Abstract
An 80-year-old man, who had been diagnosed with ulcerative colitis, was admitted due to a fever and bloody diarrhea and was treated with a glucocorticoid and azathioprine. After 5 days, he developed an impaired consciousness, headache, and neck stiffness. A sample of the colonic mucosa, blood cultures, and cerebrospinal fluid revealed Listeria monocytogenes infection. Intravenous ampicillin improved the symptoms of fever, bloody diarrhea, and headache without any neurological sequelae. Physicians should consider that Listeria enteritis complicating ulcerative colitis can cause septicemia and meningitis in immunosuppressed patients. A patient's central nervous system can avoid the effects of Listeria meningitis by an early diagnosis and appropriate treatment.
Keywords: enteritis, Listeria monocytogenes, meningitis, septicemia, ulcerative colitis
Introduction
Listeria monocytogenes (L. monocytogenes) is an uncommon pathogen that is a source of sepsis and meningitis in immunocompromised individuals such as patients with T-cell dysfunction, the elderly, patients with diabetes mellitus, and individuals receiving immunosuppressive therapy such as corticosteroids (1). Listeria infection is associated with a mortality rate of approximately 27%. Unless recognized and treated promptly, many patients who develop Listeria meningitis are left with significant neurological sequelae (2).
Ulcerative colitis is a chronic autoimmune disorder of the colonic mucosa that generally affects the colon and rectum. Patients with ulcerative colitis experience continual relapse and remittent inflammation (3). Glucocorticoids can effectively induce remission in patients with active ulcerative colitis (4). Immunomodulators (e.g., azathioprine, 6-mercaptopurine) are a key drug in the treatment of steroid-refractory and steroid-dependent ulcerative colitis (5).
There are previously reported cases of ulcerative colitis complicated by Listeria meningitis, and listeriosis has been reported as a foodborne infection (6-9). However, Listeria enteritis has rarely been confirmed as a transmission route of secondary septicemia and meningitis. This condition is difficult to diagnose and treat, and its natural course is not well known. We herein report a case of ulcerative colitis in a patient receiving immunosuppressive therapy who developed septicemia and meningitis caused by Listeria enteritis.
Case Report
An 80-year-old man, who had been diagnosed with ulcerative colitis in 2001, presented to our hospital with a 2-week history of bloody diarrhea. Oral prednisolone (20 mg/day; approximately 0.3 mg/kg) was added to the 5-aminosalicylic acid (5-ASA), which he had taken prior to this point. His symptoms gradually improved, and the prednisolone dosage was tapered for 3 months. However, he experienced a relapse of bloody diarrhea at 2 months after the discontinuation of prednisolone. Thus, treatment with oral prednisolone (30 mg/day; approximately 0.5 mg/kg) and azathioprine was initiated.
The patient was admitted 2 weeks later due to fever and persistent bloody diarrhea. On admission, his temperature was 39.0℃ and he had abdominal tenderness during the physical examination. Laboratory tests showed anemia with a hemoglobin level of 9.6 g/dL; a white blood cell count of 5,200 /μL with 91.2% neutrophils, 6.9% lymphocytes, 1.3% monocytes, 0.4% eosinophils, and 0.2% basophils; and a platelet count of 12.6×104/μL. The patient's erythrocyte sedimentation rate and C-reactive protein level were elevated at 69 mm/h (normal range, 0-10 mm/h) and 9.9 mg/dL (normal range, 0.0-0.5 mg/dL), respectively (Table).
Table.
Laboratory Findings on Admission.
| Hematology | Chemistry | ||
| WBC | 5,200/μL | CRP | 9.9 mg/dL |
| Neut | 91.2% | TP | 6.4 g/dL |
| Ly | 6.9% | Alb | 3.6 g/dL |
| Mono | 1.3% | T-bil | 0.8 mg/dL |
| Eos | 0.4% | AST | 32 IU/L |
| Baso | 0.2% | ALT | 36 IU/L |
| RBC | 405×104/μL | LDH | 320 IU/L |
| Hb | 9.6 g/dL | BUN | 37 mg/dL |
| Plt | 12.6×104/μL | Cr | 1.66 mg/dL |
| ESR | 69 mm/h | Na | 136 mEq/L |
| K | 5.6 mEq/L | ||
| Cl | 106 mEq/L |
Alb: albumin, ALT: alanine aminotransferase, AST: asparate aminotransferase, Baso: basophil, BUN: blood urea nitrogen, Cl: chloride, Cr: creatinine, CRP: C-reactive protein, Eos: eosinophil, ESR: erythrocyte sedimentation rate, Hb: hemoglobin, K: potassium, LDH: lactate dehydrogenase, Ly: lymphocyte, Mono: monocyte, Na: sodium, Neut: neutrophil, Plt: platelet, RBC: red blood cell, T-bil: total bilirubin, TP: total protein, WBC: white blood cell
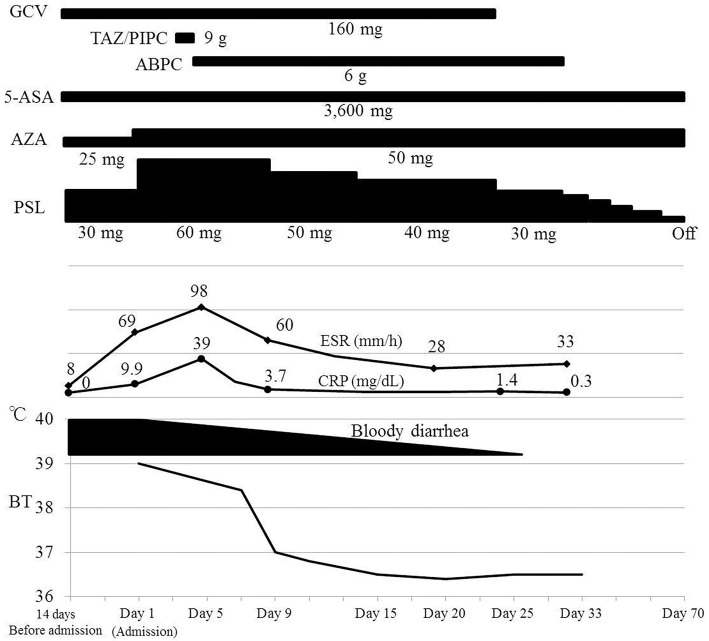
Abdominal computed tomography revealed diffuse thickening of the intestinal wall from the splenic flexure to the lower rectum. On the first day of hospitalization, we considered a diagnosis of an acute exacerbation of ulcerative colitis and initiated intravenous prednisolone (60 mg/day; approximately 1 mg/kg). He had a history of cytomegalovirus colitis; thus, ganciclovir was also administered and continued for 21 days because cytomegalovirus antigen and cytomegalovirus DNA were subsequently detected and in a sample of the colonic mucosa by the antigenemia method and a polymerase chain reaction, respectively.
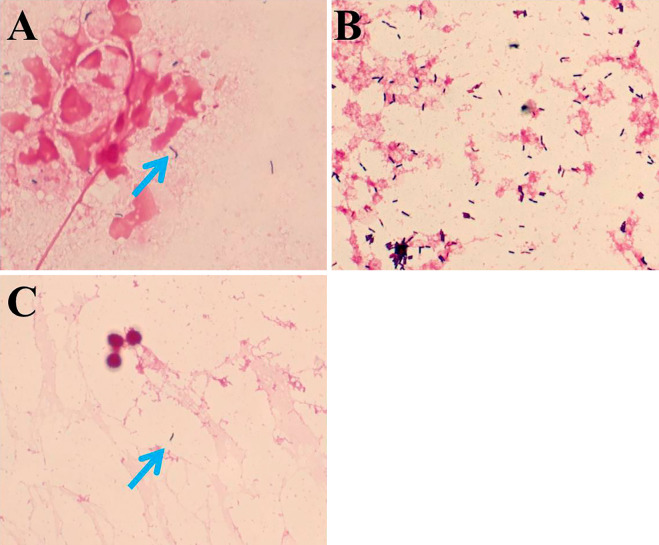
A sigmoidoscopy examination on the 4th day of admission showed a diffusely fragile and hemorrhagic mucosa with ulceration (Fig. 1A). The Mayo endoscopic score was 3 (10). A histopathological examination confirmed active ulcerative colitis with no findings of bacterial infection (Fig. 1B). We obtained a tissue sample from the colonic mucosa, subjected it to a bacteriological examination and performed blood culturing to rule out bacterial enteritis and septicemia complicating ulcerative colitis. A Gram-positive rod bacterium was detected on the smear of a colonic mucosa sample and from the blood cultures (Fig. 2A and B, respectively). Based on the characteristic formation of the bacterium, septicemia caused by Listeria enteritis complicating ulcerative colitis was suspected. However, based on the severe bowel inflammation and the patient's immunosuppressed status, the possibility of infection by intestinal bacteria, including Escherichia coli and Pseudomonas aeruginosa, could not be ruled out. Tazobactam/piperacillin was consequently administered as the empirical therapy. On the 5th day of admission, when the Gram-positive rod bacterium was identified as L. monocytogenes, the patient showed signs of meningitis (i.e., impaired consciousness, headache, and neck stiffness). Lumbar puncture was performed, which revealed a cerebrospinal fluid pressure of 140 mmH2O and a cell count of 64 /μL (polynuclear cells, 40 /μL; mononuclear cells, 12 /μL). The cerebrospinal fluid protein level was 286 mg/mL and the glucose level was 38 mg/dL. Gram staining of the cerebrospinal fluid also revealed the Gram-positive rods (Fig. 2C). We diagnosed Listeria meningitis and initiated intravenous ampicillin on the same day. His general symptoms (i.e., fever, bloody diarrhea, and headache) improved after approximately 25 days without any neurological sequelae. The erythrocyte sedimentation rate and C-reactive protein level decreased (Fig. 3). We stopped the antibiotic treatment on the 33rd day of admission after his general condition had remained stable for several days.
Figure 1.
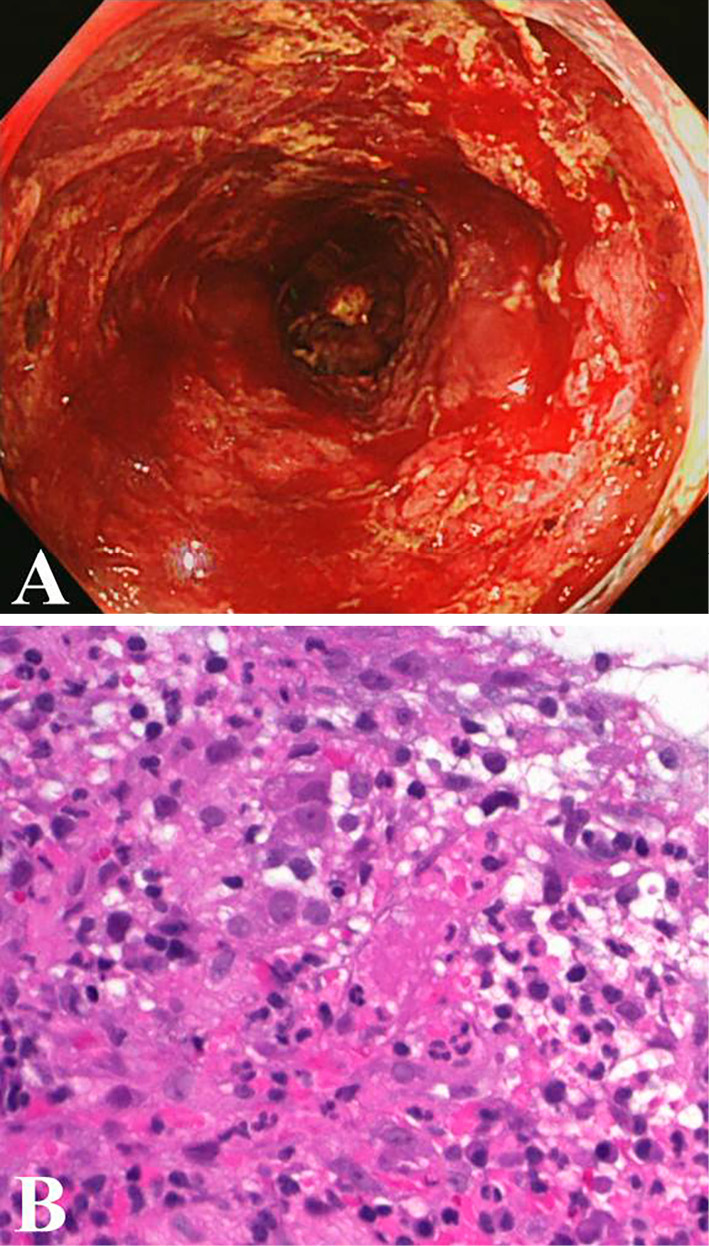
The endoscopic and pathological findings of the colon lesion on the 4th day of admission. (A) Sigmoidoscopy shows a diffusely fragile and hemorrhagic mucosa with ulceration. (B) A colon biopsy shows the colonic mucosa with inflammatory granulation tissue (Hematoxylin and Eosin staining; original magnification, 400×).
Figure 2.
Listeria monocytogenes identified from the samples (arrows) (Gram staining; original magnification, 1000×). (A) The colonic mucosa. (B) Blood cultures. (C) Cerebrospinal fluid.
Figure 3.
The clinical course of the patient from hospitalization to the improvement of listeriosis. ABPC: ampicillin, 5-ASA: 5-aminosalicylic acid, AZA: azathioprine, BT: body temperature, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, GCV: ganciclovir, PSL: prednisolone, TAZ/PIPC: tazobactam/piperacillin
After recovering from Listeria septicemia and meningitis, the patient developed Candida septicemia and pulmonary aspergilloma due to his strongly immunosuppressed status-despite the absence of abnormal findings on a chest X-ray obtained on admission. He gradually recovered with micafungin and voriconazole treatment. After a 3-month stay at our hospital his ulcerative colitis symptoms were well-controlled, and he was subsequently transferred to a hospital for recuperation to undergo rehabilitation from disuse syndrome. He was treated with an immunomodulator and 5-ASA, but not prednisolone.
Discussion
The present case suggested an important clinical issue. Listeria monocytogenes can exacerbate the inflammation in patients with ulcerative colitis, invade the bloodstream, and cause septicemia and meningitis in patients with an immunosuppressed status.
This is the first case report in which Listeria enteritis worsened ulcerative colitis and led to septicemia and meningitis. Enteritis is not a main symptom of Listeria infection, and healthy individuals with Listeria enteritis usually have mild symptoms such as self-limited febrile diarrhea (11). In some patients, acute Listeria enteritis may precede the typical symptoms of listeriosis, such as sepsis and meningitis or encephalitis (12). Pre-existing gastrointestinal disease, such as inflammatory bowel disease, may be a risk factor for L. monocytogenes infection of the gastrointestinal tract (13), and cytomegalovirus colitis and immunosuppressive medications, which are often used for ulcerative colitis may be associated with a significantly increased risk of clinical episodes of invasive listeriosis, similarly to the present case.
Lumbar puncture and cerebrospinal fluid Gram staining should be performed immediately when a patient with suspected Listeria infection presents with fever and headache. Ampicillin is the primary choice for the treatment of L. monocytogenes. If the patient has a normal renal function, aminoglycoside should be added because it enhances the effect of penicillins. Cephalosporins, which are effective for some causative bacteria of meningitis, are ineffective against L. monocytogenes (14). In patients with an impaired immune response, L. monocytogenes may reside intracellularly-despite the administration of the recommended antibiotic regimen-and patients may develop other manifestations of listeriosis (other than meningitis). For example, encephalitis and recurrence are often observed after a patient shows initial improvement (15). Hence, the mortality rate of listeriosis is significantly high, especially in at-risk patients such as the elderly and patients who are immunocompromised by immunosuppressive therapy (16, 17). In the current patient, we performed lumbar puncture and initiated ampicillin on the day of the onset of meningitis symptoms. He recovered without any permanent central nervous system damage.
In conclusion, L. monocytogenes can exacerbate inflammation in ulcerative colitis, invade the bloodstream, and cause septicemia and meningitis in patients with an immunosuppression. In the current case, Listeria enteritis was associated with ulcerative colitis flare-ups. When a patient with ulcerative colitis who is receiving immunosuppressive therapy has a history of worsening diarrhea and fever, and other symptoms, physicians should include Listeria enteritis complicating ulcerative colitis, which is often difficult to diagnose, in the differential diagnosis. By making this diagnosis, clinical measures such as close follow-up and the frequent collection of stool cultures may lead to an early diagnosis and spare a patient from the risk of septicemia and meningitis. On the other hand, in patients with active inflammation and immunosuppression, the bacteria can easily invade the bloodstream through the intestinal epithelium barrier and escape from the immune system to transgress the blood-brain barrier. Thus, Listeria monocytogenes should be considered when symptoms of meningitis are observed in elderly patients with ulcerative colitis and in patients with ulcerative colitis who are treated with immunosuppressive therapy (such as the present patient). This awareness could lead to an accurate diagnosis and the appropriate treatment of Listeria meningitis, which may preserve the function of the central nervous system.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Pagliano P, Ascione T, Boccia G, De Caro F, Esposito S. Listeria monocytogenes meningitis in the elderly: epidemiological, clinical and therapeutic findings. Infez Med 24: 105-111, 2016. [PubMed] [Google Scholar]
- 2.Hamer DH, Gorbach SL. Infectious diarrhea and bacterial food poisoning. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 7th ed Feldman M, Friedman LS, Sleisenger MH, Eds. WB Saunders, Philadelphia, PA, 2002: 1864-1913. [Google Scholar]
- 3.Song Y, Shi YH, He C, et al. Severe Henoch-Schönlein purpura with infliximab for ulcerative colitis. World J Gastroenterol 21: 6082-6087, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanauer SB. Inflammatory bowel disease. N Engl J Med 334: 841-848, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 5: CD000478, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang MH, Singh J, Ashouri N, Katz MH, Arrieta AC. Listeria meningitis after infliximab treatment of ulcerative colitis. J Pediatr Gastroenterol Nutr 50: 337-339, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Abreu C, Magro F, Vilas-Boas F, Lopes S, Macedo G, Sarmento A. Listeria infection in patients on anti-TNF treatment: report of two cases and review of the literature. J Crohns Colitis 7: 175-182, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Rana F, Shaikh MM, Bowles J. Listeria meningitis and resultant symptomatic hydrocephalus complicating infliximab treatment for ulcerative colitis. JRSM Open 5: 2054270414522223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parihar V, Maguire S, Shahin A, et al. Listeria meningitis complicating a patient with ulcerative colitis on concomitant infliximab and hydrocortisone. Ir J Med Sci 185: 965-967, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med 317: 1625-1629, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Ooi ST, Lorber B. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis 40: 1327-1332, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hof H. Listeria monocytogenes: a causative agent of gastroenteritis? Eur J Clin Microbiol Infect Dis 20: 369-373, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Barbuddhe SB, Chakraborty T. Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol 337: 173-195, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Heyderman RS. Early management of suspected bacterial meningitis and meningococcal septicaemia in immunocompetent adults-second edition. J Infect 50: 373-374, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev 10: 345-357, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guevara RE, Mascola L, Sorvillo F. Risk factors for mortality among patients with nonperinatal listeriosis in Los Angeles County, 1992-2004. Clin Infect Dis 48: 1507-1515, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Skogberg K, Syrjänen J, Jahkola M, et al. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clin Infect Dis 14: 815-821, 1992. [DOI] [PubMed] [Google Scholar]