Abstract
An evaluation of mouse red blood cell (RBC) and platelet (PLT) counting with an automated hematology analyzer was performed with three strains of mice, C57BL/6 (B6), BALB/c (BALB) and DBA/2 (D2). There were no significant differences in RBC and PLT counts between manual and automated optical methods in any of the samples, except for D2 mice. For D2, RBC counts obtained using the manual method were significantly lower than those obtained using the automated optical method (P<0.05), and PLT counts obtained using the manual method were higher than those obtained using the automated optical method (P<0.05). An automated hematology analyzer can be used for RBC and PLT counting; however, an appropriate method should be selected when D2 mice samples are used.
Keywords: automated hematology analyzer, mouse, platelet, red blood cell
Hematological analysis is an important test for the detection of disease and for monitoring the health of humans and animals. Automated hematology analyzers have been routinely used as a rapid, accurate and simple diagnostic method, not only for humans [5, 14], but also for animals [8, 15]. The three major procedures performed in red blood cell (RBC) and platelet (PLT) counting are manual phase contrast microscopy, impedance and optical light scatter/fluorescence [3]. Results produced from these three methods are not necessarily identical due to differences in PLT particle size and aggregation, and red and/or white blood cell fragmentation. Evaluations of the automated hematology analyzer using human and animal samples have been reported [1, 12]. However, despite the fact that RBC and PLT in mice are smaller in size and more numerous than in humans, evaluations of the automated hematology analyzer using murine samples have not been reported, to the best of our knowledge.
In this study, correlations between results from manual direct cell counting by microscopy and an automated hematology analyzer, including optical and impedance methods, were evaluated using commonly used three mouse strains.
C57BL/6NCrl (B6), BALB/cAnNCrlCrlj (BALB) and DBA/2NCrl (D2) inbred mice were obtained from Charles River Japan (Yokohama, Japan). Fifteen mice (five 3-week-old and ten 20-week-old male mice) from each strain were included in the study. Approximately 500 µl blood samples were collected from the axillary arteries and veins of mice under isoflurane anesthesia and placed into sample tubes (Terumo Venoject II, TERUMO Corp., Tokyo, Japan) containing ethylenediaminetetraacetic acid (EDTA-2K). Experiments were approved by the Animal Care and Use Committee of the Teikyo University. The regulations were established in compliance with the law for the humane treatment and management of animals.
RBC and PLT were counted using an automated hematology analyzer (Sysmex XE-5000 hematology analyzer, Sysmex, Kobe, Japan) in EDTA-anticoagulated whole blood samples. Measurements included RBC and PLT counts by optical and impedance methods. Samples were thoroughly inverted and then analyzed according to the manufacturer’s instructions.
Whole blood samples in EDTA were diluted with isotonic solution (Gower’s solution), and then the diluted blood was introduced into the counting chamber where the cell count was performed microscopically (E200, Nikon, Tokyo, Japan).
PLTs were counted in accordance with the brecher-chronkite method [2]. Whole blood samples were diluted with 1% ammonium oxalate solution. The isotonic balance of the diluent was such that all erythrocytes are lysed while the platelets remain intact. The dilution was mixed well and incubated at 25°C for 15 min to permit erythrocyte lysis. Following the incubation period, the dilution was mounted on a hematocytometer (ERMA Inc., Tokyo, Japan). The cells were allowed to settle and were then counted in a specific area of the hemocytometer chamber under a microscope.
Statistical analysis was performed using IBM SPSS® statistics software version 22 (Tokyo, Japan). Significant differences between the manual direct and automated counts were determined using Student’s t-test, and the coefficient of variation (CV) of the measurement results was calculated. P-values <0.05 were considered statistically significant. Correlations between both test results were evaluated using the Pearson’s correlation coefficient test.
Mean± standard deviation (SD) RBC and PLT manual and automated counts for the different methods, including optical and impedance methods, in BALB, B6 and D2 mice (n=10, 20-week-old mice each) are shown in Table 1A–C.
Table 1. Mean (± standard deviation) manual and automated counts of RBC and PLT for the different parameters, optical and impedance.
For the manual counting method, mean± SD RBC counts in BALB mice (1,092.2 ± 95.4 × 104 cells/µl) were significantly higher than those in C57BL/6 (989.6 ± 97.3 × 104 cells/µl) and D2 mice (1,003.2 ± 43.1 × 104 cells/µl) (P<0.05). There was no significant difference in RBC counts between B6 and D2 mice. Mean ± SD PLT counts in BALB mice (121.8 ± 13.3 × 104 cells/µl) were significantly higher than in B6 (104.4 ± 18.2 × 104 cells/µl) and D2 mice (107.8 ± 14.3 × 104 cells/µl) (P<0.05). There was no significant difference in PLT counts between B6 and D2 mice.
For RBC counts in BALB mice, the automated count using the impedance method generated significantly lower values (998.2 ± 25.8 × 104 cells/µl) than the manual method (1,092.2 ± 95.4 × 104 cells/µl) (P<0.05). For RBC counts in D2 mice, automated counts using the optical method (1,094.7 ± 44.1 × 104 cells/µl) were significantly higher than those using the manual method (1,003.2 ± 43.1 × 104 cells/µl) (P<0.05). There were no significant differences in counts between manual and automated methods for the BALB and B6 strains of mice (P>0.05).
For PLT in BALB B6 and D2 mice, automated counts using the impedance method (BALB: 59.9 ± 6.1 × 104 cells/µl, B6: 55.9 ± 8.9 × 104 cells/µl and D2: 47.5 ± 9.7 × 104 cells/µl) were significantly lower than those using the manual method (BALB: 121.8 ± 13.3 × 104 cells/µl, B6: 104.4 ± 18.2 × 104 cells/µl and D2: 107.8 ± 14.3 × 104 cells/µl) (P<0.05). Furthermore, PLT counts in D2 mice using the automated optical method were also lower than those using the manual method (P=0.025). The CV with the manual method was higher than that with the automated methods as shown in Table 1. Correlations between manual and automated methods using 10-, 20-week-old and five 3-week-old mice of each strain are shown in Figs. 1 and 2.
Fig. 1.
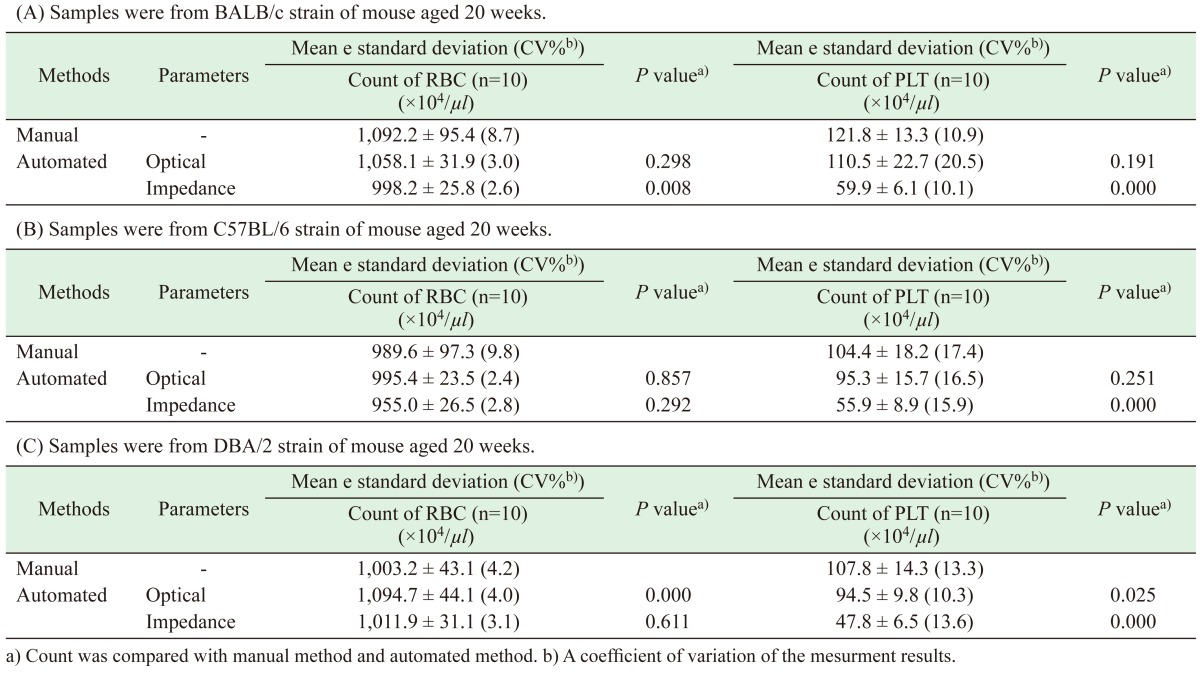
Correlation between manual and automated impedance methods for RBC assessment in BALB/c mice blood samples. Correlation coefficient: r=0.858, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=1.52X+426.
Fig. 2.
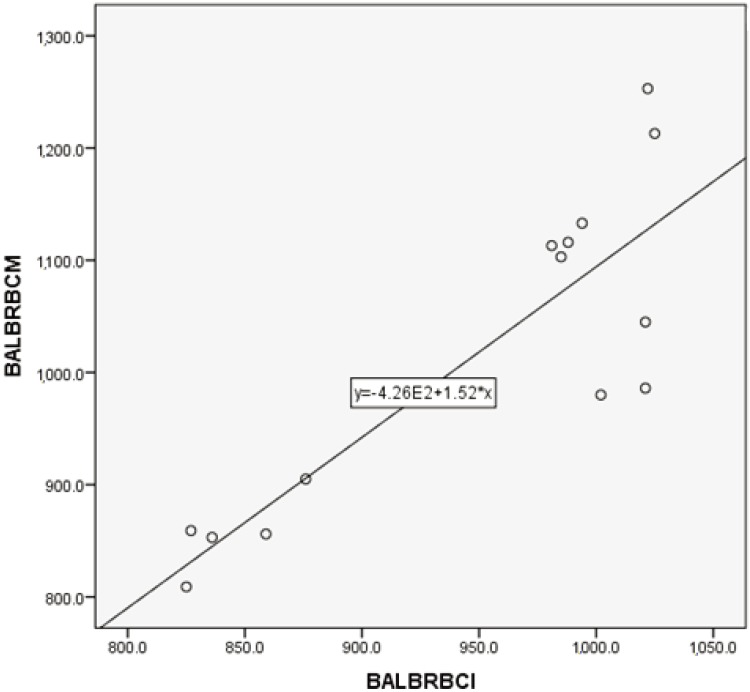
Correlation between manual and automated optical methods for RBC assessment in DBA/2 mice blood samples. Correlation coefficient: r=0.841, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=0.72X+203.
For RBC counts, the regression equation between manual (Y) and impedance (X) in BALB mice was Y=1.52X+426 (r=0.858, P<0.05), while the regression equation between manual (Y) and optical (X) in D2 mice was Y=0.72X+203 (r=0.841, P<0.05).
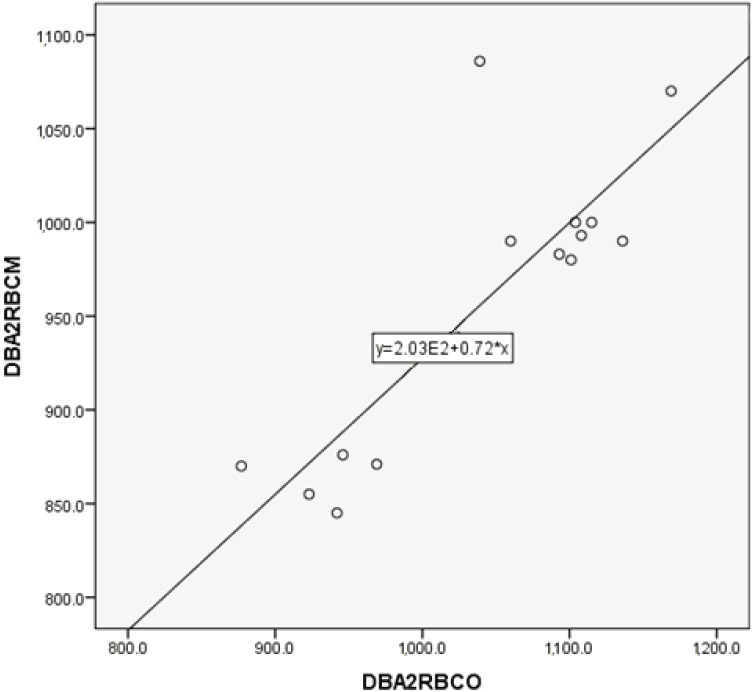
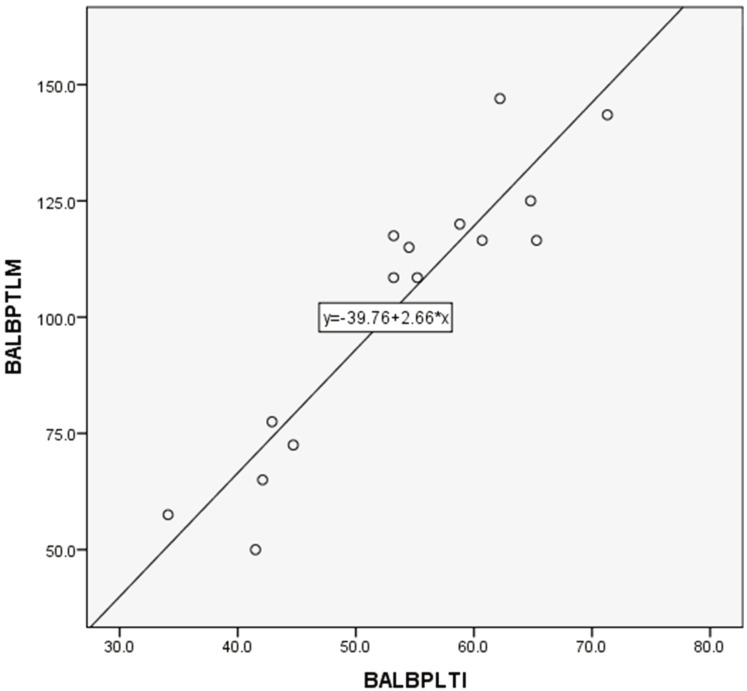
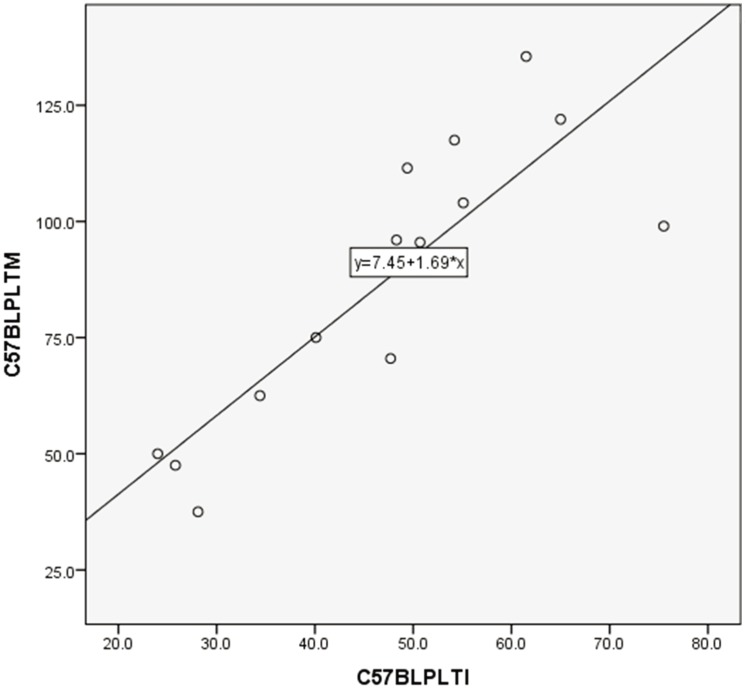
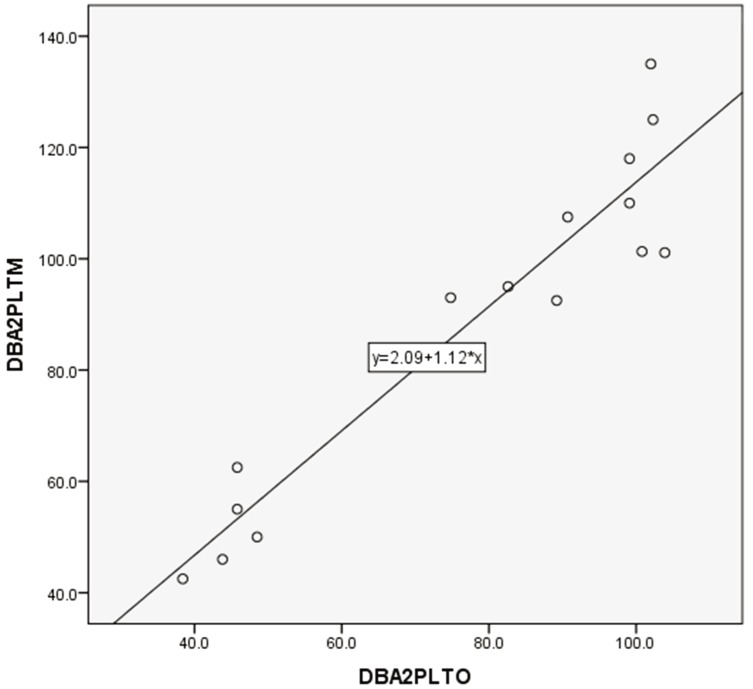
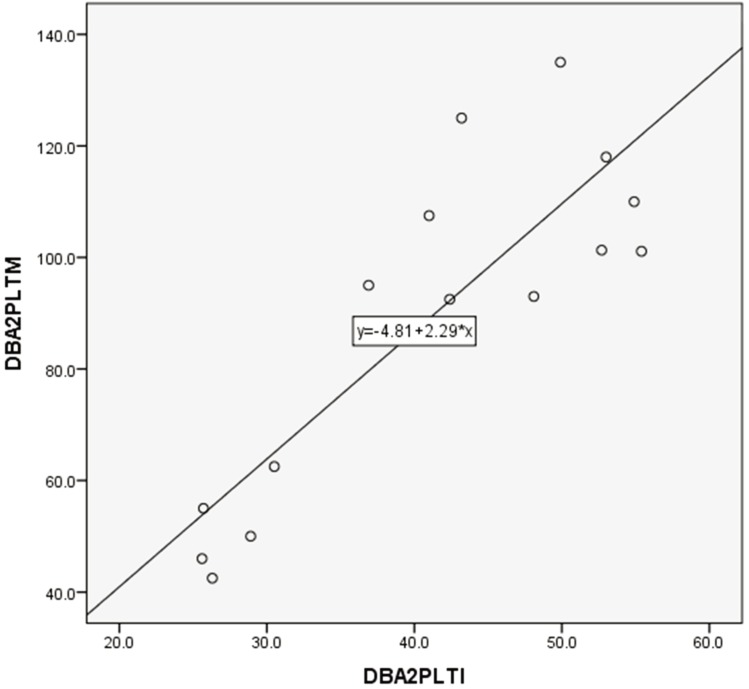
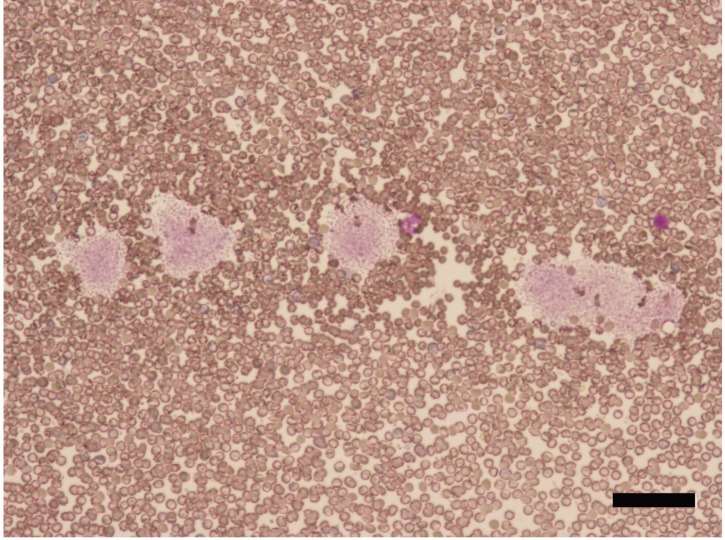
PLT counts between manual and automated impedance methods in BALB and B6 mice, between manual and automated optical methods in D2 mice, and between manual and automated impedance methods in D2 mice are shown in Figs. 3, 4, 5, 6. Regression equations were Y=2.66X+39.76 (r=0.926, P<0.05), Y=1.69X+7.45 (r=0.849, P<0.05), Y=1.12X+2.09 (r=0.95, P<0.05) and Y=2.29X+4.81 (r=0.854, P<0.05), respectively. Abnormal RBC morphology or PLT aggregation was not observed microscopically in B6 and BALB mice. In contrast, PLT clotting was observed in all blood samples from D2 mice including at 3 weeks and 20 weeks of age, as shown in Fig. 7.
Fig. 3.
Correlation between manual and automated impedance methods for PLT assessment in BALB/c mice blood samples. Correlation coefficient: r=0.926, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=2.66X+39.76.
Fig. 4.
Correlation between manual and automated impedance methods for PLT assessment in C57BL/6 mice blood samples. Correlation coefficient: r=0.849, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=1.69X+7.45.
Fig. 5.
Correlation between manual and automated optical method for PLT assessment in DBA/2 mouse blood samples. Correlation coefficient: r=0.95, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=1.12X+2.09.
Fig. 6.
Correlation between manual and automated impedance methods for PLT assessment in DBA/2 mouse blood samples. Correlation coefficient: r=0.854, P<0.05. Regression equation between manual (Y) and optical (X) methods: Y=2.29X+4.81.
Fig. 7.
Platelet clotting in blood samples from DBA/2 mice aged 20 weeks (bar=100 µm).
In this study, an evaluation of mouse red blood cell (RBC) and platelet (PLT) counting with an automated hematology analyzer, including impedance and optical methods, was performed by comparing data with that obtained by manual direct cell counting under a microscope. Fully automated analyzers have been widely used to clarify hematological parameters in both humans and animals. The automated hematology analyzer used in this study, the Sysmex XE-5000, has two modes: the optical method and impedance method. Manual phase contrast microscopy count is still currently recognized as the gold standard or reference method [3], because automated procedures are influenced by PLT clotting and cell size [3, 12]. Impedance PLT counting can provide an accurate PLT count down to 20 × 103 cells/µl; however, a major disadvantage of this method is the difficulty in distinguishing large PLT from extremely microcytic or fragmented RBC. In contrast, optical PLT counting can distinguish between small and large PLT, RBC fragments and debris [3, 12]. In particular, in the case of human blood samples, results from optical and impedance methods have revealed differences in the presence of small-sized RBC, crushed erythrocytes, large PLT, fragments of RBC and/or white blood cells, and aggregation of PLT and/or RBC [3]. It has been previously reported that the type of anticoagulant affects PLT aggregation. Blood samples had significantly lower PLT counts and more PLT aggregation in heparinized tubes than in EDTA tubes [13]; EDTA tubes were used in the present study. If blood specimens show an anomalous particle-size distribution curve in the area where PLT counts are low (exceptionally low PLT count samples), the counting method is automatically switched over to optical. In the present study, even when the method was changed to optical, the PLT count of DBA2 mice was lower compared with the manual method, suggesting that mouse strain should be taken into account when PLT is measured using an automated hematology analyzer.
Mouse blood samples, including cell size and volume, are relatively different compared with human samples. The average size of RBC in humans and mice is 7.3 and 5.8 µm in diameter [6], respectively, and the average RBC count is 3.80–5.50 × 106 cells/µl and 10.2 × 106 cells/µl [7, 9], respectively. The average size of PLT in humans and mice is 1–2 µm and 0.5 µm [11], respectively, and the average count is 150–400 × 103 cells/µl and 1,000–1,500 × 103 cells/µl, respectively [11]. Therefore, RBC and PLT sizes are larger in humans than in mice, while counts are lower in humans than in mice.
In this study, to evaluate the automated analyzer using samples from three strains of mice (BALB, B6 and D2), the results of optical and impedance methods were compared with those of the standard manual microscopic counting method. The results, including RBC and PLT counts, suggested that the automated data from the impedance method were lower than those of the manual microscopic method except with samples from B6 and D2 mice. For B6 and D2 mice samples, significant differences between automated impedance and manual microscopic methods were not observed. There were no significant differences between the automated data from the optical and manual methods except for samples from D2 mice. The RBC counts of the automated optical method were higher than those of manual method in D2 samples, while the PLT counts of the automated optical method were lower than those of the manual method. PLT from D2 mice showed a clumping tendency by microscopic analysis (data not shown) that may have caused a lower count compared with the manual method; however, RBC condition, including form, did not differ between mouse strains. Mean RBC count in D2 mice using the automated optical method was 1,094.7 ± 44.1 × 104 cells/µl, which was within the normal range, suggesting the necessity for the definition of the normal range using automated methods with D2 mice. D2 mice showed PLT clotting, the size of the clotting ranged between 50 and 100 µm (data not shown), suggesting that clotting PLT resulted in incorrect counts with the impedance method. The analytical imprecision, CV, for PLT counting was higher than that for RBC counting, which may have been caused by PLT clotting. It was reported that PLT aggregation response in the whole blood was different depending on the animal species, for example, the aggregation response against adenosine diphosphate; ADP was lower in baboons comparing with in human [10]. The anticoagulants, such as citrate, affect spontaneous aggregation of PLT in Wistar rat but not in Beagle dog [3]. These reports suggested that animal species should be taken into account to count PLT using an automated hematology analyzer.
Despite the fact that the data from manual and automated methods did not necessarily match, a correlation between RBC and PLT results from both methods was recorded. This suggests that automated data can be converted to manual data using a regression equation.
It has been reported that blood counts including RBC and PLT vary depending on several factors, such as anticoagulants, technique for obtaining a blood sample and sampling site [7, 16]. All of the samples used in the present study were collected in the same manner, using EDTA as a coagulant; therefore, the differences between strains should be due to PLT function, such as ease of clotting.
In conclusion, an automated hematology analyzer, involving optical and impedance methods, can be used as a rapid, accurate and simple diagnostic method. Sample conditions can influence the accurate assessment of RBC and PLT counts. Our data showed that PLT counts using samples from the D2 mouse strain were significantly lower compared with the manual method due to PLT clotting. This suggests that the mouse strain should be taken into consideration when performing hematological assessments.
REFERENCES
- 1.Bourgès-Abella N. H., Geffré A., Deshuillers P. L., Braun J. P., Trumel C.2014. Changes in hematology measurements in healthy and diseased dog blood stored at room temperature for 24 and 48 hours using the XT-2000iV analyzer. Vet. Clin. Pathol. 43: 24–35. doi: 10.1111/vcp.12119 [DOI] [PubMed] [Google Scholar]
- 2.Brecher G., Schneiderman M., Cronkite E. P.1953. The reproducibility and constancy of the platelet count. Am. J. Clin. Pathol. 23: 15–26. doi: 10.1093/ajcp/23.1.15 [DOI] [PubMed] [Google Scholar]
- 3.Briggs C., Harrison P., Machin S. J.2007. Continuing developments with the automated platelet count. Int. J. Lab. Hematol. 29: 77–91. doi: 10.1111/j.1751-553X.2007.00909.x [DOI] [PubMed] [Google Scholar]
- 4.Defontis M., Côté S., Stirn M., Ledieu D.2013. Optimization of Multiplate® whole blood platelet aggregometry in the Beagle dog and Wistar rat for ex vivo drug toxicity testing. Exp. Toxicol. Pathol. 65: 637–644. doi: 10.1016/j.etp.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Kotepui M., PhunPhuech B., Phiwklam N., Uthaisar K.2017. Differentiating between dengue fever and malaria using hematological parameters in endemic areas of Thailand. Infect. Dis. Poverty 6: 27–35. doi: 10.1186/s40249-017-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namdee K., Carrasco-Teja M., Fish M. B., Charoenphol P., Eniola-Adefeso O.2015. Effect of variation in hemorheology between human and animal blood on the binding efficacy of vascular-targeted carriers. Sci. Rep. 5: 11631–11644. doi: 10.1038/srep11631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemzek J. A., Bolgos G. L., Williams B. A., Remick D. G.2001. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm. Res. 50: 523–527. doi: 10.1007/PL00000229 [DOI] [PubMed] [Google Scholar]
- 8.Novacco M., Martini V., Grande C., Comazzi S.2015. Analytic errors in Sysmex-generated hematology results in blood from a dog with chronic lymphocytic leukemia. Vet. Clin. Pathol. 44: 337–341. doi: 10.1111/vcp.12270 [DOI] [PubMed] [Google Scholar]
- 9.Osei-Bimpong A., McLean R., Bhonda E., Lewis S. M.2012. The use of the white cell count and haemoglobin in combination as an effective screen to predict the normality of the full blood count. Int. J. Lab. Hematol. 34: 91–97. doi: 10.1111/j.1751-553X.2011.01365.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponschab M., van Griensven M., Heitmeier S., Laux V., Schlimp C. J., Calatzis A., Bahrami S., Redl H., Schöchl H.2016. Platelet function in baboons and humans - A comparative study of whole blood using impedance platelet aggregometry (Multiplate®). Thromb. Res. 147: 115–121. doi: 10.1016/j.thromres.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Schmitt A., Guichard J., Massé J. M., Debili N., Cramer E. M.2001. Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Exp. Hematol. 29: 1295–1302. doi: 10.1016/S0301-472X(01)00733-0 [DOI] [PubMed] [Google Scholar]
- 12.Schoorl M., Schoorl M., Oomes J., van Pelt J.2013. New fluorescent method (PLT-F) on Sysmex XN2000 hematology analyzer achieved higher accuracy in low platelet counting. Am. J. Clin. Pathol. 140: 495–499. doi: 10.1309/AJCPUAGGB4URL5XO [DOI] [PubMed] [Google Scholar]
- 13.Shojania A. M., Turnbull G.1987. Effect of heparin on platelet count and platelet aggregation. Am. J. Hematol. 26: 255–262. doi: 10.1002/ajh.2830260307 [DOI] [PubMed] [Google Scholar]
- 14.Takubo T., Tatsumi N., Satoh N., Matsuno K., Fujimoto K., Soga M., Yamagami Y., Akiba S., Sudoh T., Miyazaki M.2002. Evaluation of hematological values obtained with reference automated hematology analyzers of six manufacturers. Southeast Asian J. Trop. Med. Public Health 33Suppl 2: 62–67. [PubMed] [Google Scholar]
- 15.Tvedten H., Johansson P.2010. Feline platelet counting with prostaglandin E1 on the Sysmex XT-2000iV. Vet. Clin. Pathol. 39: 190–192. doi: 10.1111/j.1939-165X.2009.00210.x [DOI] [PubMed] [Google Scholar]
- 16.Wiedmeyer C. E., Ruben D., Franklin C.2007. Complete blood count, clinical chemistry, and serology profile by using a single tube of whole blood from mice. J. Am. Assoc. Lab. Anim. Sci. 46: 59–64. [PubMed] [Google Scholar]