Abstract
A 46-year-old woman presented with massive hematemesis, caused by the rupture of esophageal varices. The laboratory investigations showed pancytopenia, and imaging tests revealed hepatosplenomegaly and ascites. A diagnosis of systemic sarcoidosis was made based on biopsies of the liver, stomach, lungs, heart, and skin. Although fat deposition was predominant, non-caseating granuloma and cirrhotic changes were found in the liver. Non-caseating granuloma was also identified in a biopsy specimen from minute depressions of the gastric folds. This case illustrates the rare involvement of the digestive system in a case of systemic sarcoidosis.
Keywords: systemic sarcoidosis, gastric involvement, hepatic involvement, laparoscopy, esophagogastroduodenoscopy
Introduction
Sarcoidosis is a systemic inflammatory disease of unknown etiology characterized by the presence of multiple non-caseating granulomas (1). It can occur at all ages, although it usually develops before 50 years of age, with the incidence peaking at 20 to 39 years of age. In Japan, the incidence is 1.01 cases per 100,000 individuals (2). The etiology of sarcoidosis is unknown. Granulomas are typically seen in the lungs and lymph nodes, although any organ can be affected. Among organs of the alimentary system, the liver is most frequently involved, accounting for 50-90% of the patients (3-5). Most cases with hepatic involvement are asymptomatic and remain undiagnosed, and only a few cases develop progressive clinical features, such as liver cirrhosis, portal hypertension, chronic cholestasis, and liver failure (6). Apart from hepatic involvement, the prevalence of other alimentary tract lesions is low. The reported incidence of gastrointestinal lesions is 0.1% to 0.9% (7, 8).
We herein report a case of hepatic sarcoidosis presenting with esophageal variceal bleeding. Apart from liver fibrosis and portal hypertension, the patient exhibited multi-organ manifestations including the stomach, lungs, heart, and skin. Involvement of the digestive system with sarcoidosis in this case is discussed below.
Case Report
A 46-year-old woman was admitted to the hospital for massive hematemesis. Esophagogastroduodenoscopy revealed active bleeding from ruptured esophageal varices. She was treated with endoscopic variceal ligation. Computed tomography (CT) of the abdomen revealed hepatosplenomegaly and ascites. The patient was then referred to our hospital for intensive treatment and a further examination for portal hypertension.
Ten years prior to this admission, the patient had undergone abdominal ultrasound, CT, and magnetic resonance imaging (MRI) due to a diagnosis of elevated liver enzyme levels. However, the etiology could not be determined. In addition, multiple red eruptions developed on both her legs. The skin eruptions also remained undiagnosed despite a dermatological consultation. She had no history of alcoholism or blood transfusion and no family history of inherited disorders.
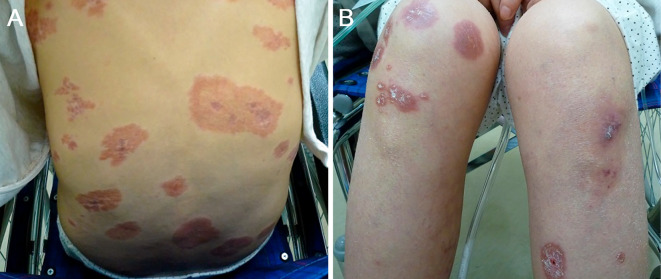
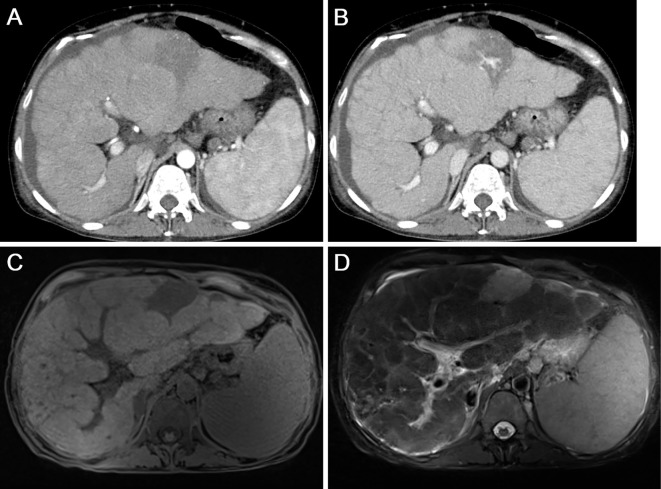
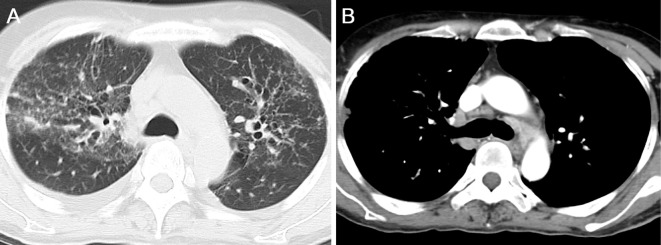
On admission, a physical examination revealed abdominal distension and bilateral edema of the lower extremities. She also had erythematous lesions on the legs, face, back, and head (Fig. 1). The liver edge was palpable approximately 5 cm below the right costal margin. An enlarged spleen was also palpable. Laboratory investigations revealed pancytopenia and elevation of hepatobiliary enzymes, with a marked increase in the alkaline phosphatase levels. Serological tests for hepatitis B and C and immunological tests, such as anti-nuclear antibody, rheumatoid factor, and anti-mitochondrial antibody, were negative. The levels of soluble interleukin 2 receptor (IL-2, 3,686 U/mL) and angiotensin-converting enzyme (ACE, 28.3 U/L) were both elevated. Abdominal CT with contrast media revealed an enlarged liver with an irregular surface and splenomegaly. Remarkably enlarged lymph nodes in the abdomen were also observed. (Fig. 2A and B). MRI revealed multiple intrahepatic nodules with heterogeneous intensities in T1-weighted images and low intensities in T2-weighted images (Fig. 2C and D). Chest CT revealed multiple small nodules and ground glass opacity bilaterally in the lungs, in addition to remarkable lymphadenopathy in the mediastinum (Fig. 3).
Figure 1.
Photographs of the skin lesions. Well-demarcated, erythematous skin lesions are seen on the back (A) and legs (B).
Figure 2.
Radiological images of the abdomen. Contrast-enhanced CT scans of the arterial phase (A) and venous phase (B) show an enlarged liver with an irregular surface and splenomegaly. On MRI, multiple intrahepatic nodules are displayed as heterogeneous intensities in T1-weighed images (C) and as low intensities in T2-weighed images (D).
Figure 3.
Chest CT images. CT images show bilateral ground glass opacities in the lungs, in addition to marked lymphadenopathy in the mediastinum.
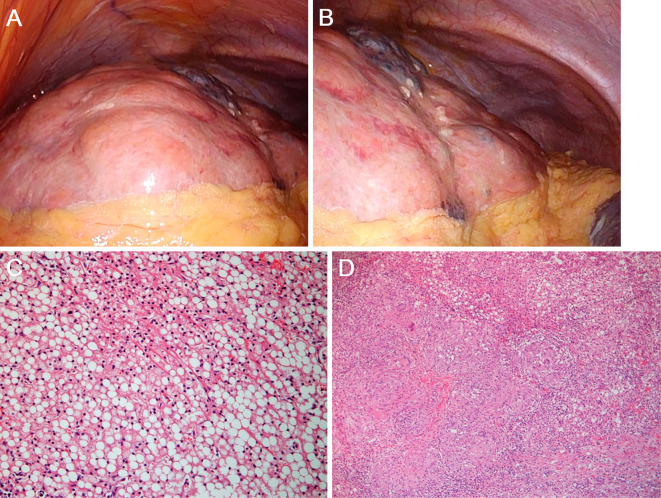
Subsequently, a laparoscopic examination was performed to arrive at a definitive diagnosis. On laparoscopy, the liver surface was irregular (Fig. 4A and B). Huge, yellow nodules 3-4 cm in diameter were observed. A histopathologic evaluation of the liver biopsy specimens revealed non-caseating granulomatous inflammation (Fig. 4C) with moderate periportal fibrosis, regenerative nodules, and significant lipid collection (Fig. 4D). Biopsies of the abdominal lymph nodes, skin lesions, and cardiac muscle revealed non-caseating granulomas as well, which led to the diagnosis of systemic sarcoidosis. Echocardiography revealed a decreased ventricular ejection fraction of 45% and ventricular aneurysms. Gallium scintigraphy revealed the myocardial uptake of gallium. Contrast-enhanced magnetic resonance imaging demonstrated delayed enhancement of the myocardium. Although atrioventricular block and thinning of the interventricular septum were absent, oral corticosteroid treatment (prednisolone, 30 mg) was administered based on the diagnosis of cardiac sarcoidosis.
Figure 4.
Macroscopic and microscopic images of the liver. Laparoscopy reveals an irregular liver surface with huge, yellow nodules 3-4 cm in diameter (A, B). Pathological, non-caseating granulomas are identified (C). Moderate periportal fibrosis, regenerative nodules, and significant lipid collection is also noted (D).
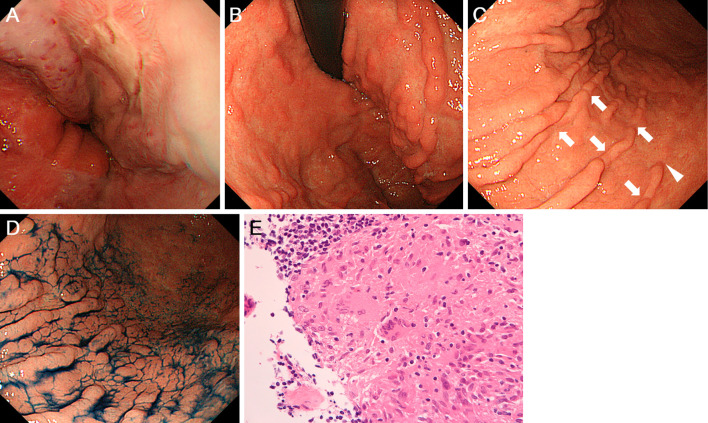
Although endoscopic variceal ligation had been performed earlier, the persistence of esophageal varices was seen on esophagogastroduodenoscopy (Fig. 5A). The varices were treated with endoscopic injection sclerotherapy with ligation. Although there were no gastric varices, multiple minute depressions were seen on the gastric folds. The mucosa of the upper gastric body showed a nodular appearance with furrow formation resembling a bamboo joint-like appearance, due to interruptions of the gastric folds (Fig. 5B). Multiple minute depressions were also noted in the middle-to-lower gastric body (Fig. 5C and D). Biopsy specimens taken from the depressions of the gastric folds contained non-caseating granulomatous inflammation, leading to the diagnosis of gastric sarcoidosis (Fig. 5E).
Figure 5.
Esophagogastroduodenoscopy images. Varices are seen in the esophagus (A). In the stomach, multiple minute depressions on the gastric folds are seen in the upper gastric body (B). Multiple minute depressions are also noted in the middle-to-lower gastric body before (C) and after indigo carmine spraying (D). Non-caseating granulomas are identified in the stomach (E).
Discussion
After the lungs and lymph nodes, the liver is the third-most commonly involved organ in systemic sarcoidosis. As described earlier, most cases with hepatic involvement are asymptomatic, and only 5-30% patients present with clinical symptoms, varying from non-specific manifestations, such as nausea and vomiting, to jaundice, abdominal pain, and hepatosplenomegaly (6). Abnormalities of liver function tests are seen in about 20-30% of the cases, whereas portal hypertension and cirrhosis are seen in less than 1% of patients (9). Portal hypertension associated with sarcoidosis was first reported in 1949 (10, 11). Although portal hypertension usually occurs in association with liver cirrhosis, in sarcoidosis patients, it might be present without evidence of cirrhotic changes in the liver (11).
The etiology of portal hypertension in liver sarcoidosis is not completely understood, and several mechanisms have been postulated. First, the small arteriovenous shunts within the granulomas may increase the portal blood flow, leading to elevation of the intrahepatic resistance (4-6). Second, the existence of sarcoid granulomas within the intrahepatic sinusoids and destruction of the sinusoidal structures likely induce increased resistance of the sinusoids. Third, presinusoidal obstruction by granulomas in the portal areas may increase the pressure of portal vein flow (3, 12). Finally, ischemic changes caused by primary granulomatous phlebitis of the portal vein and/or congestion caused by granulomatous phlebitis of the hepatic veins may result in cirrhosis and focal fibrosis, thereby increasing pre-and post-sinusoidal resistance (6, 13).
In the present case, laparoscopy revealed an irregular surface of the liver with huge yellow-colored nodules 3-4 cm in diameter. These were different from the known laparoscopic findings of liver sarcoidosis, which are small, whitish, flat lesions on the liver surface (14, 15). Furthermore, fat deposition was pathologically predominant in the liver, in addition to non-caseating granulomas. Although hepatocellular ballooning was absent in the biopsied specimen, we speculate that the leading cause of liver cirrhosis in the present case was non-alcoholic fatty liver disease rather than hepatic sarcoidosis, based on the macroscopic and microscopic features. This case therefore suggests that portal hypertension can occur in systemic sarcoidosis patients due to multifactorial causes or due to pathology that is not associated with non-caseating granulomas. A liver biopsy with or without laparoscopic observation might lead to a better understanding of the etiology of liver cirrhosis and portal hypertension in sarcoidosis patients.
The administration of corticosteroids is the standard treatment for advanced-stage sarcoidosis with life-threatening manifestations; however, the role of corticosteroids in the treatment of hepatic sarcoidosis is unknown. It has been reported that the use of corticosteroids leads to partial improvement in liver function tests. However, corticosteroids do not prevent the development of portal hypertension or liver cirrhosis (6). In the present case, we administered corticosteroid therapy, as the lungs, heart, and skin were involved. The clinical course of this patient needs to be carefully followed, particularly the portal hypertension and liver cirrhosis. The treatment options for refractory hepatic sarcoidosis include ursodeoxycholic acid and immunomodulators like azathioprine, methotrexate, hydroxychloroquine, and infliximab (16). However, the efficacy of these agents for the treatment of hepatic manifestations in sarcoidosis patients has not been fully evaluated. In patients who develop liver failure, liver transplantation is the only definitive treatment (17), although recurrence of the disease in the allograft has been reported (18, 19).
The gastrointestinal manifestations in systemic sarcoidosis remain unclear due to the rarity of involvement of these organs. With regard to gastric sarcoidosis, two major forms have been reported: gastric ulcer and gastric wall rigidity (7). Gastric ulcer formation is believed to be a result of localized infiltration with non-caseating granulomas. Diffuse infiltration throughout the gastric wall leads to thickening of the gastric folds, narrowing of the gastric lumen, and gastric wall rigidity. The latter has a manifestation similar to that of diffuse gastric cancer, i.e. gastric linitis plastica (8, 20). In the present case, interruptions of the gastric folds and multiple furrow formation on the folds were observed in the gastric body, resembling a bamboo joint-like appearance. Of note: multiple minute depressions on the gastric folds are seen in patients with Crohn's disease (21, 22). Swollen longitudinal folds transversed by erosive fissures or linear furrows, which are most frequently found at the gastric body and cardia of patients with Crohn's disease, have been referred to as a bamboo joint-like appearance. Since non-caseating granuloma formation is the major pathological feature in Crohn's disease as well, granuloma formation in the present patient may result in a similar macroscopic morphology to that of Crohn's disease. However, since our assumption is based on a single case, further studies are required to determine whether or not a bamboo joint-like appearance on the gastric body is a specific feature of gastric sarcoidosis.
In summary, we herein presented a case of systemic sarcoidosis involving the liver, stomach, heart, lungs, and skin. The patient presented with unusual morphology and gastric involvement, showing a bamboo joint-like appearance in the gastric body. A liver biopsy specimen revealed that fat deposition was predominant, in addition to non-caseating granuloma and cirrhotic changes. An in-depth evaluation by a biopsy via esophagogastroduodenoscopy and laparoscopy might help clarify the pathophysiology of the involvement of the digestive system in patients with systemic sarcoidosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 357: 2153-2165, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 31: 372-379, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Maddrey WC, Johns CJ, Boitnott JK, et al. Sarcoidosis and chronic hepatic disease: a clinical and pathologic study of 20 patients. Medicine (Baltimore) 49: 375-395, 1970. [DOI] [PubMed] [Google Scholar]
- 4.Devaney K, Goodman ZD, Epstein MS, et al. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol 17: 1272-1280, 1993. [PubMed] [Google Scholar]
- 5.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol 103: 3184-3192, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Blich M, Edoute Y. Clinical manifestations of sarcoid liver disease. J Gastroenterol Hepatol 19: 732-737, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara T, Hirahara N, Hyakudomi R, et al. Early gastric cancer associated with gastric sarcoidosis. Int Surg 100: 949-953, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fireman Z, Sternberg A, Yarchovsky Y, et al. Multiple antral ulcers in gastric sarcoid. J Clin Gastroenterol 24: 97-99, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Tan CB, Rashid S, Rajan D, et al. Hepatic sarcoidosis presenting as portal hypertension and liver cirrhosis: case report and review of the literature. Case Rep Gastroenterol 6: 183-189, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mino RA, Murphy AI Jr, Livingstone RG. Sarcoidosis producing portal hypertension; treatment by splenectomy and splenorenal shunt. Ann Surg 130: 951-957, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivonye C, Elhammali B, Henriques-Forsythe M, et al. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol 26: 508-509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valla D, Pessegueiro-Miranda H, Degott C, et al. Hepatic sarcoidosis with portal hypertension. A report of seven cases with a review of the literature. Q J Med 63: 531-544, 1987. [PubMed] [Google Scholar]
- 13.Moreno-Merlo F, Wanless IR, Shimamatsu K, et al. The role of granulomatous phlebitis and thrombosis in the pathogenesis of cirrhosis and portal hypertension in sarcoidosis. Hepatology 26: 554-560, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda H, Horie R, Zen K, et al. Hepatobiliary sarcoidosis: Typical appearance in laparoscopy. Digestive Endoscopy 20: 162-165, 2008. [Google Scholar]
- 15.Kataoka M, Nakata Y, Hiramatsu J, et al. Hepatic and splenic sarcoidosis evaluated by multiple imaging modalities. Intern Med 37: 449-453, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Judson MA. The treatment of pulmonary sarcoidosis. Respir Med 106: 1351-1361, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Ayyala US, Padilla ML. Diagnosis and treatment of hepatic sarcoidosis. Curr Treat Options Gastroenterol 9: 475-483, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Neuberger J, Jothimani D. Long-term immunosuppression for prevention of nonviral disease recurrence. Transplant Proc 37: 1671-1674, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag 12: 1623-1634, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korsager S. Sarcoidosis of the stomach. A case report. Scand J Respir Dis 60: 24-28, 1979. [PubMed] [Google Scholar]
- 21.Fujiya M, Sakatani A, Dokoshi T, et al. A bamboo joint-like appearance is a characteristic finding in the upper gastrointestinal tract of Crohn's disease patients: a case-control study. Medicine (Baltimore) 94: e1500, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hokama A, Nakamura M, Ihama Y, et al. Notched sign and bamboo-joint-like appearance in duodenal Crohn's disease. Endoscopy 40 (Suppl 2): E151, 2008. [DOI] [PubMed] [Google Scholar]