficacy, safety, and patient satisfaction should be considered in the future.
Author's disclosure of potential Conflicts of Interest (COI).
Takahiro Tosaki: Honoraria, Eli Lilly, Astellas, AstraZeneca, Mitsubishi Tanabe, MSD and Takeda; Research funding, Mitsubishi Tanabe, Daiichi Sankyo and Takeda. Hideki Kamiya: Honoraria, Eli Lilly, Ono, Novartis, Astellas, MSD, Sanofi and Mitsubishi Tanabe; Research funding, Johnson & Johnson, Daiichi Sankyo, Eli Lilly, Kyowa Hakko Kirin, Taisho Toyama, MSD, Mitsubishi Tanabe, Sanofi, Takeda, Japan Tobacco, Novo Nordisk and Ono. Yoshiro Kato: Research funding, Johnson & Johnson, Daiichi Sankyo, Eli Lilly, Kyowa Hakko Kirin, Taisho Toyama, MSD, Mitsubishi Tanabe, Sanofi, Takeda, Japan Tobacco, Novo Nordisk and Ono. Masaki Kondo: Research funding, Johnson & Johnson, Daiichi Sankyo, Eli Lilly, Kyowa Hakko Kirin, Taisho Toyama, MSD, Mitsubishi Tanabe, Sanofi, Takeda, Japan Tobacco, Novo Nordisk and Ono. Jiro Nakamura: Honoraria, Kyowa Hakko Kirin, Ono, Pfizer, Eli Lilly, Novartis, Sanofi, MSD, Taisho Toyama, Mitsubishi Tanabe, Astellas and Shionogi; Research funding, Johnson & Johnson, Daiichi Sankyo, Eli Lilly, Kyowa Hakko Kirin, Taisho Toyama, MSD, Mitsubishi Tanabe, Sanofi, Takeda, Japan Tobacco, Novo Nordisk and Ono.
References
- 1.Tanaka M, Ito H, Nemoto A, et al. Relationship between the history of intermittent treatment for type 2 diabetes mellitus and the risk of diabetic vascular complications. J Jpn Diabetes Soc 58: 100-108, 2015. [Google Scholar]
- 2.Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 23: 112-119, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med 25: 284-290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care 20: 1512-1517, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother 38: 1357-1362, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Oishi M, Yamazaki K, Okuguchi F, et al. Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002-2011 in Japan (JDDM32). J Diabetes Investig 5: 581-587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanasaki K, Shi S, Kanasaki M, et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 63: 2120-2131, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Leibowitz G, Cahn A, Bhatt DL, et al. Impact of treatment with saxagliptin on glycaemic stability and β-cell function in the SAVOR-TIMI 53 study. Diabetes Obes Metab 17: 487-494, 2015. [DOI] [PubMed] [Google Scholar]
- 9.McKeage K. Trelagliptin: first global approval. Drugs 75: 1161-1164, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Biftu T, Sinha-Roy R, Chen P, et al. Omarigliptin (MK-3102): a novel long-acting DPP-4 inhibitor for once-weekly treatment of type 2 diabetes. J Med Chem 57: 3205-3212, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Ishi H, Bradley C, Riazi A, Barendse S, Yamamoto T. Japanese version of the Diabetes Treatment Satisfaction Questionaire (DTSQ): translation and clinical evaluation. J Clin Exp Med 192: 809-814, 2000. [Google Scholar]
- 12.Inagaki N, Onouchi H, Maezawa H, et al. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol 3: 191-197, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Gantz I, Chen M, Mirza A, et al. Effect of MK-3102, a novel once-weekly DPP-4 inhibitor, over 12 weeks in patients with type 2 diabetes mellitus. 2012. Abstract 101. [Google Scholar]
- 14.Hauber AB, Tunceli K, Yang JC, et al. A survey of patient preferences for oral antihyperglycemic therapy in patients with type 2 diabetes mellitus. Diabetes Ther 6: 75-84, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saisho Y, Itoh H. Relationship between treatment satisfaction and intension to drop out in outpatients with type 2 diabetes. J Jpn Diabetes Soc 55: 768-773, 2012. [Google Scholar]
- 16.Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes attitudes, wishes and needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 30: 767-777, 2013. [DOI] [PubMed] [Google Scholar]