Abstract
Interstitial lung disease (ILD) has rarely been reported as a manifestation of giant cell arteritis (GCA). We herein report a unique case of GCA in a 76-year-old woman who presented with ILD as an initial manifestation of GCA. Ten years before admission, she had been diagnosed with granulomatous ILD of unknown etiology. Corticosteroid therapy induced remission. One year after the cessation of corticosteroid therapy, she was admitted with a persistent fever. After admission, she developed left oculomotor paralysis. Positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography (18F-FDG PET/CT) proved extremely useful in establishing the diagnosis. Our case promotes awareness of GCA as a possible diagnosis for granulomatous ILD with unknown etiology.
Keywords: giant cell arteritis, interstitial lung disease, granulomatous ILD, 18F-FDG PET/CT, vasculitis-associated ILD
Introduction
Giant cell arteritis (GCA) is characterized by inflammation of large- and medium-sized vessels. Common symptoms are a fever, fatigue, headache, and jaw claudication (1). Documented involvement of the lungs in GCA is rare, but interstitial lung disease (ILD) has been reported as an uncommon clinical manifestation of GCA (2). The first case of a patient with GCA who presented with ILD was reported in 1982 by Karam et al. (3) However, there have been no reported cases of ILD preceding the onset of the other common symptoms of GCA. We herein report a case of a patient with GCA who had ILD as an initial manifestation. Our study also highlights the usefulness of positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography (18F-FDG PET/CT) in the diagnosis of GCA.
Case Report
A 77-year-old Japanese woman was admitted to our hospital for a fever that had persisted for 2 weeks. Prior to admission, she had been administered a 7-day course of antibiotics for a urinary tract infection. However, her fever persisted, and her general condition gradually deteriorated. She was referred to our hospital.
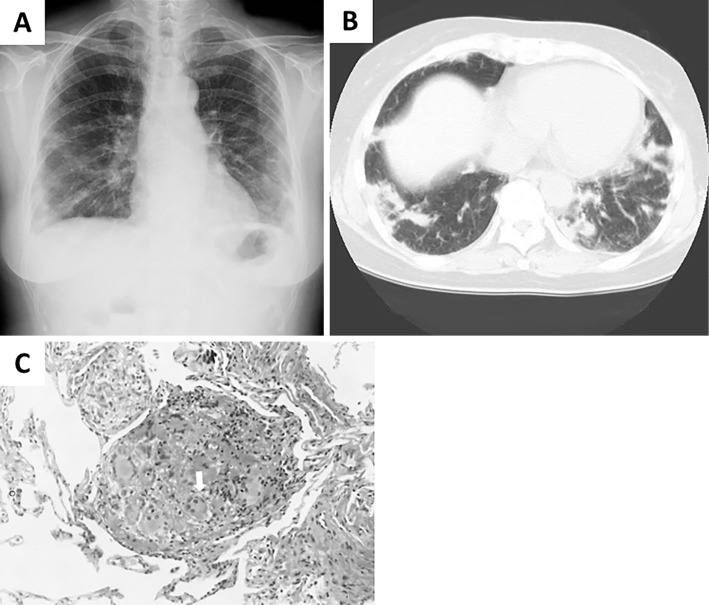
Her medical history consisted of ILD of unknown etiology, which had been treated with corticosteroid therapy for 10 years. At that time, she had presented with progressive shortness of breath without any systemic manifestations. She did not show any signs of disease involvement in the upper respiratory tract or the kidneys. Chest CT revealed poorly defined nodules and peribronchial and subpleural areas of consolidation, primarily in the lower zone. The patient underwent a transbronchial lung biopsy of the right upper lobe and right lower lobe, which revealed interstitial pneumonia with granuloma (Fig. 1). The laboratory findings were normal, including negative results for autoantibodies, antineutrophil cytoplasmic antibodies (ANCA), and the interferon gamma (IFNγ) release assay. With possible causes of ILD excluded, she was diagnosed with ILD of unknown etiology. Her oxygenation continued to deteriorate, and she began corticosteroid therapy [prednisolone, 60 mg/day (1 mg/kg/day)]. After the initiation of steroid therapy, her oxygen saturation improved, and she achieved remission. Her corticosteroid dose was subsequently reduced over the course of nine years. She had stopped taking corticosteroids a year prior to her most recent admission to our hospital. She was otherwise healthy and neither smoked nor drank alcohol, although she had a history of asbestos exposure.
Figure 1.
Diagnostic radiological and histopathological findings related to ILD of unknown etiology. (A) A chest radiograph obtained 10 years ago, showing multiple bilateral nodules. (B) A chest CT scan obtained 10 years ago, showing poorly defined nodules, and peribronchial and subpleural areas of consolidation. (C) Histopathological findings of lung biopsy specimens. Multiple, multinucleated giant cells (white arrow) are observed with inflammatory mononuclear cell infiltration, which is compatible with a granuloma (Hematoxylin and Eosin staining, ×400).
On admission, her body temperature was 38.5℃, blood pressure was 120/84 mmHg, and heart rate was 77 bpm (regular rhythm). A physical examination revealed no remarkable findings, including normal chest sounds. There was no temporal tenderness and no limb girdle tenderness or weakness. However, she had lost approximately 3 kg of body weight in 2 months. The laboratory findings showed a normal white blood cell count (6,200 /μL), low hemoglobin (7.5 g/dL), and elevated platelet count (48.4×104 /μL). Her C-reactive protein level was elevated to 21.3 mg/dL. All other data were normal, including negative results for autoantibodies, tumor markers, and multiple bacteriological cultures (Table). Chest X-ray, chest CT, and abdominal CT revealed no remarkable abnormalities, including hepatosplenomegaly or enlarged lymph nodes. To examine a possible diagnosis of malignant lymphoma and an autoinflammatory disease such as Castleman's disease, we decided to conduct an 18F-FDG PET/CT examination.
Table.
Laboratory Data on Admission.
| <Peripheral blood> | LDH | 235 U/L | <Urinalysis> | ||
| WBC | 6,200 /μL | CK | 50 U/L | protein | 30 mg/dL |
| Seg | 83 % | BUN | 15.9 mg/dL | glucose | (-) |
| Eosi | 1 % | Cre | 0.55 mg/dL | ketone body | (-) |
| Baso | 0 % | Na | 141 mEq/L | occult blood | (-) |
| Mono | 7 % | K | 5.0 mEq/L | urobilinogen | (2+) |
| Lymp | 9 % | Cl | 106 mEq/L | nitrate | (-) |
| RBC | 260×104/μL | Ca | 8.0 mg/dL | <Urinary sediment> | |
| Hb | 7.5 g/dL | <Serological tests> | red blood cell | 1-5 /HPF | |
| Hct | 23.6 % | CRP | 21.3 mg/dL | white blood cell | <1 /HPF |
| MCV | 89.8 fL | KL-6 | 145 pg/mL | epithelial cell | (-) |
| MCH | 28.8 pg | PCT | 0.11 ng/mL | cast | 1-10 /WF |
| MCHC | 31.6 % | IgG | 1,838 mg/dL | Bacteria | (-) |
| PLT | 48.4×104/μL | IgA | 422 mg/dL | <Bacteria test> | |
| ESR | 119 mm/h | IgM | 54 mg/dL | Blood culture | (-) |
| <Coagulation> | IgG4 | 20.4 mg/dL | Urine culture | (-) | |
| PT-INR | 1.30 | sIL-2R | 759 U/mL | ||
| APTT | 30 s | CEA | 1.0 ng/mL | ||
| Fibrinogen | 935 mg/dL | CA19-9 | 2.4 U/mL | ||
| FDP | 13 μg/mL | CA125 | 13.9 U/mL | ||
| D-Dimer | 0.8 μg/mL | ANA | (-) | ||
| <Blood chemistry> | MPO-ANCA | <1.0 | |||
| Total protein | 7.5 g/dL | PR3-ANCA | <1.0 | ||
| Albumin | 2.5 g/dL | β-D-glucan | <0.5 ng/mL | ||
| AST | 33 U/L | Aspergillus Ab | (-) | ||
| ALT | 20 U/L | IGRAs | (-) | ||
| ALP | 355 U/L | ||||
WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, Hct: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, PLT: platelet, ESR: erythrocyte sedimentation rate, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin, FDP: fibrin/fibrinogen degradation products, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, CK: creatine kinase, BUN: blood urea nitrogen, Cre: creatinine, CRP: C-reactive protein, KL-6: Krebs von den Lungen-6, PCT: procalcitonin, Ig: immunoglobulin, sIL-2R: soluble interleukin-2 receptor, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, CA125: carbohydrate antigen 125, ANA: anti-nuclear antibody, MPO-ANCA: myeroperoxidase antineutrophil cytoplasmic antibody, PR3-ANCA: proteinase3 antineutrophil cytoplasmic antibody, IGRAs: interferon-gamma release assays
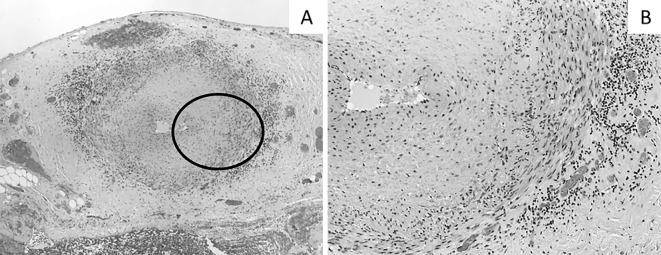
During hospitalization, she suddenly developed isolated oculomotor paralysis in her left eye. Brain MRI revealed no structural disease. 18F-FDG PET/CT showed an FDG uptake in her aortic wall and aortic branches (Fig. 2). These findings were compatible with vasculitis affecting large- and medium-sized arteries and suggested GCA as the most likely diagnosis. At this time, she satisfied only two out of the five diagnostic criteria of GCA (4), namely her age and high erythrocyte sedimentation rate. To meet the criteria for a definitive diagnosis, a temporal artery biopsy was needed. To prevent loss of vision, we immediately administered corticosteroid therapy [prednisolone, 55 mg/day (1 mg/kg/day)], and a temporal artery biopsy was performed 7 days later. Her biopsy specimens showed inflammatory mononuclear cell infiltration in the adventitia (Fig. 3). There were no granulomas or multinucleated giant cells, which are characteristic findings in GCA (5, 6). We assumed that the corticosteroid therapy administered prior to the biopsy had masked these typical findings of GCA (7). Elastic tissue staining (EVG stain) revealed focal destruction of the internal elastic lamina, which was specific for GCA. Therefore, we concluded that this result did not exclude this potential diagnosis and that she satisfied three out of the five diagnostic criteria of GCA. This diagnosis explained her constitutional symptoms, including a fever, weight loss, and anemia. Her slightly elevated soluble interleukin-2 receptor (sIL-2R) were also able to be explained by GCA (8). Extraocular motility disorders occur in approximately 5% of patients with GCA (9), and ischemic damage or inflammatory intracranial aneurysm could have been the cause of her oculomotor paralysis. Based on these observations, we made a definitive diagnosis of GCA.
Figure 2.
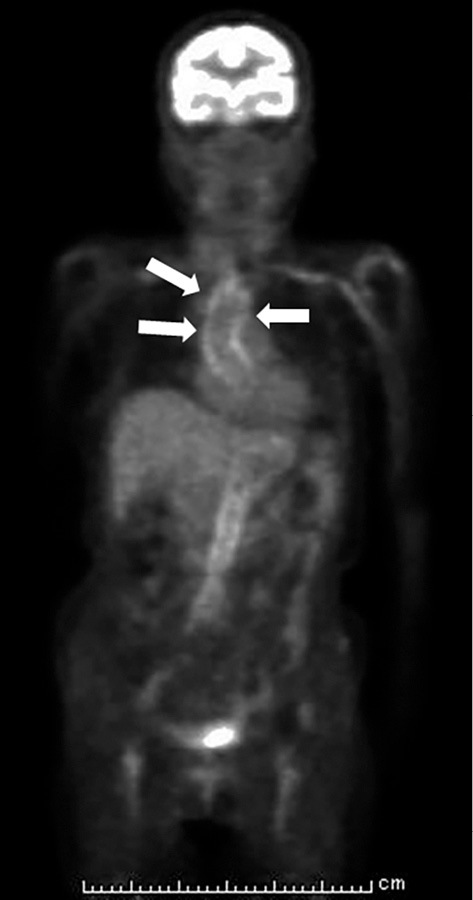
Coronal section of 18F-FDG PET/CT. Abnormal FDG uptakes were observed in the patient’s aortic wall and aortic branches (white arrow).
Figure 3.
Histopathological findings in the temporal artery. (A) Inflammatory mononuclear cells infiltrate the adventitia [Hematoxylin and Eosin (H&E) staining, ×40]. (B) A magnified image of the adventitia. Multinucleated giant cells are not observed in our specimen (H&E staining, ×100).
Her symptoms completely resolved following re-administration of corticosteroid therapy (10). The dose was reduced to 30 mg/day until 6 weeks, after which the dose was tapered by 5 to 10 mg/month to a maintenance dose. Two years after discharge, the patient has remained well. Her GCA-related symptoms disappeared with no sign of recurrence.
Discussion
This study revealed two important clinical findings. First, ILD may precede the common systemic symptoms in patients with GCA. Second, 18F-FDG PET/CT is a useful alternative diagnostic imaging modality for GCA.
Our patient was the first case of GCA to present with ILD as an initial manifestation. We concluded that ILD represented the characteristic lung involvement of GCA for the following reasons: First, although extremely rare, reported pathological findings of lung tissues in GCA share the characteristics findings of granulomatous inflammation (3), which were compatible with our patient's specimen taken 10 years previously. Second, the clinical manifestation of granulomatous ILD in this patient resolved immediately after the administration of corticosteroid therapy. The common etiologies of granulomatous ILD responsive to steroid therapy are sarcoidosis, hypersensitive pneumonitis, and vasculitis-associated ILD. The clinical findings in this patient have never supported the diagnosis of sarcoidosis or hypersensitive pneumonitis. Therefore, in terms of an exclusive diagnosis, vasculitis-associated ILD is the most likely etiology of granulomatous ILD in this patient. At the time of admission, she did not present with symptoms of ILD. We do not believe this presentation excludes the diagnosis of previous ILD as the manifestation of GCA, as some patients have been reported to exhibit symptomatic episodes that occurred months or years apart and affected different anatomical regions (11). We believe that the long-term use of corticosteroid therapy by this patient kept her GCA in remission and accounts for the interval between the initial ILD symptoms and general symptoms of GCA. Although noted 10 years early, we believe that granulomatous ILD in this patient is likely to have been an initial manifestation of GCA.
18F-FDG PET/CT strongly supported our diagnostic assessment of GCA in this patient. The criteria for the diagnosis of GCA consists of five symptoms: >50 years of age at disease onset, new onset of localized headache, temporal artery tenderness or reduced pulse, elevated erythrocyte sedimentation rate (Westergren) ≥50 mm/h, and abnormal temporal artery biopsy findings demonstrating mononuclear infiltration or granulomatous inflammation (4). These criteria do not include any radiological assessments. Recently, imaging modalities, such as magnetic resonance imaging or angiography, conventional angiography, Doppler ultrasound, and 18F-FDG PET/CT, have been suggested to support the diagnosis of GCA (10). 18F-FDG PET/CT has been suggested to be more useful for the evaluation of large vessels than other imaging modalities (12). In an initial study of 25 patients with GCA and 13 patients with polymyalgia rheumatic (PMR), the thoracic vascular FDG uptake had a sensitivity of 56% for the diagnoses of GCA or PMR and a specificity of 98% (13).
In our case, there were no typical symptoms of GCA at the initial presentation. Therefore, it was difficult to make a definitive diagnosis. To examine the possible diagnosis of lymphoma and other autoinflammatory diseases, we performed an 18F-FDG PET/CT examination. Fortunately, this helped greatly. The 18F-FDG PET/CT results facilitated a prompt diagnosis and an early introduction of the appropriate treatment, which prevented the patient's vision loss. Therefore, we suggest using 18F-FDG PET/CT for the diagnosis of GCA, especially in patients with atypical clinical presentations.
We have reported the first case of a patient who presented with ILD as an initial manifestation of GCA. Lung involvement in GCA patients has rarely been reported, but our case promotes the awareness of GCA as a possible diagnosis for granulomatous ILD with unknown etiology.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Chew SS, Kerr NM, Denesh-Meyer HV. Giant cell arteritis. J Clin Neurosci 16: 1263-1268, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Kramer M, Melzer E, Nesher G, Sonnenblick M. Pulmonary manifestations of temporal arteritis. Eur J Respir Dis 71: 430-433, 1987. [PubMed] [Google Scholar]
- 3.Karam GH, Fulmer JD. Giant cell arteritis presenting as interstitial lung disease. Chest 82: 781-784, 1982. [DOI] [PubMed] [Google Scholar]
- 4.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 33: 1122-1128, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Ness T, Bley TA, Schmidt WA, Lamprecht P. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int 110: 376-385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lie JT. Illustrated histopathological classification criteria for selected vasculitis syndromes. American College of Pheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum 33: 1074-1087, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Davies CG, May DJ. The role of temporal artery biopsies in giant cell arteritis. Ann R Coll Surg Engl 93: 4-5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvarani C, Boiardi L, Macchioni P, et al. Role of peripheral CD8 lymphocytes and soluble IL-2 receptor in predicting the duration of corticosteroid treatment in polymyalgia rheumatica and giant cell arteritis. Ann Rheum Dis 54: 640-644, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caselli R, Hunder GG. Neurologic complications of giant cell (temporal) arteritis. Semin Neurol 14: 349-353, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Ninan J, Lester S, Hill C. Giant cell arteritis. Clin Rheumatol 30: 169-188, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Kermani TA, Warrington KJ, Cuthbertson D, et al. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 42: 1213-1217, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel AK, Meller J, Vosshenrich R, et al. Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis 63: 1507-1510, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blockmans D, Stroobants S, Maes A. Positron emission tomography in giant cell arteritis and polymyalgia rheumatic: evidence for inflammation of the aortic arch. Am J Med 108: 246-249, 2000. [DOI] [PubMed] [Google Scholar]