Abstract
Burdock (BD) is a common vegetable with many pharmacological properties. However, few studies have examined the effect of BD on exercise performance and physical fatigue. We aimed to evaluate the potential beneficial effects of BD on fatigue and ergogenic functions following physical challenge in mice. Methods: Male ICR mice were divided into four groups to receive either vehicle, or BD at 348.5, 697 or 1,742.5 mg/kg/day, by daily oral gavage for 4 weeks. Exercise performance and fatigue were evaluated from forelimb grip strength, exhaustive swimming time, and post-exercise levels of physical fatigue-related biomarkers serum lactate, ammonia, glucose, and creatine kinase (CK). Results: BD supplementation elevated endurance and grip strength in a dose-dependent manner. It also significantly decreased lactate, ammonia, and CK levels after physical challenge. In addition, BD supplementation had few subchronic toxic effects. Conclusions: Supplementation with BD has a wide spectrum of bioactive effects, including health promotion, performance improvement, and fatigue reduction.
Keywords: anti-fatigue, burdock, endurance, grip strength, toxicity
Fatigue is characterized by three major aspects: physiological effects, psychological effects, and disease [2]. Physical fatigue is commonly associated with elevated stress levels caused by a modern lifestyle, and is also related to the exercise-reduced maximal force-generating capacity of muscle [46]. It alters performance, leading to decreased muscular power and endurance, decreased motor skill performance, and diminished physical and mental function [39]. The exhaustion theory suggests that during exercise, many energy sources, such as glucose and liver glycogen, will be exhausted, leading to physical fatigue [37]. Other biomarkers, such as lactate, ammonia, blood urea nitrogen (BUN), and glucose, are also widely used to evaluate fatigue [20, 35]. Regular exercise improves the body’s functionality, but strenuous sports are responsible for the accumulation of reactive oxygen and lipid peroxides, which damage organs and also lead to fatigue [22, 32]. Fatigue is worthy of attention, as it may cause various disorders related to the bio-regulatory and immune systems. These disorders also cause reductions in exercise intensity, or even interruption of activity [33].
Arctium lappa, known as “Niubang” in Chinese, has been used in China and some western countries for over 3,000 years. Its therapeutic uses have been documented in The Compendium of Materia Medica (Bencao Gangmu in Chinese) written in the Ming Dynasty by Li Shizhen, the most important and famous figure in the history and development of traditional Chinese medicine [45]. Commonly known as burdock (BD), this plant is promoted as a healthy and nutritive food in Chinese societies. It is a perennial herb in the Compositae family, storing most of its nutrients during its first year of growth. Many studies have confirmed that BD has antitumor [3], antioxidative [29], anti-inflammatory [24], antibacterial [43], prebiotic [26], and anti-HIV properties [41], inhibits NO production [30], and prevents diabetic complications [42]. BD has also been demonstrated to have many other beneficial effects on health and disease prevention.
However, few studies have directly addressed the effect of BD on physiological fatigue. Our present study aims to use in vivo tests in mice to explore whether a drink containing BD, taken after exercise, can delay fatigue, reduce recovery time, improve athletic performance, with minimal toxic effects, promoting the use of such supplements in sports. Therefore, we evaluated the potential ergogenic and anti-fatigue effects of BD by using our previously established in vivo platform [18, 20, 21].
MATERIALS AND METHODS
Materials
BD supplement, a brown color liquid with a characteristic odor, was purchased from Kagro Biotech. Co., Ltd. (Kaohsiung, Taiwan) and provided by Professor Fu-An Chen (Tajen University, Taiwan). BD was prepared using good manufacturing practice and hazard analysis and critical control point-qualified manufacturing. To ensure precise and accurate dosing of animals, heat-sterilized BD extract was lyophilized by freeze-drying to obtain a powder extract. The crude powder extract was stored at −80°C until use. In this study, the human BD dose was 1.7 g/day (as lyophilized powder), equivalent to a daily recommended dose of 200 ml water/day. The mouse BD dose (348.5 mg/kg) was calculated from the human equivalent dose (HED) based on body surface area, using the following formula from the US Food and Drug Administration, assuming a human body weight of 60 kg: HED of 1.7 g/60 kg body weight=0.02833 g/kg. Therefore, 0.02833 g/kg × 12.3=a mouse dose of 348.5 mg/kg. A conversion coefficient of 12.3 was used to account for differences in body surface area between mice and humans, as we described previously [11].
Analysis of total BD polyphenol content was performed spectrophotometrically, using a Folin-Ciocalteu reagent based on a colorimetric redox reaction [15]. BD antioxidant activity was estimated from 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity [5, 10]. Hazardous chemical contaminants (pesticide residues and heavy metals) and biological contaminants were determined by a Taiwan Food and Drug Administration (TFDA) accredited company (ABM International Lab Inc. Pingtung, Taiwan).
Specifications from the BD certificate of analysis for total polyphenol content and antioxidant activity were ≥150 µg/ml and ≥70% DPPH-scavenging activity, respectively. Release limits of 310 pesticide residues and five heavy metals (Pb, Cd, Hg, Cu and As) were all undetectable. Regarding microbiological analysis: total plate counts, and counts for yeast and mold, E. coli, Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella spp., all met TFDA criteria.
Animals and treatment
We purchased male specific pathogen-free ICR mice (6 weeks old), from BioLASCO (A Charles River Licensee Corp., Yi-Lan, Taiwan). Experimental animals were given 2 weeks to acclimatize to the environment and diet. All animals were provided with a standard laboratory diet (No. 5001; PMI Nutrition International, Brentwood, MO, U.S.A.) and distilled water ad libitum, and maintained at a regular light cycle (12-hr light/dark), at room temperature (24 ± 2°C) and 60–70% humidity. Bedding was changed and cleaned twice per week. All animal experiments conformed to the Institutional Animal Care and Use Committee (IACUC) of the National Taiwan Sport University, and the study conformed to guidelines in the protocol IACUC-10309 approved by the IACUC ethics committee.
Animals were randomly separated into four groups (10 mice/group) for oral gavage treatment once a day for 4 weeks: (1) vehicle (distilled water); (2) 348.5 mg/kg BD (BD-1X); (3) 697 mg/kg BD (BD-2X); and (4) 1,742.5 mg/kg BD (BD-5X). The vehicle group received the same volumes of solution as the BD groups, calculated based on individual body weight (BW), and received the vehicle at a constant dosage volume of 10 ml/kg throughout the treatment period. Food intake and water consumption were monitored daily, and BW was recorded weekly.
Forelimb grip strength
We used a low-force testing system (Model-RX-5, Aikoh Engineering, Nagoya, Japan) to measure forelimb grip strength, as previously described [18, 21, 35].
Swimming exercise performance test
Mice were pretreated with the vehicle, or 348.5, 697, or 1,742.5 mg BD/kg for 28 continuous days, followed by an exhaustive swimming test commencing 1 hr after the last treatment. Details of the swimming exercise in the forced swimming test were as previously described, to evaluate endurance [21, 35]. The swim-to-exhaustion exercise test involved mice carrying constant loads corresponding to 5% BW. The swimming endurance time was recorded from the start of the test to exhaustion, determined by observing loss of coordinated movements and failure to return to the surface within 7 sec.
Determination of fatigue-associated biochemical variables
The effects of BD on the following fatigue-associated biochemical indices were evaluated post exercise: serum lactate, ammonia, glucose, creatine kinase (CK), and BUN activity. Mice underwent a 15-min swimming test without weight loading, 1 hr after the last treatment. Blood samples were then immediately collected from the submandibular duct of pretreated mice, and then centrifuged at 1,500 ×g and 4°C for 10 min for serum preparation [20, 35]. Serum lactate, ammonia, glucose, CK and BUN levels were determined on the same day, using an autoanalyzer (Hitachi 7060, Hitachi, Tokyo, Japan).
Tissue glycogen determination
Glucose is stored as glycogen, which mostly exists in the liver and muscle tissues. The mice were pretreated with vehicle, BD-1X, BD-2X and BD-5X for 28 continuous days. All mice were killed 1 hr after the last treatment. After blood collection and sacrifice, the liver and muscle tissues were excised and weighed for glycogen content analysis.
Glycogen analysis was performed as previously described, with some modifications [21]. For each mouse, 100 mg of liver and muscle was finely cut, weighed, and homogenized in 0.5 ml cold 10% perchloric acid. After centrifugation for 15 min at 15,000 ×g and 4°C, the supernatant was carefully decanted and incubated on ice for analysis. Standard glycogen (Sigma) or tissue extract (30 µl) was added to 96-well micro-plates, and 200 µl iodine-potassium iodide reagent was added to each well to measure iodine binding to glycogen. An amber-brown compound developed immediately after the reaction. An ELISA reader (Tecan Infinite M200, Tecan Austria, Salzburg, Austria) with wavelength 460 nm was used to measure the absorbance after resting the material for 10 min [38].
Histological staining of tissues
Tissues were collected (liver, kidney, heart and muscle), weighed, and immediately fixed in 10% formalin. Heart tissue was cut transversely to obtain ventricular sections and four-chamber cross-sections, and the liver and muscle tissues (soleus) were minced, embedded in paraffin, and then stained with hematoxylin and eosin (H&E). Tissues were then examined under a light microscope equipped with a CCD camera (BX-51, Olympus, Tokyo, Japan) by a veterinary pathologist [21].
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by one-way ANOVA and the Duncan test, to test for significant differences between the treatment groups. The Cochran-Armitage test for dose-effect trend analysis was also used, with SAS 9.0 software (SAS Inst., Cary, NC, U.S.A.). Values for P<0.05 were considered statistically significant.
RESULTS
Subacute toxicity of BD supplementation with general characteristics
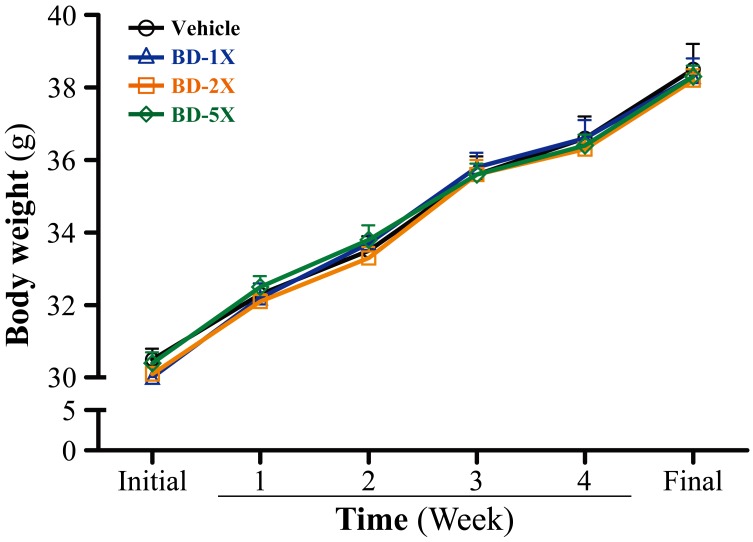
We evaluated the general characteristics of the mice and subchronic toxic effects of BD supplementation, by observing behavior, food consumption, growth curves, organ weight, and histopathology. BW was unaltered in all groups over the duration of 4 weeks (Fig. 1). In addition, daily intake of diet and water did not differ in the vehicle and BD treatment groups. Organ weights, including the liver, muscle, kidney, testis, epididymal fat pad (EFP), and brown adipose tissue (BAT), did not differ between groups (Table 1).
Fig. 1.
The effect of BD supplementation on growth. Data are mean ± SEM, n=10.
Table 1. General characteristics of the experimental groups.
| Characteristic | Vehicle | BD-1X | BD-2X | BD-5X | Trend analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 30.5 ± 0.3 | 30.0 ± 0.3 | 30.1 ± 0.2 | 30.4 ± 0.3 | 0.8597 |
| Final BW (g) | 38.5 ± 0.7 | 38.3 ± 0.5 | 38.2 ± 0.3 | 38.3 ± 0.3 | 0.4544 |
| Food intake (g/mouse/day) | 6.2 ± 0.1a,b) | 6.4 ± 0.1b) | 6.4 ± 0.2b) | 6.0 ± 0.1a) | 0.3241 |
| Water intake (ml/mouse/day) | 8.3 ± 0.3 | 8.6 ± 0.3 | 8.6 ± 0.3 | 8.2 ± 0.3 | 0.6170 |
| Liver (g) | 1.99 ± 0.05 | 1.98 ± 0.03 | 1.98 ± 0.04 | 2.00 ± 0.03 | 0.8300 |
| Muscle (g) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.4304 |
| Kidney (g) | 0.59 ± 0.01 | 0.59 ± 0.02 | 0.59 ± 0.01 | 0.59 ± 0.01 | 0.9820 |
| Heart (g) | 0.21 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.4464 |
| Lung (g) | 0.29 ± 0.02 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.02 | 0.7800 |
| EFP (g) | 0.51 ± 0.04 | 0.51 ± 0.04 | 0.52 ± 0.04 | 0.54 ± 0.04 | 0.3095 |
| Relative liver weight (%) | 5.17 ± 0.09 | 5.19 ± 0.14 | 5.20 ± 0.10 | 5.21 ± 0.08 | 0.7025 |
| Relative muscle weight (%) | 1.01 ± 0.02 | 1.01 ± 0.03 | 1.01 ± 0.03 | 1.01 ± 0.02 | 0.2564 |
| Relative kidney weight (%) | 1.55 ± 0.05 | 1.53 ± 0.05 | 1.55 ± 0.02 | 1.55 ± 0.03 | 0.7361 |
| Relative heart weight (%) | 0.56 ± 0.02 | 0.59 ± 0.02 | 0.57 ± 0.01 | 0.58 ± 0.02 | 0.3909 |
| Relative lung weight (%) | 0.75 ± 0.04 | 0.77 ± 0.03 | 0.79 ± 0.03 | 0.79 ± 0.04 | 0.6889 |
| Relative EFP weight (%) | 1.32 ± 0.09 | 1.31 ± 0.16 | 1.35 ± 0.10 | 1.40 ± 0.07 | 0.3838 |
Data are mean ± SEM, n=10. Data in the same line followed by different letters [a or b] differ significantly with P<0.05, determined by one-way ANOVA. Vehicle: water; BD-1X: 348.5 mg/kg BD; BD-2X: 697 mg/kg BD; BD-5X: 1,742.5 mg/kg BD; EFP: epididymal fat pads.
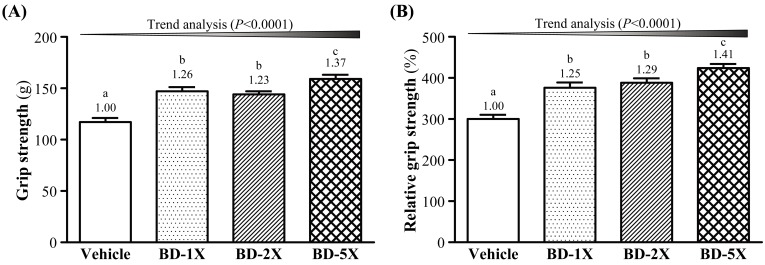
Effect of BD supplementation on forelimb grip strength
Forelimb grip strength is a routine physical examination test. As shown in Fig. 2A, BD-1X, BD-2X and BD-5X treatments significantly increased forelimb grip strength by 1.26-, 1.23- and 1.37-fold (all P<0.0001), respectively, compared to vehicle treatment. Therefore, we divided grip strength by BW to determine relative grip strength, and still found higher grip strength for BD-treated groups than for vehicle groups (Fig. 2B), with significant trend findings.
Fig. 2.
Effect of BD supplementation on forelimb grip strength. (A) grip strength; (B) relative grip strength. Data are mean ± SEM, n=10. Bars with different letters (a, b, c) are significantly different with P<0.05, determined by one-way ANOVA. Vehicle (water); 348.5 mg/kg BD (BD-1X); 697 mg/kg BD (BD-2X); 1,742.5 mg/kg BD (BD-5X).
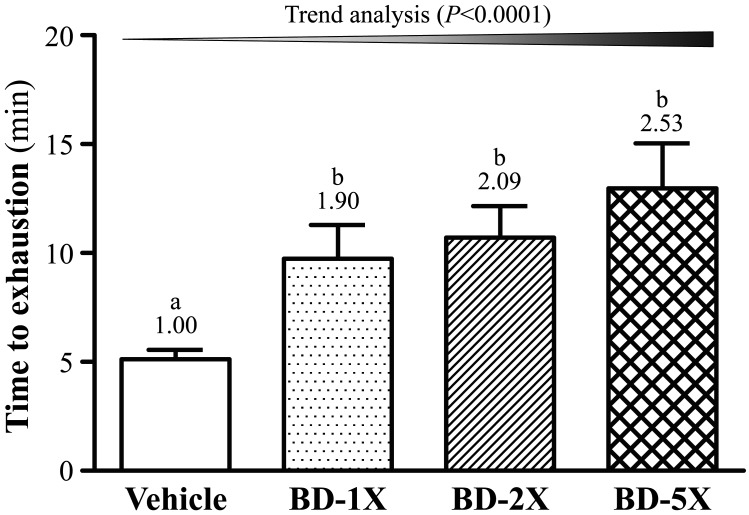
Effect of BD supplementation on exhaustive swimming test
Exercise endurance is an important variable in evaluating anti-fatigue treatment. A key indicator to assess the efficacy of anti-fatigue treatment is exercise endurance ability, determined by an exhaustive swimming test (Fig. 3). BD-1X, BD-2X and BD-5X treatments significantly increased swimming time by 1.90- (P=0.0365), 2.09- (P=0.0124) and 2.53-fold (P=0.0007), respectively, compared to vehicle treatment. In the trend analysis, maximal swimming time increased with BD treatment, in a dose-dependent manner (P<0.0001).
Fig. 3.
Effect of BD supplementation on exhaustive swimming test. Data are mean ± SEM, n=10. Bars with different letters (a, b) are significantly different with P<0.05, determined by one-way ANOVA.
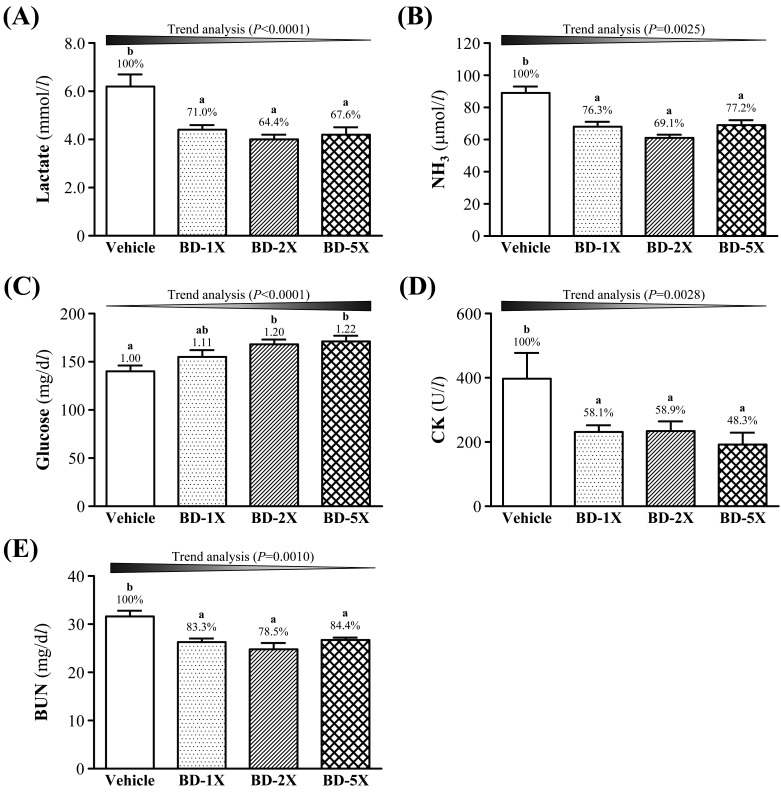
Effect of BD supplementation on exercise fatigue-related indicators after acute exercise
The status of muscle fatigue after exercise can be evaluated by key biochemical indicators, including lactate, ammonia, glucose, and BUN [17, 21, 28]. We found that vehicle treatment increased serum lactate levels by approximately 29.0% (P=0.0005), 35.6% (P<0.0001) and 32.4% (P=0.0001), compared to BD-1X, BD-2X and BD-5X treatments, respectively, after acute exercise challenge (Fig. 4A). BD-1X, BD-2X and BD-5X treatments decreased serum ammonia levels, by 23.7, 30.9 and 22.8% (all P<0.0001), respectively, compared to vehicle treatment (Fig. 4B). BD-2X and BD-5X treatment increased post-exercise serum glucose levels by 1.20- (P=0.0023) and 1.22-fold (P=0.0009), respectively, compared to vehicle treatment (Fig. 4C). BD-1X treatment slightly increased blood glucose levels compared to vehicle treatment, however, this was not a significant difference compared to either the vehicle or the other BD treatments (P>0.05). BD-1X, BD-2X and BD-5X treatments decreased CK levels by 16.7- (P=0.0005), 21.5- (P<0.0001) and 15.6-fold (P=0.0009), respectively, compared to vehicle treatment (Fig. 4D). BD-1X, BD-2X, and BD-5X treatments significantly decreased BUN levels by 16.7- (P=0.0005), 21.5- (P<0.0001) and 15.6-fold (P=0.0009), respectively, compared to vehicle treatment (Fig. 4E).
Fig. 4.
Effect of BD supplementation on serum (A) lactate; (B) ammonia; (C) glucose; (D) creatine kinase (CK) and (E) blood urea nitrogen (BUN) levels after acute exercise challenge. Data are mean ± SEM, n=10. Bars with different letters (a, b) are significantly different with P<0.05, determined by one-way ANOVA.
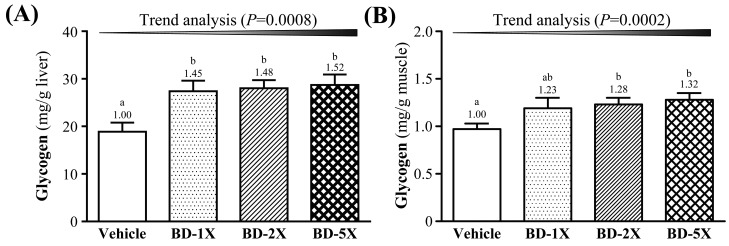
Effect of BD supplementation on tissue glycogen level
The three levels of BD supplementation increased hepatic glycogen levels by 1.45- (P=0.0050), 1.48- (P=0.0028), and 1.52-fold (P=0.0014), respectively, compared to vehicle treatment (Fig. 5A). BD-2X and BD-5X treatments increased post-exercise muscle glycogen levels by 1.28- (P=0.0207) and 1.32-fold (P=0.0079), compared to vehicle treatment (Fig. 5B). Post-exercise muscle glycogen levels were significantly different between BD-2X and BD-5X treatments, but BD-1X treatment did not induce a significant difference in glycogen levels, compared to either the vehicle or the other BD groups. Muscle glycogen levels did increase in the BD-1X group compared to the vehicle group, however, this was not a significant difference (P>0.05).
Fig. 5.
Effect of BD supplementation on hepatic and muscle glycogen level. Data are mean ± SEM, n=10. Bars with different letters (a, b) are significantly different with P<0.05, determined by one-way ANOVA.
Subacute toxicity of BD supplementation with biochemistry and histopathology evaluation
In Table 2, vehicle and BD-1X treatments increased AST and ALT levels compared to BD-2X and BD-5X supplementation. Regarding kidney function: the biochemistry of urea, creatinine, and uric acid (UA) can reflect renal damage. However, vehicle treatment increased serum UA levels by 1.32- (P=0.0088), 1.25- (P=0.0279) and 1.33-fold (P=0.0069), and increased BUN levels by 1.12- (P=0.0292), 1.13- (P=0.0214) and 1.13-fold (P=0.0242), compared to BD-1X, BD-2X and BD-5X treatments, respectively (Table 2).
Table 2. Biochemical analysis of vehicle and BD treatment groups at the end of the experiment.
| Parameter | Vehicle | BD-1X | BD-2X | BD-5X | Trend analysis |
|---|---|---|---|---|---|
| UA (mg/dl) | 1.21 ± 0.09b) | 0.92 ± 0.08a) | 0.97 ± 0.03a) | 0.91 ± 0.08a) | 0.0165 |
| BUN (mg/dl) | 24.9 ± 1.2b) | 22.9 ± 0.6a) | 22.1 ± 0.8a) | 22.1 ± 0.5a) | 0.0260 |
| TC (mg/dl) | 141 ± 5 | 135 ± 3 | 136 ± 5 | 136 ± 7 | 0.7251 |
| TG (mg/dl) | 154 ± 5 | 130 ± 5 | 136 ± 7 | 132 ± 14 | 0.2516 |
| LDH (U/l) | 346 ± 29b) | 319 ± 23a,b) | 298 ± 27a,b) | 273 ± 21a) | 0.0288 |
| AST (U/l) | 79 ± 7b) | 77 ± 4b) | 62 ± 3a) | 59 ± 2a) | <0.0001 |
| ALT (U/l) | 46 ± 3b) | 42 ± 2b) | 34 ± 2a) | 35 ± 2a) | <0.0001 |
| ALK-P (U/l) | 62 ± 3 | 52 ± 5 | 55 ± 4 | 56 ± 4 | 0.4712 |
| CPK (U/l) | 208 ± 51b) | 200 ± 21b) | 173 ± 28a,b) | 102 ± 24a) | 0.0047 |
| ALB (g/dl) | 3.4 ± 0.04a) | 3.44 ± 0.07a) | 3.53 ± 0.04a,b) | 3.62 ± 0.11b) | <0.0001 |
| TP (g/dl) | 4.8 ± 0.0a) | 4.8 ± 0.1a) | 4.9 ± 0.1a) | 5.1 ± 0.0b) | <0.0001 |
| CREA (mg/dl) | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.0572 |
| GLU (mg/dl) | 170 ± 7 | 163 ± 7 | 166 ± 5 | 168 ± 4 | 0.9791 |
Data are mean ± SEM, n=10. Values in the same row with different superscript letters (a, b) differ significantly with P<0.05, determined by one-way ANOVA. UA: uric acid; BUN: blood urea nitrogen; TC: total cholesterol; TG: triacylglycerol; LDH: lactate dehydrogenase; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALK-P: alkaline phosphatase; CPK: Creatine phosphokinase; ALB: albumin; TP: total protein; CREA: Creatinine; GLU: glucose.
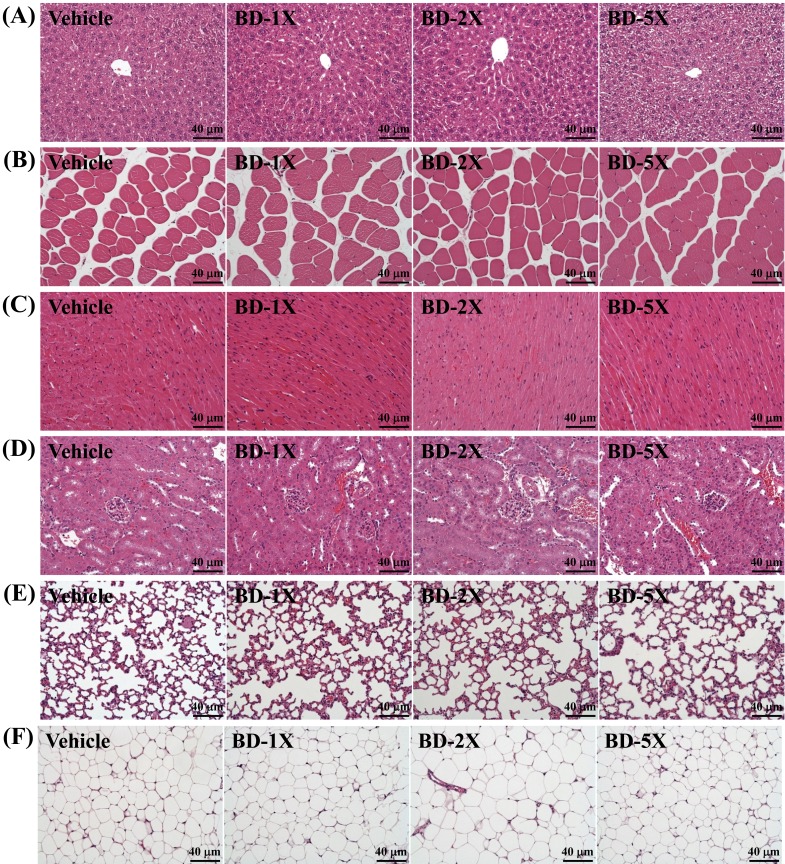
In addition, no differences were observed between the four groups (Vehicle, BD-1X, BD-2X and BD-5X) according to histological observations of the liver, muscle, heart, kidney, lung, and epididymal fat pad. Thus, BD was not harmful, regardless of the administered dosage (Fig. 6).
Fig. 6.
Effect of BD supplementation on morphology of (A) liver; (B) skeletal muscle; (C) heart; (D) kidney; (E) lung; and (F) epididymal fat pad in mice. Specimens were photographed by light microscopy (H&E stain, magnification: ×200; Scale bar, 40 µm).
DISCUSSION
Safety is a concern when considering the use of specific extracts or herbs as plant-derived nutritional, medicinal or health-care products. However, limited toxicological reference is available regarding their safety. We assessed the subacute toxic effects of BD by observing mouse behavior, weight (growth), food consumption, organ weight, clinical biochemistry, and histopathology, after 28-day BD repeated-dose supplementation. Previously, BD toxicity did not result in any significant treatment-related changes in behavior, weight, food consumption, organ weight, hematology, clinical biochemistry, and urinalysis [12]. In this study, the no-observed-adverse-effect level of BD could provide optimized dosages for physiological benefit without risk to health, for pursuing the preservation of health.
Forelimb grip strength is a routine physical examination test that reflects the overall health of the musculoskeletal system, and can also be used to evaluate motor-associated coordination and adaption in neurological studies [1]. Previous study has shown a positive correlation between grip strength and anthropometric factors such as age, weight, body mass index, and waist circumference [8]. Our previous study showed that muscle strength positively correlated with forelimb grip strength [16]. In this study, we found the grip of the BD-5X group was greater than the other groups. Therefore, the results indicated that long-term BD supplementation could benefit grip strength when no training protocol is implemented. Our previous reports have shown that long-term supplementation with polyphenols, such as resveratrol and curcumin, also improves the grip strength of untrained animals [20, 21].
Our experimental results (Fig. 3) indicated that physical fatigue could be ameliorated according to swimming time extension with 4-week BD treatment. This phenomenon is consistent with a previous study, in which administration of arctigenin, a bioactive lignan found in BD, efficiently improved endurance running time on the treadmill in mice [36]. Another study confirmed that arctigenin efficiently enhanced the endurance swimming of sedentary SD rats by elevating the antioxidant capacity of the skeletal muscles via two antioxidant pathways: AMPK/PGC1α/PPARα in mitochondria and AMPK/p53/Nrf2 in the cell nucleus [40].
In exhaustive and high intensity exercise, excessive production of reactive oxygen species may induce oxidative stress and tissue damage [4], and the removal of excess free radicals may alleviate exercise-induced oxidative stress and body fatigue. Previous studies have reported that the free radical scavenging activity of BD is associated with its phenolic properties [9, 31]. Oral BD treatment may help reduce oxidative stress, decrease muscle damage, and improve exercise performance. Our data show that administration of BD extract extends endurance time to exhaustion of mice, therefore indicating that BD extract reduces fatigue and enhances exercise tolerance.
Glucose is transported to skeletal muscle to fulfil energy requirements, but anaerobic glycolysis occurs and results in lactate accumulation during high intensity exercise. Increased lactate levels lower pH, resulting in various biochemical and physiological side effects affecting glycolysis, phosphofructokinase, and muscular contractions caused by calcium ion release [6]. Levels of another important metabolite, ammonia, significantly increase with intense or prolonged exercise. Muscle fatigue is associated with the deamination of adenine nucleotides, and increased AMP deamination coincides with decreased phosphocreatine levels and pH, as well as failure of the contraction process. Peripheral and central fatigue levels are related to increased ammonia levels during exercise [7]. BUN is an important metabolite formed by protein degradation after intensive exercise [21]. Glucose, a breakdown product of tissue glycogen, is released as a circulating substrate and used as energy after intense exercise [13]. Exercise and muscle contractions increase glucose uptake by skeletal muscles via an insulin-independent mechanism [25]. During exercise, efficient utilization of glucose is an important index for performance maintenance. For incremental exhaustive exercise, several indicators are used to evaluate muscle and liver injury, such as CK [19]. Exercise performance is determined by energy storage and supply.
Catabolized fat and carbohydrates are considered the main sources of energy during exercise in skeletal muscles, and glycogen is the predominant source of glycolysis for energy production. The muscle content of glycogen is a limiting factor of prolonged exercise, and nutritional interventions could be beneficial for increasing or maintaining liver or muscle glycogen content, before or during exercise [14, 18]. Therefore, glycogen storage directly affects exercise ability [44]. Arctigenin, which is abundantly present in BD, can significantly increase Adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) activation, regulating glycogen synthesis and efficiently increasing sedentary rodent treadmill endurance via AMPK phosphorylation enhancement [40]. Our results showed a significant increase in tissue glycogen storage with BD supplementation, which could enhance endurance performance.
In the present study, we observed the beneficial effects of BD using an exhaustive exercise challenge, and measured other physiological effects after 4 weeks of BD supplementation. However, intensive or exhaustive exercise will induce oxidative stress, such as the production of reactive oxygen species and free radicals, which injure cells and tissues [23, 27]. Such injury includes lipid peroxidation, which destroys membrane permeability, cell organization, and DNA integrity and function. Important enzymes such as LDH, CK, myoglobin, AST and ALT are also released into serum, and are considered biomarkers of tissue injury under high-intensity exercise challenge [34]. BD supplementation produces no side effects in vivo and exhibits potential for applications in measuring blood glucose, UA and hyperlipidemia, according to our in vivo data.
Histological data related to the pathological effects of BD is quite limited, especially with respect to the indicated bioactive doses. Our results also showed that the liver showed no lesions or pathological changes attributable to BD treatment (Fig. 6). Hypertrophy and hyperplasia were not observed in heart cardiomyocytes or rhabdomyocytes of the gastrocnemius muscle. Furthermore, no lung inflammatory conditions were observed. Histological examination of organs showed no apparent damage in any mice.
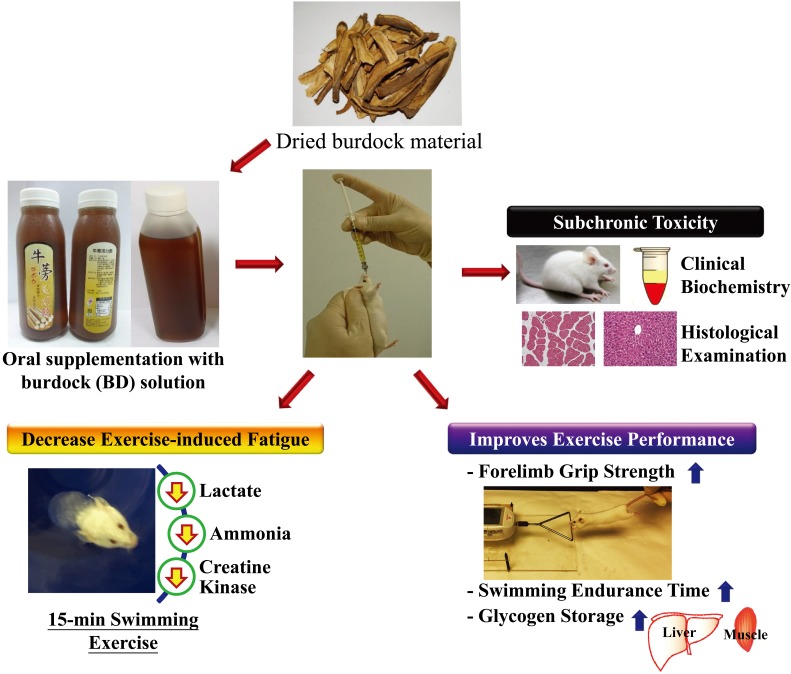
In summary, here we provide evidence-based results to show that BD can improve physically-induced fatigue and elevate exercise performance in mice, as shown in the Appendix Fig. We found that BD supplementation significantly improved post-exercise physiological indicators and exercise performance, including grip strength and endurance time, by increasing muscle glycogen content. BD ameliorated exercise-related increases in levels of biomarkers such as lactate and ammonia. It decreases levels of CK, a muscle injury biomarker, and can be supplemented as optimized and reasonable doses for physiological benefits. Therefore, BD could help to ameliorate exercise-induced fatigue and safely contribute to the promotion of health.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
The corresponding authors acknowledge the Ministry of Science and Technology of Taiwan (grant no. MOST-103-2622-H-127-001-CC3) for financial support of this study. The authors thank Mr. John Ring (National Taiwan University, Taipei City, Taiwan) for his careful editing of the manuscript and Dr. Chien-Chao Chiu for conducting histological examinations.
Appendix
Appendix figure
REFERENCES
- 1.Al-Obaidi S., Al-Sayegh N., Nadar M.2014. Smoking impact on grip strength and fatigue resistance: implications for exercise and hand therapy practice. J. Phys. Act. Health 11: 1025–1031. doi: 10.1123/jpah.2011-0357 [DOI] [PubMed] [Google Scholar]
- 2.Ament W., Verkerke G. J.2009. Exercise and fatigue. Sports Med. 39: 389–422. doi: 10.2165/00007256-200939050-00005 [DOI] [PubMed] [Google Scholar]
- 3.Awale S., Lu J., Kalauni S. K., Kurashima Y., Tezuka Y., Kadota S., Esumi H.2006. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 66: 1751–1757. doi: 10.1158/0008-5472.CAN-05-3143 [DOI] [PubMed] [Google Scholar]
- 4.Bessa A. L., Oliveira V. N., Agostini G. G., Oliveira R. J., Oliveira A. C., White G. E., Wells G. D., Teixeira D. N., Espindola F. S.2016. Exercise Intensity and Recovery: Biomarkers of Injury, Inflammation, and Oxidative Stress. J. Strength Cond. Res. 30: 311–319. doi: 10.1519/JSC.0b013e31828f1ee9 [DOI] [PubMed] [Google Scholar]
- 5.Blois M. S.1958. Antioxidant determinations by the use of a stable free radical. Nature 181: 1199–1200. doi: 10.1038/1811199a0 [DOI] [Google Scholar]
- 6.Cairns S. P.2006. Lactic acid and exercise performance : culprit or friend? Sports Med. 36: 279–291. doi: 10.2165/00007256-200636040-00001 [DOI] [PubMed] [Google Scholar]
- 7.Carvalho-Peixoto J., Alves R. C., Cameron L. C.2007. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 32: 1186–1190. doi: 10.1139/H07-091 [DOI] [PubMed] [Google Scholar]
- 8.Chen C. C., Ringenbach D. R., Snow M.2014. Treadmill walking effects on grip strength in young men with Down syndrome. Res. Dev. Disabil. 35: 288–293. doi: 10.1016/j.ridd.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 9.Chen F. A., Wu A. B., Chen C. Y.2004. The influence of different treatments on the free radical scavenging activity of burdock and variations of its active components. Food Chem. 86: 479–484. doi: 10.1016/j.foodchem.2003.09.020 [DOI] [Google Scholar]
- 10.Chen F. A., Wu A. B., Shieh P., Kuo D. H., Hsieh C. Y.2006. Evaluation of antioxidant activity of Ruellia Tuberosa. Food Chem. 94: 14–18. doi: 10.1016/j.foodchem.2004.09.046 [DOI] [Google Scholar]
- 11.Chen W. C., Huang W. C., Chiu C. C., Chang Y. K., Huang C. C.2014. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 46: 1517–1524. doi: 10.1249/MSS.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadhaniya P., Patel C., Muchhara J., Bhadja N., Mathuria N., Vachhani K., Soni M. G.2011. Safety assessment of a solid lipid curcumin particle preparation: acute and subchronic toxicity studies. Food Chem. Toxicol. 49: 1834–1842. doi: 10.1016/j.fct.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Fujii N., Jessen N., Goodyear L. J.2006. AMP-activated protein kinase and the regulation of glucose transport. Am. J. Physiol. Endocrinol. Metab. 291: E867–E877. doi: 10.1152/ajpendo.00207.2006 [DOI] [PubMed] [Google Scholar]
- 14.Gejl K. D., Hvid L. G., Frandsen U., Jensen K., Sahlin K., Ørtenblad N.2014. Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med. Sci. Sports Exerc. 46: 496–505. doi: 10.1249/MSS.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 15.Horng C. T., Tsai M. L., Hsueh C. W., Hsu S. Y., Wang H. Y., Chen F. A.2013. Antioxidant activity of Arctium Lappa L. and its effect on biochemical parameters in exercised rats. Asian J. Chem. 25: 1970–1974. [Google Scholar]
- 16.Hsu Y. J., Huang W. C., Chiu C. C., Liu Y. L., Chiu W. C., Chiu C. H., Chiu Y. S., Huang C. C.2016. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients 8: E648. doi: 10.3390/nu8100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C. C., Tsai S. C., Lin W. T.2008. Potential ergogenic effects of L-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp. Gerontol. 43: 571–577. doi: 10.1016/j.exger.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Huang C. C., Hsu M. C., Huang W. C., Yang H. R., Hou C. C.2012. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012: 364741. doi: 10.1155/2012/364741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C. C., Tseng T. L., Huang W. C., Chung Y. H., Chuang H. L., Wu J. H.2014. Whole-body vibration training effect on physical performance and obesity in mice. Int. J. Med. Sci. 11: 1218–1227. doi: 10.7150/ijms.9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W. C., Lin C. I., Chiu C. C., Lin Y. T., Huang W. K., Huang H. Y., Huang C. C.2014. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 6: 2681–2696. doi: 10.3390/nu6072681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W. C., Chiu W. C., Chuang H. L., Tang D. W., Lee Z. M., Wei L., Chen F. A., Huang C. C.2015. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 7: 905–921. doi: 10.3390/nu7020905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins R. R.1993. Exercise, oxidative stress, and antioxidants: a review. Int. J. Sport Nutr. 3: 356–375. doi: 10.1123/ijsn.3.4.356 [DOI] [PubMed] [Google Scholar]
- 23.Kan N. W., Huang W. C., Lin W. T., Huang C. Y., Wen K. C., Chiang H. M., Huang C. C., Hsu M. C.2013. Hepatoprotective effects of Ixora parviflora extract against exhaustive exercise-induced oxidative stress in mice. Molecules 18: 10721–10732. doi: 10.3390/molecules180910721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knipping K., van Esch E. C., Wijering S. C., van der Heide S., Dubois A. E., Garssen J.2008. In vitro and in vivo anti-allergic effects of Arctium lappa L. Exp. Biol. Med. (Maywood) 233: 1469–1477. doi: 10.3181/0803-RM-97 [DOI] [PubMed] [Google Scholar]
- 25.Krook A., Wallberg-Henriksson H., Zierath J. R.2004. Sending the signal: molecular mechanisms regulating glucose uptake. Med. Sci. Sports Exerc. 36: 1212–1217. doi: 10.1249/01.MSS.0000132387.25853.3B [DOI] [PubMed] [Google Scholar]
- 26.Li Y., Shi W., Li Y., Zhou Y., Hu X., Song C., Ma H., Wang C., Li Y.2008. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environ. Toxicol. Pharmacol. 26: 13–21. doi: 10.1016/j.etap.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 27.Manabe Y., Miyatake S., Takagi M., Nakamura M., Okeda A., Nakano T., Hirshman M. F., Goodyear L. J., Fujii N. L.2012. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS ONE 7: e52592. doi: 10.1371/journal.pone.0052592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni W., Gao T., Wang H., Du Y., Li J., Li C., Wei L., Bi H.2013. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 150: 529–535. doi: 10.1016/j.jep.2013.08.055 [DOI] [PubMed] [Google Scholar]
- 29.Pari L., Prasath A.2008. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem. Biol. Interact. 173: 77–83. doi: 10.1016/j.cbi.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 30.Park S. Y., Hong S. S., Han X. H., Hwang J. S., Lee D., Ro J. S., Hwang B. Y.2007. Lignans from Arctium lappa and their inhibition of LPS-induced nitric oxide production. Chem. Pharm. Bull. (Tokyo) 55: 150–152. doi: 10.1248/cpb.55.150 [DOI] [PubMed] [Google Scholar]
- 31.Predes F. S., Ruiz A. L., Carvalho J. E., Foglio M. A., Dolder H.2011. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement. Altern. Med. 11: 25. doi: 10.1186/1472-6882-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts C. K., Barnard R. J.2005. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 98: 3–30. doi: 10.1152/japplphysiol.00852.2004 [DOI] [PubMed] [Google Scholar]
- 33.Shang H., Cao S., Wang J., Zheng H., Putheti R.2009. Glabridin from Chinese herb licorice inhibits fatigue in mice. Afr. J. Tradit. Complement. Altern. Medicines 7: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skenderi K. P., Kavouras S. A., Anastasiou C. A., Yiannakouris N., Matalas A. L.2006. Exertional Rhabdomyolysis during a 246-km continuous running race. Med. Sci. Sports Exerc. 38: 1054–1057. doi: 10.1249/01.mss.0000222831.35897.5f [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M., Baba Y., Kataoka Y., Kinbara N., Sagesaka Y. M., Kakuda T., Watanabe Y.2008. Effects of (-) -epigallocatechin gallate in liver of an animal model of combined (physical and mental) fatigue. Nutrition 24: 599–603. doi: 10.1016/j.nut.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 36.Tang X., Zhuang J., Chen J., Yu L., Hu L., Jiang H., Shen X.2011. Arctigenin efficiently enhanced sedentary mice treadmill endurance. PLoS ONE 6: e24224. doi: 10.1371/journal.pone.0024224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Zhang H. L., Lu R., Zhou Y. J., Ma R., Lv J. Q., Li X. L., Chen L. J., Yao Z.2008. The decapeptide CMS001 enhances swimming endurance in mice. Peptides 29: 1176–1182. doi: 10.1016/j.peptides.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 38.Wang S. Y., Huang W. C., Liu C. C., Wang M. F., Ho C. S., Huang W. P., Hou C. C., Chuang H. L., Huang C. C.2012. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 17: 11864–11876. doi: 10.3390/molecules171011864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu R. E., Huang W. C., Liao C. C., Chang Y. K., Kan N. W., Huang C. C.2013. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 18: 4689–4702. doi: 10.3390/molecules18044689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu R. M., Sun Y. Y., Zhou T. T., Zhu Z. Y., Zhuang J. J., Tang X., Chen J., Hu L. H., Shen X.2014. Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol. Sin. 35: 1274–1284. doi: 10.1038/aps.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia Z. Q., Costa M. A., Pélissier H. C., Davin L. B., Lewis N. G.2001. Secoisolariciresinol dehydrogenase purification, cloning, and functional expression. Implications for human health protection. J. Biol. Chem. 276: 12614–12623. doi: 10.1074/jbc.M008622200 [DOI] [PubMed] [Google Scholar]
- 42.Xu Z., Wang X., Zhou M., Ma L., Deng Y., Zhang H., Zhao A., Zhang Y., Jia W.2008. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytother. Res. 22: 97–101. doi: 10.1002/ptr.2273 [DOI] [PubMed] [Google Scholar]
- 43.Yayli N., Yaşar A., Güleç C., Usta A., Kolayli S., Coşkunçelebi K., Karaoğlu S.2005. Composition and antimicrobial activity of essential oils from Centaurea sessilis and Centaurea armena. Phytochemistry 66: 1741–1745. doi: 10.1016/j.phytochem.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 44.Young A. J., Castellani J. W.2001. Exertion-induced fatigue and thermoregulation in the cold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 128: 769–776. doi: 10.1016/S1095-6433(01)00282-3 [DOI] [PubMed] [Google Scholar]
- 45.Yu B. S., Yan X. P., Xiong J., Xin Q.2003. Simultaneous determination of chlorogenic acid, forsythin and arctiin in Chinese traditional medicines preparation by reversed phase-HPLC. Chem. Pharm. Bull. (Tokyo) 51: 421–424. doi: 10.1248/cpb.51.421 [DOI] [PubMed] [Google Scholar]
- 46.Zhang X. L., Ren F., Huang W., Ding R. T., Zhou Q. S., Liu X. W.2010. Anti-fatigue activity of extracts of stem bark from Acanthopanax senticosus. Molecules 16: 28–37. doi: 10.3390/molecules16010028 [DOI] [PMC free article] [PubMed] [Google Scholar]