Abstract
Objective
Smoking is common in patients with allergic diseases. The aim of this study was to evaluate the cross-sectional association between the current smoking status and total and specific Immunoglobulin E (IgE) levels in Korean adults.
Methods
Data were obtained from the 2010 Korean National Health and Nutrition Examination Survey, a national cross-sectional study. We analyzed the data of subjects whose smoking status and serum IgE levels were of acceptable quality.
Results
A total of 1,963 subjects (1,118 never smokers, 340 ex-smokers, and 505 current smokers) were included. The total IgE levels and specific IgE levels to house dust mite Dermatophagoides farinae (Df), cockroach, and dog allergens in never smokers were significantly (p<0.0001) lower than in ex-smokers or current smokers. After adjusting for other variables, current smokers independently had significantly higher levels of total IgE and cockroach-specific IgE than ex-smokers or never smokers. The proportions of subjects with total IgE ≥150 kU/L and specific IgE ≥0.35 kU/L to Df-specific IgE were significantly (p value for trend <0.05) increased in ex-smokers and current smokers. The total IgE levels and IgE levels specific to Df, cockroaches, and dogs significantly (p value for trend <0.05) and proportionally increased with increasing numbers of cigarettes smoked daily.
Conclusion
Smoking was associated with elevated total IgE levels and IgE levels specific to Df, cockroach, and dog allergens in a cumulative, dose-dependent manner. Furthermore, current smoking status was an independent risk factor for elevated total IgE levels and IgE levels specific to cockroach allergen.
Keywords: tobacco smoke, immunoglobulin E
Introduction
Immunoglobulin E (IgE) is a key mediator in allergic diseases such as allergic rhinitis, allergic asthma, and atopic dermatitis caused by increased Th2 immune response. IgE antibodies specific to foreign proteins or glycoproteins are produced by B cells in the process of sensitization (1). IgE sensitizes mast cells and basophils by binding to high-affinity receptor for IgE (FcεRI) expressed at the surface of these cells (2). When IgE-FcεRI complexes are crosslinked by allergens, these cells degranulate and release vasoactive amines, lipid mediators, chemokines, and other cytokines (2). Several genes associated with asthma and other allergic disease have been identified through genome-wide association studies (3). However, environmental factors appear to be more important than genetic factors in determining whether or not an atopic individual will develop an allergic disease. The prevalence of allergic diseases has increased worldwide over the last few decades (4). Many common environmental factors are associated with these trends (4). Changes towards a westernized, urban, and affluent lifestyle appear to be accompanied by an increasing prevalence of aeroallergen sensitization and atopic diseases (5-7).
Smoking is a leading cause of preventable death, contributing to approximately 58,000 deaths annually in South Korea (8). Tobacco smoke contains carcinogens and high levels of carbon monoxide and nitrogen oxide. It has numerous adverse health effects (9). Smoking is common in patients with allergic diseases. They show increased levels of total and specific IgE (10). Asthmatic patients who are active smokers and who have parents or close friends who smoke are more likely to have asthma-associated symptoms (11). In the Korean general population, a history of smoking is significantly associated with the prevalence of sensitization to house dust mite Dermatophagoides farinae (Df), but not with total IgE levels (12). There are no clear-cut results regarding the effects of smoking exposure on allergic sensitization or total IgE levels (10), although active or passive tobacco smoke exposure has been found to reduce the risk of allergic sensitization and allergic rhinitis in adults (13-15). However, data regarding the association between total IgE levels or IgE levels specific to common allergens and the current smoking status or daily smoking amount in the general population are limited.
The aim of this study was to evaluate the association between a current smoking status and IgE levels in Korean adults based on a cross-sectional study. We also determined the potential effects of smoking status or the amount of smoking on total IgE levels and sensitization to common allergens in this population.
Materials and Methods
Study population
This study was based on data obtained from the 2010 Korean National Health and Nutrition Examination Survey (KNHANES), a national cross-sectional study conducted by the Korea Center for Disease Control and Prevention, Ministry of Health and Welfare in South Korea. The sampling units were households selected via a complex, stratified, multistage probability cluster survey. Its sample included all non-institutionalized civilians aged ≥18 years of South Korea. The probability of being sampled was weighted for each participant. All participants completed a four-part questionnaire exploring health, health behavior, health examinations, and nutrition. All questionnaires were administered by medical doctors and trained interviewers in person at mobile examination centers or at the participant's home. Written informed consent was obtained from all subjects. The Korean Centers for Disease Control and Prevention obtained written informed consent to analyze the subject sera. The study was approved by the Institutional Review Board (IRB) of Korea Centers for Disease Control and Prevention (IRB: 2010-02CON-21-C).
Data collection
All candidate participants were informed that they had been randomly selected to voluntarily participate in a national representative survey conducted by the Ministry of Health and Welfare of Korea in 2010. They could refuse to participate under the National Health Enhancement Act supported by the National Statistics Law of Korea. Health examinations were conducted in 2010, including medical history-taking, a physical examination, anthropometric measurements, and biochemical data. A questionnaire was used to explore health-related behaviors.
Sociodemographic characteristics including age (years), sex (percentage of individuals of male sex for), education level (percentage of individuals completing middle school or higher education), household income (percentage of households on the lowest 25% of family income), habitation (percentage of subjects living in an urban area), and spouse presence (percent of individuals having a spouse) were obtained from a self-reported questionnaire. Exercise was defined as walking regularly at least five times per week for at least 30 minutes each time or engaging in moderate exercise at least five times per week for at least 30 minutes at a time or vigorous exercise at least three times per week for at least 20 minutes at a time during the survey period. The frequency of alcohol consumption was classified into two categories: <1 time per a month, and ≥1 time per a month. The prevalence of asthma, allergic rhinitis, and atopic dermatitis was assessed based on physician's diagnoses.
Trained medical staff performed physical examinations in accordance with standardized procedures. Body weight and height were measured for subjects wearing light indoor clothing without shoes to the nearest 0.1 kg and 0.1 cm, respectively. The body mass index (BMI) was calculated as the individual's weight in kilograms divided by the square of the height (in meters). The waist circumference was measured using a measuring tape (seca 200, seca Deutschland, Hamburg, Germany) to the nearest 0.1 cm in a horizontal plane at the midpoint between the iliac crest and the costal margin at the end of normal expiration. The percentage of wholebody fat was measured using dual-energy X-ray absorptionmetry (QDR 4500A, Hologic, Waltham, USA) at mobile examination centers operated by licensed and trained technicians following a standard protocol. After a 12-hour overnight fast, blood samples were obtained from antecubital veins, centrifuged, refrigerated at the examination sites, and transferred on ice to a central laboratory in Seoul on the same day.
Definition of smokers and the amount of smoking
Based on their smoking habit, participants were considered nonsmokers (never smoked or smoked <5 packs in their lifetime) or smokers (smoked ≥5 packs in their lifetime). Smokers were divided into ex-smokers and current smokers depending on whether or not they were currently smoking. For current smokers, the average daily amount of cigarettes smoked was determined via self-reported questionnaires.
Total and specific IgE levels
The total IgE level was measured using an ImmunoCAP100 (Phadia, Uppsala, Sweden) and an immunoradiometric assay with a 1470 WIZARD gamma counter (PerkinElmer, Turku, Finland). The specific IgE levels against Df, cockroach, and dog allergens were measured with the same method. The detection ranges of total IgE and specific IgE levels were 2.00 - 5,000.00 kU/L and ≤100.0 kU/L, respectively. Total IgE levels of ≤2.00 kU/L and ≥5,000.00 kU/L were scored as 1.99 kU/L and 5,000.01 kU/L, respectively. A specific IgE level of ≥100.0 kU/L was scored as 100.01 kU/L. We defined sensitization to an allergen as a total IgE level ≥150 kU/L. We defined specific sensitization to Df, cockroach, and dog allergens as a specific IgE level ≥0.35 kU/L.
Statistical analyses
Data are expressed as frequencies, percentages, or means with standard deviations. One way analyses of variance and chi-squared tests were used to compare the mean values for continuous variables and percentages for categorical variables. A linear-by-linear association test was employed to explore the associations between sensitization to total or specific IgE and smoking status or the amount of smoking. Multivariate logistic regression analyses were performed to estimate the associations between serum IgE levels and smoking status. We first analyzed non-adjusted variables (Model 1) and then adjusted for age and sex (Model 2). We then (Model 3) adjusted for the variables in Model 2 plus BMI, drinking habit, exercise habit, income, and medical history of asthma, allergic rhinitis, and atopic dermatitis. The SAS version 9.1 software program (SAS Institute, Cary, USA) for Windows was used for all analyses. Statistical significance was considered when a p value was less than 0.05.
Results
Clinical characteristics and IgE levels in subjects by smoking status
The baseline characteristics of the study population are shown in Table 1. A total of 1,963 subjects were divided into groups of non-smokers (n=1,118), ex-smokers (n=340), and current smokers (n=505) based on the self-reported questionnaires regarding their smoking status. In the total population, men were commonly found to be ex-smokers or current smokers than women. A higher percentage of wholebody fat was associated with never smokers. Ex- or current smokers were more likely to have higher frequency of alcohol drinking and higher education than never smokers. There were no significant differences in the presence of a physician-diagnosed history of asthma, allergic rhinitis, or atopic dermatitis among the three smoking habit groups.
Table 1.
Clinical Characteristics and IgE Levels of Subjects Based on Smoking Status.
| Characteristics | Non-smoker (n=1,118) | Ex-smoker (n=340) | Current smoker (n=505) | p value (ANOVA) |
|---|---|---|---|---|
| Male, % | 20 (1.5) | 84.8 (2.7) | 89.5 (1.8) | <0.0001 |
| Age, years | 44.6±0.72 | 47.8±1.1 | 43.4±0.8 | 0.0113 |
| BMI, kg/m2 | 23.5±0.1 | 24.1±0.2 | 24.0±0.2 | 0.0153 |
| WC, cm | 78.9±0.4 | 84.0±0.79 | 83.5±0.5 | <0.0001 |
| Whole body fat, % | 32.0±0.3 | 25.2±0.5 | 23.8±0.4 | <0.0001 |
| Frequency of alcohol ≥ one month, % | 45.1 (2.0) | 68.6 (3.3) | 80.6 (2.2) | <0.0001 |
| Exercising group | 20.6 (1.7) | 30.9 (3.2) | 27.9 (2.5) | 0.0036 |
| Living place (urban), % | 77.8 (3.3) | 77.2 (4.2) | 78.8 (3.8) | 0.8813 |
| Education>12 years, % | 66.5 (2.1) | 71.7 (2.9) | 74.5 (2.6) | 0.0198 |
| Income (lowest quartile), % | 20.8 (2.0) | 12.9 (2.6) | 19.7 (2.5) | 0.0553 |
| Spouse, % | 65.6 (1.9) | 80.8 (2.5) | 63.5 (2.7) | <0.0001 |
| Physician-diagnosed allergic rhinitis, % | 15.3 (1.3) | 14.9 (2.1) | 14.3 (2.0) | 0.9101 |
| Physician-diagnosed asthma, % | 2.7 (0.6) | 3.1 (1.4) | 2.2 (0.7) | 0.8081 |
| Physician-diagnosed atopic dermatitis, % | 3.0 (3.7) | 2.5 (1.5) | 2.7 (0.8) | 0.9315 |
| Total IgE, kU/L | 68.1 (61.9-75.0) | 114.4 (96.0-136.4) | 159.2 (133.1-190.4) | <0.0001 |
| IgE to Dermatophagoides farina, kU/L | 0.17 (0.14-0.20) | 0.28 (0.21-0.37) | 0.41 (0.30-0.55) | <0.0001 |
| IgE to cockroach, kU/L | 0.08 (0.07-0.09) | 0.11 (0.09-0.14) | 0.17 (0.14-0.21) | <0.0001 |
| IgE to dog, kU/L | 0.026 (0.023-0.028) | 0.035 (0.029-0.042) | 0.045 (0.037-0.054) | <0.0001 |
All values are expressed as number (%), mean ± SD, or geometric mean (95% confidence interval).
BMI: body mass index, WC: waist circumference, IgE: immunoglobulin E
The total serum IgE levels ranged from 1.9 to 500.1 kU/L. The mean value of total IgE was 99.5 kU/L in all subjects. The total IgE levels in never smokers (geographic mean value, 68.1 kU/L) were significantly (p<0.0001) lower compared than those in ex-smokers (geographic mean value, 114.4 kU/L) or current smokers (geographic mean value, 159.2 kU/L). The specific IgE levels to Df, cockroach, and dog allergens in ex-smokers or current smokers were significantly (p<0.0001) higher than those in never smokers.
Factors associated with elevated total and specific IgE levels
We adjusted for variables that might influence the association between smoking status and serum IgE levels (Table 2). Without adjusting for any variables, ever-smokers showed significantly higher levels of total IgE levels and IgE levels specific to Df, cockroach, and dog allergens (Model 1) than non-smokers. After adjusting for age and sex, the total IgE levels and IgE levels specific to cockroach allergen were significantly higher in current smokers than in ex-smokers or never smokers (Model 2). There was no significant difference in the IgE levels specific to Df or dog allergen among the three groups. After adjusting for the variables in Model 2 plus BMI, drinking habit, exercise habit, income, and medical history of asthma, allergic rhinitis, and atopic dermatitis, current smokers had independently higher (p<0.05) levels of total and cockroach-specific IgE levels than ex-smokers or never smokers (Model 3).
Table 2.
Multivariate Adjusted Smoking Status and Total or Specific IgE Level.
| Total IgE (kU/L) | IgE for Df (kU/L) | IgE for cockroach (kU/L) | IgE for dog (kU/L) | |
|---|---|---|---|---|
| Model 1 | ||||
| Non-smoker | 68.1 (61.9-75) | 0.17 (0.14-0.2) | 0.08 (0.07-0.09) | 0.026 (0.023-0.028) |
| Ex-smoker | 114.4 (96-136.4) | 0.28 (0.21-0.37) | 0.11 (0.09-0.14) | 0.035 (0.029-0.042) |
| Current smoker | 159.2 (133.1-190.4) | 0.41 (0.3-0.55) | 0.17 (0.14-0.21) | 0.045 (0.037-0.054) |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Model 2 | ||||
| Non-smoker | 82.8 (74-92.5) | 0.22 (0.18-0.27) | 0.09 (0.08-0.11) | 0.029 (0.025-0.033) |
| Ex-smoker | 88.5 (73.7-106.3) | 0.21 (0.16-0.29) | 0.09 (0.07-0.11) | 0.031 (0.025-0.038) |
| Current smoker | 123.8 (101.3-151.4) | 0.28 (0.2-0.38) | 0.13 (0.1-0.16) | 0.038 (0.03-0.048) |
| p value | 0.0031 | 0.4243 | 0.0283 | 0.1483 |
| Model 3 | ||||
| Non-smoker | 80.6 (72.3-89.8) | 0.2 (0.17-0.25) | 0.09 (0.08-0.1) | 0.028 (0.024-0.032) |
| Ex-smoker | 87.5 (71.9-106.4) | 0.22 (0.16-0.31) | 0.09 (0.07-0.11) | 0.031 (0.025-0.04) |
| Current smoker | 120.1 (98.6-146.4) | 0.28 (0.21-0.39) | 0.12 (0.1-0.15) | 0.038 (0.03-0.048) |
| p value | 0.004 | 0.295 | 0.0428 | 0.1244 |
All values are expressed as geometric mean (95% confidence interval). Statistically significant values are shown in bold. Non-adjusted variables were used in Model 1. Variables in Model 1 plus age and sex were used in Model 2. Variables in Model 2 plus BMI, drink, exercise, income, and medical history of asthma, allergic rhinitis, and atopic dermatitis were used in Model 3.
Df: Dermatophagoidesfarina, IgE: immunoglobulin E
Trends for total and specific IgE levels according to the smoking status and number of cigarettes smoked
Table 3 shows the total and specific IgE levels for Df, cockroach, and dog allergens according to the current smoking status. The proportion of subjects with total IgE ≥150 kU/L and specific IgE ≥0.35 kU/L to Df was significantly (p value for trend <0.05) higher in ex-smokers and current smokers than in non-smokers. However, there was no significant trend for sensitization to cockroach or dog allergens in never smokers, ex-smokers, or current smokers.
Table 3.
Trends of Sensitization for Total and Specific IgE according to Smoking Status.
| Total IgE (≥150 kU/L) | IgE for Df (≥0.35 kU/L) | IgE for cockroach (≥0.35 kU/L) | IgE for dog (≥0.35 kU/L) | |
|---|---|---|---|---|
| Coefficient (95% CI) | ||||
| Non-smoker | Reference | Reference | Reference | Reference |
| Ex-smoker | 1.108 (0.743, 1.651) | 1.200 (0.784, 1.838) | 0.810 (0.515, 1.275) | 2.476 (1.256, 4.879) |
| Current smoker | 1.702 (1.148, 2.524) | 1.482 (1.028, 2.137) | 0.990 (0.673, 1.458) | 2.119 (1.035, 4.339) |
| p value for trend | 0.0062 | 0.0316 | 0.9269 | 0.0658 |
Df: Dermatophagoidesfarina, IgE: immunoglobulin E
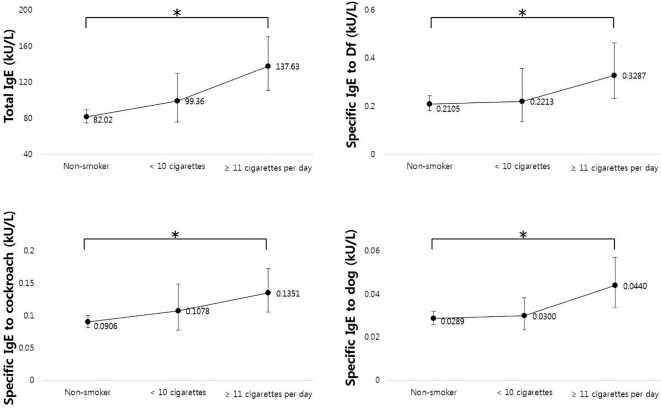
The results of linear-by-linear association test according to the number of cigarettes smoked per day are shown in Figure. We divided the subjects into three groups: non-smokers, current smokers with average <10 cigarettes per day, and current smokers with average ≥10 cigarettes per day. The total IgE levels and IgE levels specific to Df, cockroach, and dog allergens proportionally and significantly (p value for trend <0.05) increased with increasing numbers of cigarettes smoked daily.
Figure.
IgE levels according to the average number of cigarettes smoked daily. Total IgE levels, IgE levels specific to Df, cockroach, and dog allergens were analyzed using a linear-by-linear association test (* p<0.05 for trend). Df: Dermatophagoides farinae, IgE: immunoglobulin E
Discussion
We investigated the association between current smoking status and total IgE levels in general Korean adults. The total IgE and IgE levels specific to Df, cockroach, and dog allergens were significantly higher in ex-smokers and current smokers than those in never smokers. In multivariate analyses, current smoking habit was an independent risk factor for high total IgE levels and IgE levels specific to cockroaches. Furthermore, the total IgE levels and IgE levels specific to Df increased with increasing numbers of cigarettes smoked daily.
Cigarette smoke contains many toxic substances and strong pro-inflammatory stimuli. It is widely recognized as a significant risk factor for allergic and respiratory diseases (16). Active smoking and passive exposure are both risk factors for asthma and allergic rhinitis in adolescents (17). In patients with asthma, current smoking is associated with a lower pulmonary function and frequent symptoms of asthma, such as recent wheezing and recent exercise-induced wheezing (18). In addition, active smoking and passive exposure to smokers were associated with increased prevalence of atopic dermatitis in a meta-analysis (19). According to these reports, an epidemiologically strong association may exist between tobacco smoking and allergic diseases. However, a few studies have shown that smoking does not alter nasal symptoms or the nasal-specific quality of life in patients with allergic rhinitis (10, 20). Sustained smoking is negatively associated with the development of allergic sensitization to aeroallergens (21). Such negative associations might be due to the immunosuppressive effect of smoking (21). Therefore, the impact of smoking on the pathogenesis and symptoms of allergic diseases remains inconclusive. The mechanisms involved in such associations should be further elucidated.
Tobacco smoke has many harmful effects on the immune system, including humoral and cellular immunity (22). It is well documented that cigarette smoking causes an accumulation of neutrophils and macrophages in human lung tissues and induces Th17-type airway inflammation with airway remodeling (23). Furthermore, tobacco cigarette smoke exposure reduced the allergen-induced Th2 response and development of airway hyperreactivity in mice (24). The Th1 response might be augmented under conditions of cigarette smoke-related neutrophil inflammation with underlying allergic inflammation. However, factors such as cigarette smoke may initiate Th2/eosinophilic inflammation in the pathogenesis of allergic diseases. Exposure to cigarette smoke was found capable of enhancing Th2-driven airway inflammation and delaying inhalational tolerance in a murine model of allergic asthma (25). In steroid-naïve asthmatics, smoking can attenuate age-related decreases in the IgE level and maintain eosinophilic inflammation (26).
Moerloose et al. showed that smoke and ovalbumin (OVA) exposure augmented the OVA-specific IgE levels in mice (27). In addition, the numbers of dendritic cells were increased in mice exposed to combined OVA and cigarette smoke, which supports the notion that dendritic cells are important in the induction and maintenance of eosinophilic airway inflammation (28). Furthermore, cigarette smoke exposure can enhance OVA-specific IgE levels and antigen-induced mast cell activation, thereby exacerbating airway inflammation and remodeling (29). Although smoking alone induces Th17-type-related inflammation-characterized neutrophils and macrophages, chronic exposure to tobacco smoke in OVA-induced allergic mice enhances mast cell activation and allergic airway inflammation (29, 30). Our present study showed that smoking was associated with elevated total IgE levels. This increase in the total IgE levels was correlated with a cumulative dose-response effect of smoking exposure. Few data have shown that cigarette smoke can suppress IgE-mediated degranulation and cytokine release in murine mast cells (31). Our findings are compatible with those of a systematic review and meta-analysis report showing that active smoking is associated with an elevated total IgE level and increased risk of sensitization to common allergens (32-34).
Allergies to American house dust mite Df, cockroaches, and dogs are associated with allergic rhinitis and asthma. Smoking increases the permeability of the bronchus and may also increase the access of antigens to antigen-presenting cells, which can promote sensitization to allergens (35). Lanckacker et al. demonstrated that cigarette smoke and ubiquitous environmental allergens, such as house dust mites, can synergistically result in pronounced Th2-driven airway inflammation in mice (36). Our results also indicated that tobacco smoke exposure was associated with sensitization to common environmental allergens, such cockroaches and dogs, which is consistent with the results of previous studies (34, 35). Yao et al. showed that elevated serum cotinine levels were significantly associated with IgE sensitization to cockroaches, with a potential dose-dependent relationship (34). However, little is known about the possible dose effect of tobacco smoke exposure on allergic sensitization or the effect of tobacco exposure regarding different allergens. Further understanding of how the components of tobacco smoke influence the immune response is necessary.
Smoking remains one of the most prevalent modifiable risk factors for chronic disease in adults. Despite improved understanding in pathophysiology and continual advances in disease management, a small subgroup of allergic diseases remain partially controlled or refractory to standard treatment. Smoking cessation as a confounding factor in asthma, allergic rhinitis, and atopic dermatitis can result in healthier patients by making patients more responsive to medical treatments (37). Qiaoling et al. showed that tobacco smoke exposure and the serum IgE levels (total or specific IgE) are significantly correlated with the clinical response to allergy immunotherapy in children with asthma and allergic rhinitis (38).
Several limitations associated with the present study warrant mention. First, the study design was cross-sectional; we were therefore unable to determine the cause-and-effect association in these analyses. Second, we did not obtain any data regarding allergic symptoms or disease status, such as eosinophil counts, spirometric parameters, and exhaled nitric oxide levels, which should be included in future analyses to determine the clinical significance of our findings. Third, the study is subject to recall bias, as physician-diagnosed allergic rhinitis, asthma, and atopic dermatitis were self-reported. Fourth, secondhand smoking was not included in this survey and analysis.
In conclusion, we found that smoke exposure was associated with elevated total IgE levels and IgE levels specific to Df, cockroach, and dog allergens in a cumulative dose-dependent manner. In addition, we found that current smoking status was an independent risk factor for elevated total IgE levels and IgE levels specific to cockroach allergen. Therefore, exposure to tobacco smoke should be minimized in order to reduce the risk of allergic sensitization.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gadermaier E, Levin M, Flicker S, Ohlin M. The human IgE repertoire. Int Arch Allergy Immunol 163: 77-91, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol 6: 761-771, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev 242: 31-50, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Yang SN, Hsieh CC, Kuo HF, et al. The effects of environmental toxins on allergic inflammation. Allergy Asthma Immunol Res 6: 478-484, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douwes J, Pearce N. Asthma and the westernization ‘package'. Int J Epidemiol 31: 1098-1102, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy 60: 1357-1360, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Von Hertzen LC, Haahtela T. Asthma and atopy - the price of affluence? Allergy 59: 124-137, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Lamichhane DK, Park SG, et al. Association between second-hand smoke and psychological well-being amongst non-smoking wageworkers in Republic of Korea. Ann Occup Environ Med 28: 49, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CM, Corey CG, Rostron BL, Apelberg BJ. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health 15: 390, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousquet PJ, Cropet C, Klossek JM, Allaf B, Neukirch F, Bousquet J. Effect of smoking on symptoms of allergic rhinitis. Ann Allergy Asthma Immunol 103: 195-200, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Mak KK, Ho RC, Day JR. The associations of asthma symptoms with active and passive smoking in Hong Kong adolescents. Respir Care 57: 1398-1404, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Kang JW, Baek SH, Rha MS, Kim JH. The effects of alcohol consumption and smoking on allergy risk in Korean adults. Am J Rhinol Allergy 28: e35-e39, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Saulyte J, Regueira C, Montes-Martinez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS Med 11: e1001611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topp R, Thefeld W, Wichmann HE, Heinrich J. The effect of environmental tobacco smoke exposure on allergic sensitization and allergic rhinitis in adults. Indoor Air 15: 222-227, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hancox RJ, Welch D, Poulton R, et al. Cigarette smoking and allergic sensitization: a 32-year population-based cohort study. J Allergy Clin Immunol 121: 38-42.e3, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Romero Palacios PJ. [Asthma and tobacco smoke]. Arch Bronconeumol 40: 414-418, 2004(in Spanish). [PubMed] [Google Scholar]
- 17.Gomez M, Vollmer WM, Caceres ME, Jossen R, Baena-Cagnani CE. Adolescent smokers are at greater risk for current asthma and rhinitis. Int J Tuberc Lung Dis 13: 1023-1028, 2009. [PubMed] [Google Scholar]
- 18.Yoo S, Kim HB, Lee SY, et al. Effect of active smoking on asthma symptoms, pulmonary function, and BHR in adolescents. Pediatr Pulmonol 44: 954-961, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Kantor R, Kim A, Thyssen J, Silverberg JI. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol 75: 1119-1125. e1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bousquet PJ, Fabbro-Peray P, Janin N, et al. Pilot study assessing the impact of smoking on nasal-specific quality of life. Allergy 59: 1015-1016, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy 56: 328-332, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2: 372-377, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Floreani AA, Rennard SI. The role of cigarette smoke in the pathogenesis of asthma and as a trigger for acute symptoms. Curr Opin Pulm Med 5: 38-46, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Tilp C, Bucher H, Haas H, Duechs MJ, Wex E, Erb KJ. Effects of conventional tobacco smoke and nicotine-free cigarette smoke on airway inflammation, airway remodelling and lung function in a triple allergen model of severe asthma. Clin Exp Allergy 46: 957-972, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Van Hove CL, Moerloose K, Maes T, Joos GF, Tournoy KG. Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir Res 9: 42, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasaki T, Matsumoto H, Nakaji H, et al. Smoking attenuates the age-related decrease in IgE levels and maintains eosinophilic inflammation. Clin Exp Allergy 43: 608-615, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Moerloose KB, Pauwels RA, Joos GF. Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. Am J Respir Crit Care Med 172: 168-172, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lambrecht BN, Hoogsteden HC, Pauwels RA. Dendritic cells as regulators of the immune response to inhaled allergen: recent findings in animal models of asthma. Int Arch Allergy Immunol 124: 432-446, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Kim DY, Kwon EY, Hong GU, Lee YS, Lee SH, Ro JY. Cigarette smoke exacerbates mouse allergic asthma through Smad proteins expressed in mast cells. Respir Res 12: 49, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min MG, Song DJ, Miller M, et al. Coexposure to environmental tobacco smoke increases levels of allergen-induced airway remodeling in mice. J Immunol 178: 5321-5328, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Mortaz E, Folkerts G, Engels F, Nijkamp FP, Redegeld FA. Cigarette smoke suppresses in vitro allergic activation of mouse mast cells. Clin Exp Allergy 39: 679-687, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Accordini S, Janson C, Svanes C, Jarvis D. The role of smoking in allergy and asthma: lessons from the ECRHS. Curr Allergy Asthma Rep 12: 185-191, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Feleszko W, Ruszczynski M, Jaworska J, Strzelak A, Zalewski BM, Kulus M. Environmental tobacco smoke exposure and risk of allergic sensitisation in children: a systematic review and meta-analysis. Arch Dis Child 99: 985-992, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Yao TC, Chang SW, Hua MC, et al. Tobacco smoke exposure and multiplexed immunoglobulin E sensitization in children: a population-based study. Allergy 71: 90-98, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Jarvis D, Chinn S, Luczynska C, Burney P. The association of smoking with sensitization to common environmental allergens: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol 104: 934-940, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Lanckacker EA, Tournoy KG, Hammad H, et al. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J 41: 1189-1199, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Johnson KS, Tankersley MS. Smoking cessation toolbox for allergists. Ann Allergy Asthma Immunol 103: 271-278; quiz 279,-281, 336, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Li M, Yue W, et al. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol 164: 210-217, 2014. [DOI] [PubMed] [Google Scholar]