Abstract
A 78-year-old man presented with acute-onset fever and dyspnea. He had been taking Sho-seiryu-to for allergic rhinitis. A chest radiograph showed diffuse bilateral ground-glass opacities with subpleural sparing, crazy-paving pattern, and traction bronchiectasis. The patient's bronchoalveolar lavage fluid was bloody and transbronchial lung biopsy specimens showed alveolitis, organizing pneumonia, and type 2 alveolar epithelial cell proliferation. There were no clinical and laboratory findings suggestive of respiratory tract infection or connective tissue disease. Based on the clinical course and the exclusion of other etiologies, Sho-seiryu-to-induced pneumonitis with diffuse alveolar hemorrhage was considered. The patient's pneumonitis resolved after the discontinuation of the drug and the administration of systemic corticosteroid therapy.
Keywords: drug induced pneumonitis, diffuse alveolar hemorrhage, herbal medicine, Sho-seiryu-to, Xiao-Qing-Long-Tang
Introduction
Various drug-induced pulmonary parenchymal diseases have been reported, including interstitial pneumonia, eosinophilic pneumonia, pulmonary edema, and diffuse alveolar hemorrhage (DAH). Some drugs, such as amiodarone, cyclophosphamide, tetracycline, propylthiouracil, and penicillamine, are known to cause DAH (1, 2). However, DAH is rarely reported in patients using herbal medicines (3). We herein describe a case of pneumonitis with DAH that was probably caused by a herbal medicine, Sho-seiryu-to.
Case Report
A 78-year-old man was referred to our hospital due to fever, which had persisted for 6 days, followed by 1 day of dyspnea. He had a 50 pack-year history of smoking and his medical history included spinal canal stenosis and colonic polyp. One month previously, he had diagnosed by a primary care physician with allergic rhinitis and was prescribed Sho-seiryu-to, a herbal medicine, which he took for 17 days. The patient took no other drugs.
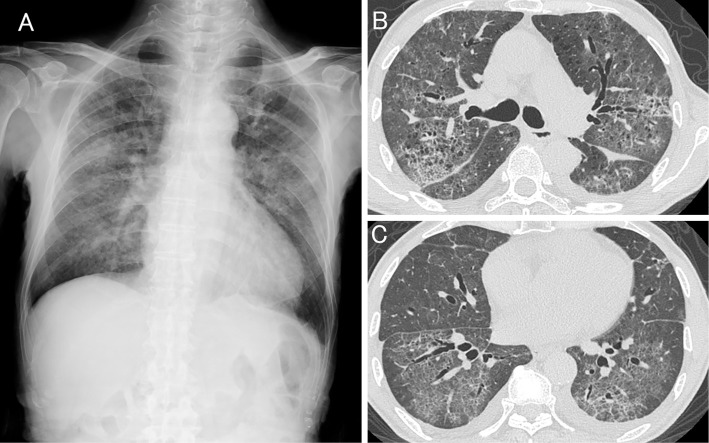
On admission, the patient was tachypneic with a respiratory rate of 30 breaths per minute. His body temperature was 37.1℃. Chest auscultation revealed bilateral fine crackles on both lungs. No physical findings suggestive of connective tissue disease or congestive heart failure (pitting edema of lower extremities and jugular venous distention) were observed. Chest radiography showed diffuse bilateral ground-glass opacities (Fig. 1A). High-resolution computed tomography (HRCT) of the chest revealed diffuse ground-glass opacities with subpleural sparing, interlobular septal thickening, a crazy-paving appearance and traction bronchiectasis (Fig. 1B and C). Laboratory tests showed elevated serum levels of lactate dehydrogenase (LDH), C-reactive protein and Krebs von den Lungen-6 (KL-6) and hypoxia. The patient's serum was negative for all autoantibodies. A peripheral blood lymphocyte stimulation test against Sho-seiryu-to was negative. A pulmonary function test showed restrictive impairment. The patient's bronchoalveolar lavage (BAL) fluid was bloody (Fig. 2) and neutrophilia, lymphocytosis and eosinophilia were observed in the cell differential count of the BAL fluid. No microorganisms grew on a BAL fluid culture. The results of a urinalysis were normal. These data are summarized in Table. The electrocardiography and echocardiography findings were normal. No microorganisms grew on a sputum culture. The patient's serum antibody titers against Mycoplasma pneumoniae, Chlamydia psittaci, Chlamydia pneumoniae, parainfluenza virus, respiratory syncytial virus and adenovirus were not elevated. A nasal swab was negative for influenza A and B virus antigens, and patient's urine was negative for Pneumococcal and Legionella pneumophila serotype 1 antigens. A transbronchial lung biopsy specimen obtained from the left lower lobe showed alveolitis, organizing pneumonia, and the proliferation of type 2 alveolar epithelial cells (Fig. 3).
Figure 1.
A chest radiograph showing diffuse bilateral ground-glass opacities (A). High-resolution computed tomography of the chest confirms the presence of diffuse ground-glass opacities with subpleural sparing, interlobular septal thickening, a crazy-paving appearance and traction bronchiectasis (B, C). Small amounts of pleural effusion and emphysema are also seen on both upper lobes.
Figure 2.

The gross appearance of the bronchoalveolar lavage fluid was bloody.
Table.
Laboratory Findings on Admission.
| Hematology | Immunology | Pulmonary function tests | ||||||||
| WBC | 7,800 | /μL | CRP | 4.7 | mg/dL | VC | 2.01 | L | ||
| Neutrophil | 86.6 | % | KL-6 | 526 | U/mL | %VC | 68.8 | % | ||
| Lymphocyte | 8.3 | % | SP-D | 154 | ng/mL | FEV1.0 | 1.95 | % | ||
| Monocyte | 2.3 | % | Rheumatoid factor | <5 | IU/mL | FEV1.0% | 97.0 | % | ||
| Basophil | 0.1 | % | anti-CCP antibody | <0.6 | U/mL | DLco | 8.45 | mL/min/mmHg | ||
| Eosinophil | 1.9 | % | anti-nuclear antibody | <40 | U/mL | %DLco | 103.0 | % | ||
| Hb | 13.9 | g/dL | anti-ds-DNA-IgG antibody | <1.2 | IU/mL | BAL fluid | ||||
| PLT | 33.6 | ×104/μL | anti-SS-A antibody | <1.0 | U/mL | Total cell counts | 2.72 | ×105/mL | ||
| Biochemistry | anti-SS-B antibody | <1.0 | U/mL | Neutrophil | 45.0 | % | ||||
| Alb | 2.6 | g/dL | anti-Sm antibody | <1.0 | U/mL | Lymphocyte | 17.5 | % | ||
| AST | 44 | IU/L | anti-RNP antibody | <2.0 | U/mL | Eosinophil | 14.0 | % | ||
| ALT | 25 | IU/L | MPO-ANCA | <1.0 | U/mL | Macrophage | 19.5 | % | ||
| LDH | 585 | IU/L | PR3-ANCA | <1.0 | U/mL | Others | 4.0 | % | ||
| BUN | 12 | mg/dL | anti-GBM antibody | <2.0 | U/mL | CD4/CD8 | 0.2 | |||
| Cre | 0.7 | mg/dL | LST (SI) | 137 | % | Culture | negative | |||
| Na | 133 | mEq/L | Arterial blood gas analysis (oxygen 2 L/min) | Urinalysis | ||||||
| K | 3.7 | mEq/L | pH | 7.387 | Protein | negative | ||||
| Cl | 98 | mEq/L | PaCO2 | 48.2 | Torr | Occult bood | negative | |||
| BNP | 117.6 | pg/mL | PaO2 | 58.0 | Torr | Casts | negative | |||
WBC: white blood cells, Hb: hemoglobin, PLT: platelet, Alb: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, Cre: creatinine, BNP: brain natriuretic peptide, CRP: C-reactive protein, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, anti-CCP antibody: anti-cyclic citrullinated peptide antibody, anti-ds-DNA-IgG antibody: anti-double stranded-DNA-IgG antibody, MPO-ANCA: myeloperoxidase anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase3 antineutrophil cytoplasmic antibody, GBM: glomerular basement membrane, LST: lymphocyte stimulation test against Sho-seiryu-to, SI: stimulation index, PaCO2: arterial carbon dioxide tension, PaO2: arterial oxygen tension, VC: vital capacity, FEV1.0: forced expiratory capacity in one second, DLco: diffusing capacity for carbon monoxide
Figure 3.
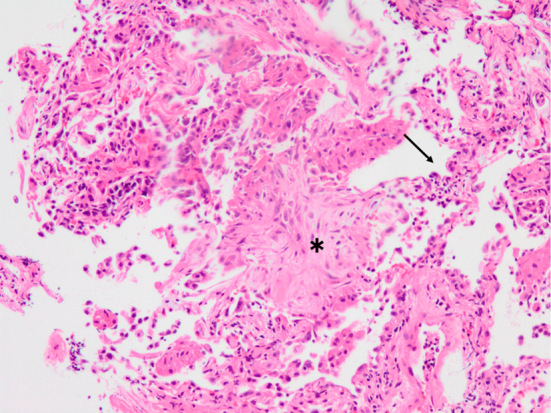
A transbronchial lung biopsy specimen shows alveolitis; organizing pneumonia (asterisk); proliferation of type 2 alveolar epithelial cells (arrow) (Hematoxylin and Eosin staining, ×200).
Based on the chest HRCT findings, the bloody appearance of the BAL fluid, the clinical course of the appearance of pneumonitis after the initiation of Sho-seiryu-to usage and the exclusion of other etiologies of pneumonitis, (such as respiratory tract infections and connective tissue diseases), the patient was diagnosed with Sho-seiryu-to-induced pneumonitis with DAH. Sho-seiryu-to was discontinued immediately upon admission and systemic corticosteroid therapy with intravenous methylprednisolone (1,000 mg per day for 3 days) followed by oral prednisolone (30 mg per day) was introduced after a bronchoscopic examination. Thereafter, his symptoms, respiratory condition, and chest radiologic findings began to gradually improve and resolved within a month. The serum levels of LDH and KL-6 normalized after treatment. Peripheral eosinophilia was not seen observed during the disease course. Oral prednisolone was tapered and was discontinued without a recurrence of pneumonitis.
Discussion
In the present case, pneumonitis with DAH developed after the initiation of Sho-seiryu-to treatment and without the administration of other drugs. In addition, no clinical or laboratory findings suggestive of respiratory tract infection, connective tissue disease, or congestive heart failure were observed. There have been several case reports on Sho-seiryu-to-induced pneumonitis (4-6). Similarly, in the present case, the diagnosis of Sho-seiryu-to-induced pneumonitis with DAH was the most plausible; however, iron staining of the BAL fluid was not preformed and the transbronchial lung biopsy (TBLB) specimens did not show evidence of DAH.
Drug-induced DAH can be divided into two types according to the pathophysiology (1, 2). One type is a small-vessel, vasculitis-like process that is anti-neutrophil cytoplasmic antibody (ANCA)-positive; the other is accompanied by acute lung injury, such as diffuse alveolar damage. ANCA-related DAH has been known to be induced by propylthiouracil, penicillamine, and tetracyclines; on the other hand, DAH accompanied by acute lung injury has been linked to amiodarone, cyclophosphamide, gemcitabine, and gefitinib (1, 2, 7, 8). In the present case, the patient was ANCA-negative and DAH developed with an accompanying acute lung injury, which was demonstrated as organizing pneumonia on histological examination.
Sho-seiryu-to is a herbal medicine that is thought to have anti-allergic and anti-inflammatory effects and which is usually used for the treatment of common cold and allergic rhinitis. A review of the literature revealed only 3 case reports of drug-induced pneumonitis caused by Sho-seiryu-to (4-6). In those cases, the drug was indeed used to treat common cold or allergic rhinitis and the duration from the initiation of treatment to the onset of pneumonitis ranged from 3 to 11 days. A peripheral blood lymphocyte stimulation test against Sho-seiryu-to was positive in 2 of the 3 reported cases. A BAL fluid analysis, which was performed in one case, revealed lymphocytosis, eosinophilia, and a decreased CD4/CD8 ratio. One of these reported cases showed marked peripheral blood eosinophilia and an eosinophilic pneumonia pattern on CT; however, a bronchoscopic examination was not performed. Pneumonitis only resolved after the discontinuation of Sho-seiryu-to in 1 case. Systemic corticosteroid therapy was required for the resolution of pneumonitis in the other 2 cases.
In pneumonitis caused by herbal medicines (such as Sho-saiko-to), the interval between the initiation of treatment with the herbal medicine and the onset of pneumonitis was usually approximately 2 months (9). Physicians should be aware that drug-induced pneumonitis can occur secondarily to the administration of herbal medicines within a period of less than 1 month, as is exemplified by our case. The previously reported lung injury patterns of herbal medicine-induced pneumonitis include hypersensitivity pneumonitis, diffuse alveolar damage, and eosinophilic pneumonia (10, 11). Reports of DAH caused by herbal medicines are rare (3). To the best of our knowledge, this was the first case report of pneumonitis with DAH that was precipitated by the use of Sho-seiryu-to.
One report stated that pneumonitis was induced by another herbal medicine, Sho-saiko-to due to an allergic or immunologic reaction to Ogon, which is one of the components of the drug (12). Sho-seiryu-to, which our patient took, does not include Ogon. Nevertheless, previous studies have demonstrated that Sho-seiryu-to can suppress Th2 reactions (13, 14) and result in the promotion of Th1 reactions in mice. This mechanism might have been involved in the development of pneumonitis caused by Sho-seiryu-to; however, the specific component that triggered these effects remains to be identified.
In conclusion, we herein reported a case of pneumonitis with DAH that was likely caused by a herbal medicine, Sho-seiryu-to. Physicians should consider the possibility of drug-induced pneumonitis in patients who develop respiratory symptoms after using herbal medicines, even if the interval from the initiation of treatment with the drug to the onset of symptoms is less than 1 month.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Flieder DB, Travis WD. Pathologic characteristics of drug-induced lung disease. Clin Chest Med 25: 37-45, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Camus P, Bonniaud P, Fanton A, Camus C, Baudaun N, Foucher P. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med 25: 479-519, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Iida Y, Takano Y, Ishiwatari Y, et al. Diffuse alveolar hemorrhage associated with Makyo-kanseki-to administration. Intern Med 55: 3321-3323, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hata Y, Uehara H. A case where herbal medicine Sho-seiryu-to induced interstitial pneumonitis. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 43: 23-31, 2005(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 5.Suzuki T, Higa M, Takahashi M, Saito S, Kikuchi N, Yamamuro W. A case of Sho-seiryu-to-induced pneumonia with a marked increase in peripheral eosinophils. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 44: 578-582, 2006(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 6.Wada H, Inoue S, Ozaki Y, Kitamura S, Ueda K, Nagatani Y. A case of Syo-seiryu-to-induced interstitial pneumonia. Nihon Kyoubu Rinsyou (Jpn J Chest Dis) 75: 197-202, 2016(in Japanese, Abstract in English). [Google Scholar]
- 7.Nagashima O, Tajima K, Ito J, et al. A case of non-small cell lung cancer accompanied with hemorrhage after chemotherapy including gemcitabine. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 44: 215-219, 2006(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 8.Sakoda Y, Kitasato Y, Kawano Y, Mizuta Y, Takata S, Kawasaki M. A case of alveolar hemorrhage caused by gefitinib. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 49: 506-510, 2011(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 9.Sato A, Toyoshima M, Kondo A, Ohta K, Sato H, Ohsumi A. Pneumonitis induced by the herbal medicine Sho-saiko-to in Japan. Nihon Kyobu Shikkan Gakkai Zasshi (J Jpn Respir Soc) 35: 391-395, 1997(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10.Tomioka H, Hashimoto K, Ohnishi H, et al. An autopsy case of interstitial pneumonia probably induced by Sho-saiko-to. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 37: 1013-1018, 1999(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 11.Matsushima H, Takayanagi N, Tokunaga D, et al. CT findings of drug-induced pneumonitis--characteristic CT findings by histological group and subgroup. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 42: 145-152, 2014(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 12.Toyoshima M, Chida K, Suda T, Harada M. A case of pneumonitis caused by Seisin-renshi-in, herbal medicine. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 46: 31-34, 2008(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 13.Nagai T, Arai Y, Emori M, et al. Anti-allergic activity of a Kampo (Japanese herbal) medicine “Sho-seiryu-to (Xiao-Qing-Long-Tang)” on airway inflammation in a mouse model. Int Immunopharmacol 4: 1353-1365, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Wang SD, Lin LJ, Chen CL, et al. Xiao-Qing-Long-Tang attenuates allergic airway inflammation and remodeling in repetitive Dermatogoides pteronyssinus challenged chronic asthmatic mice model. J Ethnopharmacol 142: 531-538, 2012. [DOI] [PubMed] [Google Scholar]