Abstract
The study was aimed to investigate biofilm forming ability of Mycoplasma hyopneumoniae and to determine the minimum biofilm eradication concentrations of antibiotics. Biofilm forming ability of six strains of M. hyopneumoniae was examined using crystal violet staining on coverslips. The results demonstrated an apparent line of biofilm growth in 3 of the strains isolated from swine with confirmed cases of enzootic pneumonia. BacLight bacterial viability assay revealed that the majority of the cells were viable after 336 hr of incubation. Moreover, M. hyopneumoniae persists in the biofilm after being exposed to 10 fold higher concentration of antibiotics than the minimum inhibitory concentrations in planktonic cells. To the best of our knowledge, this is the first report of biofilm formation in M. hyopneumoniae. However, comprehensive studies on the mechanisms of biofilm formation are needed to combat swine enzootic pneumonia caused by resistant M. hyopneumoniae.
Keywords: biofilm, eradication, inhibition, Mycoplasma hyopneumoniae
Enzootic pneumonia is the most significant chronic respiratory disease of swine causing high morbidity, low mortality and contributes to significant economic losses to the commercial swine production industry [17]. Although Mycoplasma hyopneumoniae is the primary pathogen responsible for swine enzootic pneumonia, opportunistic bacteria, such as Pasteurella multocida are involved in the full development of the disease [27]. Mycoplasmas are a distinct genus of bacteria that are characterized by a single plasma membrane, without a rigid cell wall, and inability to synthesize essential biomolecules [26]. In spite of several studies on the host cell interaction mechanisms in M. pneumoniae and related species [2, 21,22,23,24], limited information is available on the contributing factors for the pathogenicity of mycoplasmas and mechanisms of survival and/or persistence in the host [15, 16].
Biofilms are adherent bacterial aggregates that are encased in an extrapolymeric matrix composed of DNA, protein, lipid and polysaccharide [7]. Biofilm formation is one of the mechanisms for the survival of a number of bacterial strains in the environment [10]. It is known to provide natural protection which subsequently resulted in increased abilities of bacterial resistance to antibiotics and disinfectants. Biofilms can also cause recalcitrance to antibiotics either due to reducing antibiotic penetration and altering the microenvironment. Phenotypic heterogeneity, adaptive response, and the presence of bacterial persister cells are also mentioned as mechanisms of antibiotic resistance in bacterial biofilms [26]. Studies on M. pulmonis showed that the lytic effect of gramicidin, a small antimicrobial peptide, is prevented due to biofilm formation [25].
Despite the use of many antibiotics in pigs, more than 60% of the pig farms in Korea are infected with M. hyopneumoniae. We have been studying the pathogenesis of mycoplasmas with the objective of finding an effective treatment [5, 11, 12, 19]. Despite a wealth of information on other species of mycoplasma and bacteria [4, 13,14,15,16], biofilm formation and its effect on antimicrobials used for the treatment of enzootic pneumonia caused by M. hyopneumoniae in pigs is not studied. Therefore, the current study was designed to investigate biofilm forming ability of M. hyopneumoniae and the effect of biofilm formation on the susceptibility of M. hyopneumoniae to antibiotics.
M. hyopneumoniae was isolated from tracheobronchial swabs of pigs with confirmed cases of enzootic pneumonia from a commercial farm in the Republic of Korea according to a previous method [19]. Briefly, samples were cultured in Friis broth medium (FBM) until a color change was observed and then, it was confirmed by PCR analysis of the culture [28]. The titer of M. hyopneumoniae culture was expressed as a color changing unit per milliliter (CCU/ml). A semi-quantitative screening assay of biofilm formation in 96-well plates were conducted on 40 strains of M. hyopneumoniae identified from the farm, according to a previous method [16]. Accordingly, 3 strong biofilm forming strains (M-IS-1, M-IS-2 and M-IS-3), one non-biofilm strain (M-IS-4) and two reference strains (ATCC) of M. hyopneumoniae were included for crystal violet assay on glass coverslips.
Biofilm growth on 22 mm2 glass coverslips and staining with crystal violet was conducted with slight modifications of previous methods [16, 18]. Eight-milliliter pre-warmed FBM was added in 50 ml conical tubes (Corning, CL, U.S.A.) and sterile coverslips were placed vertically, at the center. The medium was inoculated with a 1:100 dilution of a 20 hr planktonic culture and left at 37°C for 336 hr with shaking at 100 RPM. Non-adherent cells were removed by rinsing in Phosphate buffer saline (PBS). The coverslips were then stained with 0.5% crystal violet solution for 30 min. Finally, biofilm growth and structure were examined microscopically.
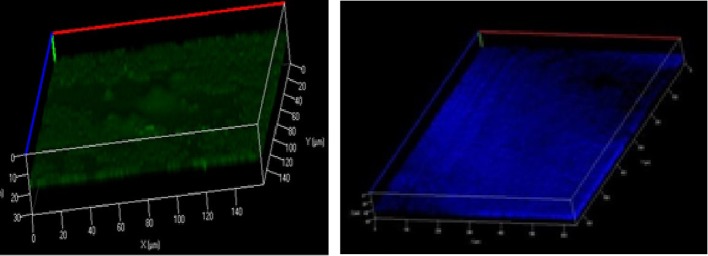
Confocal laser scanning microscopy was used to determine the architecture of the biofilm. For this purpose, biofilms grown on glass coverslips were stained with BacLight bacterial viability assay kit (Molecular Probes). SYTO9 and propidium iodide stained cells were analyzed using confocal laser scanning microscopy with an excitation wavelength and an emission filter of 488 nm vs 500–550 nm and 568 nm vs 580–650 nm, respectively. Accordingly, green coloration denotes the presence of live cells, however; the red color indicates that cells are dead and/or has damaged membranes. In addition, Calcofluor White (Sigma) stain (excitation of 405 nm and an emission filter of 580–650 nm) was used to examine the presence of extracellular polysaccharide, which stains as blue [16].
Minimal inhibitory concentrations (MICs) of colistin (CL), enrofloxacin (ENR), marbofloxacin (MAR), tylosin (TYL), florfenicol (FF), gentamicin (GM) and oxytetracycline (OTC) (Sigma Chemical Co., St. Louis, MO, U.S.A.) for planktonic cultures of M. hyopneumoniae were determined using a broth microdilution method [9]. Whereas, the minimum biofilm eradication concentrations (MBECs) were determined on the Calgary Biofim Device (CBD), according to the manufacturer’s instructions with slight modifications. Briefly, the trough of the CBD was filled with 22 ml fresh FBM and inoculated with approximately 106 CCU of M. hyopneumoniae. The lid, with 96 pegs for bacterial adherence and uniform biofilm formation, was placed in the trough and incubated at 37°C with 95% relative humidity. Samples were incubated for 336 hr with constant shaking at 10 rpm (Gyro rotary shaker, model C2, New Brunswick Scientific, San Francisco, CA, U.S.A.).
A duplicate, serial twofold dilutions of the antibiotics in FBM used in the determination of MIC were made in 96-well plate. Lid pegs with bacterial biofilm were rinsed in sterile PBS and placed in the 96-well plate containing the antibiotics and incubated at 37°C for 336 hr. The lid was removed, rinsed with PBS, placed in another 96-well plate containing only sterile FBM. The lid was then sonicated for 5 min to remove the biofilm (Aquasonic, model 250; VWR Scientific, Buffalo Grove, IL, U.S.A.). A new plate cover was added and the plates were incubated at 37°C for 336 hr. The presence of viable bacteria was determined by the color change of the broth media. The growth of bacteria in a particular well indicates the regrowth of bacteria as a result of surviving biofilm and the MBEC value was taken as the lowest dilution for the bacteria fail to regrow [3].
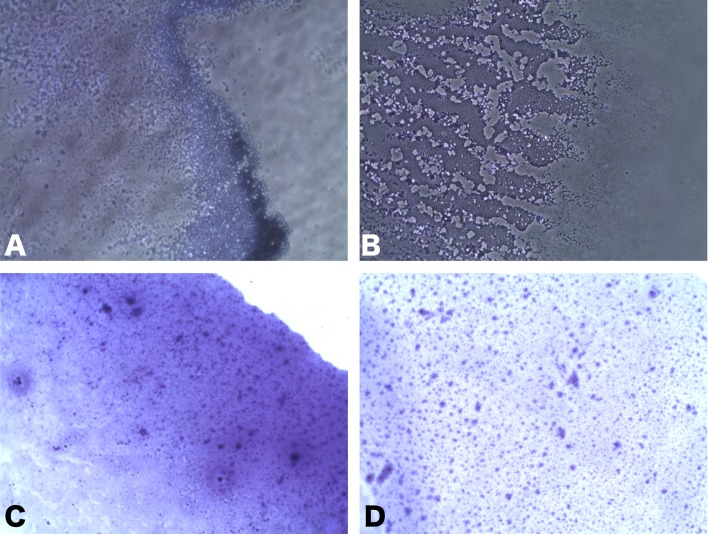
Among the six strains assessed, the M-IS-1, M-IS-2 and M-IS-3 showed a clear and consistent biofilm formation on coverslips after 336 hr of incubation. All of the biofilm forming strains (M-IS-1, M-IS-2, and M-IS-3) showed similar biofilm structure. Crystal violet staining on coverslips revealed an apparent line of biofilm growth at the air/liquid interface (Fig. 1). However, the non-biofilm forming strains (for example, strain M-IS-4) could not adhere on the glass coverslips (Fig. 2). Confocal imaging of M. hyopneumoniae biofilms stained with backlight viability stain revealed a green fluorescence, indicating that the majority of cells were viable with an intact membrane, after 336 hr of incubation (Fig. 3-Left). The proportion of dead cells or cells with damaged membrane were about 30 % of the total. Moreover, Calcofluor white staining demonstrated a clear polysaccharide layer covering the biofilm, stained as blue (Fig. 3-Right).
Fig. 1.
Crystal violet stained M. hyopneumoniae biofilms (MI-S-3) on coverslips indicating biofilm growth at the air/liquid interface (10–100 × magnification).
Fig. 2.
Crystal violet staining of non-biofilm forming M. hyopneumoniae (MI-S-4) on coverslips. The M. hyopneumoniae could not adhere to the coverslip after washing with PBS (100× magnification).
Fig. 3.
Confocal images of BacLight stained viable cells (MI-S-3) and green coloration denotes the presence of live cells (Left). Calcofluor White stained MI-S-3 showing blue stained polysaccharide surrounding the biofilm layer (Right).
The MICs and MBCEs of selected antibiotics against planktonic and biofilm cultures of M. hyopneumoniae and the non-biofilm forming standard strains are summarized in Table 1. The planktonic cultures were susceptible to almost all of the antibiotics tested. However, three strains of M. hyopneumoniae (M-IS-1, M-IS-2 and M-IS-3) survived in the biofilm at concentrations which are 10 fold higher than the MICs in planktonic cultures. Moreover, we have observed that the antibiotics failed to inhibit polysaccharide production in M. hyopneumoniae biofilm cells, even at significantly higher concentrations than the MICs as compared to the planktonic cells.
Table 1. MICs (MBECs) (µg/ml) of antibiotics against different strains of M. hyopneumoniae.
| Designated strain | ENR | MAR | CL | TYL | FF | GM | OTC |
|---|---|---|---|---|---|---|---|
| M-ATTC 21774 | 0.03 | 0.5 | 1 | 0.03 | 0.12 | 0.25 | 0.12 |
| M-ATTC 25156 | 0.06 | 0.5 | 1 | 0.03 | 0.25 | 0.5 | 0.12 |
| M-IS-1 | 0.06 | 1 | 0.5 | 0.06 | 0.25 | 0.25 | 0.5 |
| (32) | (256) | (512) | (32) | (128) | (512) | (32) | |
| M-IS-2 | 0.06 | 1 | 2 | 0.039 | 0.5 | 0.25 | 0.5 |
| (>2,048) | (256) | (512) | (128) | (512) | (512) | (32) | |
| M-IS-3 | 0.06 | 1 | 1 | 0.03 | 0.25 | 0.5 | 0.5 |
| (32) | (512) | (256) | (256) | (1,024) | (256) | (256) | |
| M-IS-4 | 0.03 | 1 | 1 | 0.06 | 0.5 | 0.5 | 0.5 |
ENR: enrofloxacin, MAR: marbofloxacin, CL: colistin, TYL: tylosin, FF: florfenicol, GM: gentamicin and OTC: oxytetracycline.
From a total of 6 strains, three of them (M-IS-1, M-IS-2 and M-IS-3) exhibited biofilm formation with significant variability. Clear biofilm structure with channels and stacks were observed similar to previous studies in other mycoplasma species [15, 16], Staphylococcus epidermidis and Pseudomonas aeruginosa [6]. Despite variation in passage number, consistent biofilm formation was observed in these three isolates. Two of them had a relatively low passage number compared to the reference strains. Previous studies on Actinobacillus pleuropneumoniae showed that increased passage number could contribute to loss of biofilm forming ability and variation in adhering abilities of the pathogen [1]. In addition, a rough colony of M. hyopneumoniae with a star shape grown in FB agar plate was observed under light microscope, only in the biofilm forming strains. Earlier studies on other mycoplasma species demonstrated that rough colony types may have been associated with biofilm formation [16]. Therefore, passage number and colony nature could affect the biofilm forming ability of M. hyopneumoniae.
McAuliffe et al. [16] demonstrated the absence of a significant difference in the planktonic and biofilm MICs. In the current study, however; the MBECs of the antibiotics in biofilm cells were significantly higher than the MICs in planktonic cells. Cells of M. hyopneumoniae in a biofilm were at least 500 times more resistant to the tested antibiotics than their planktonic counterparts. Similar to previous findings in E. coli, Staphylococcus aureus, M. gallisepticum and P. aeruginosa [3, 4], M. hyopneumoniae grown in biofilm conditions were much less vulnerable to antibiotics than were the planktonic cells suggesting that M. hyopneumoniae biofilms are more likely resistant to commonly used antibiotics. This could be due to the protective exopolysaccharide layer in biofilm cultures which may reduce the penetration to antibiotics [15]. Biofilm growth is also indicated in the persistence of mycoplasmas in the environment [16]. This improved resistance suggests that some mycoplasma strains may form biofilms in vivo, thus inhibiting the activity of antimicrobial agents and creating a prolonged infection. Our findings showed that higher concentrations of the antibiotics above the MIC failed to inhibit polysaccharide production in biofilm cells, which could contribute to recalcitrance to the antibiotics. On the contrary, studies on S. epidermidis demonstrated that subinhibitory concentrations of antibiotics can result in phenotypic variations, especially on adhesion and polysaccharide production [8, 16, 20].
To the best of our knowledge, this is the first report of biofilm formation in M. hyopneumoniae. This is a preliminary study on biofilm formation of M. hyopneumoniae and the findings indicated the ability of M. hyopneumoniae to form biofilm under stated conditions. The study also revealed the contribution of biofilms to the persistence of M. hyopneumoniae in the presence of higher concentrations of selected antibiotics. This will be an important consideration in antibacterial therapy against M. hyopneumoniae infection. However, comprehensive characterization of M. hyopneumoniae biofilm, how it adheres to the surface and initiates biofilm formation is needed to combat swine enzootic pneumonia caused by resistant M. hyopneumoniae.
Acknowledgments
This research was funded in part by the Cooperative research program for agricultural science and technology development (Project No. PJ01128901), the National foundation of Korea (2016R1A2B4013507), and by the Korean institute of planning and evaluation of technology (314082-3) funded by the Ministry of agriculture, food, and rural affairs, South Korea.
REFERENCES
- 1.Archambault M., Harel J., Gouré J., Tremblay Y. D., Jacques M.2012. Antimicrobial susceptibilities and resistance genes of Canadian isolates of Actinobacillus pleuropneumoniae. Microb. Drug Resist. 18: 198–206. doi: 10.1089/mdr.2011.0150 [DOI] [PubMed] [Google Scholar]
- 2.Balish M., Krause D. C.2002. Molecular Biology and Pathogenicity of Mycoplasmas. (Herrmann, R, Razin, S. eds.), Kluwer Academic/Plenum, New York. [Google Scholar]
- 3.Ceri H., Olson M. E., Stremick C., Read R. R., Morck D., Buret A.1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Yu S., Hu M., Han X., Chen D., Qiu X., Ding C.2012. Identification of biofilm formation by Mycoplasma gallisepticum. Vet. Microbiol. 161: 96–103. doi: 10.1016/j.vetmic.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 5.Damte D., Suh J. W., Lee S. J., Yohannes S. B., Hossain M. A., Park S. C.2013. Putative drug and vaccine target protein identification using comparative genomic analysis of KEGG annotated metabolic pathways of Mycoplasma hyopneumoniae. Genomics 102: 47–56. doi: 10.1016/j.ygeno.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Dunne W. M., Jr.2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15: 155–166. doi: 10.1128/CMR.15.2.155-166.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemming H. C., Wingender J.2010. The biofilm matrix. Nat. Rev. Microbiol. 8: 623–633. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca A. P., Extremina C., Fonseca A. F., Sousa J. C.2004. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J. Med. Microbiol. 53: 903–910. doi: 10.1099/jmm.0.45637-0 [DOI] [PubMed] [Google Scholar]
- 9.Hannan P. C. T.2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 31: 373–395. doi: 10.1051/vetres:2000100 [DOI] [PubMed] [Google Scholar]
- 10.Hu Q., Han X., Zhou X., Ding S., Ding C., Yu S.2010. Characterization of biofilm formation by Riemerella anatipestifer. Vet. Microbiol. 144: 429–436. doi: 10.1016/j.vetmic.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 11.Hwang M. H., Damte D., Cho M. H., Kim Y. H., Park S. C.2010. Optimization of culture media of pathogenic Mycoplasma hyopneumoniae by a response surface methodology. J. Vet. Sci. 11: 327–332. doi: 10.4142/jvs.2010.11.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang M. H., Damte D., Lee J. S., Gebru E., Chang Z. Q., Cheng H., Jung B. Y., Rhee M. H., Park S. C.2011. Mycoplasma hyopneumoniae induces pro-inflammatory cytokine and nitric oxide production through NFκB and MAPK pathways in RAW264.7 cells. Vet. Res. Commun. 35: 21–34. doi: 10.1007/s11259-010-9447-5 [DOI] [PubMed] [Google Scholar]
- 13.Justice-Allen A., Trujillo J., Corbett R., Harding R., Goodell G., Wilson D.2010. Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. J. Dairy Sci. 93: 192–202. doi: 10.3168/jds.2009-2474 [DOI] [PubMed] [Google Scholar]
- 14.Kornspan J. D., Tarshis M., Rottem S.2011. Adhesion and biofilm formation of Mycoplasma pneumoniae on an abiotic surface. Arch. Microbiol. 193: 833–836. doi: 10.1007/s00203-011-0749-y [DOI] [PubMed] [Google Scholar]
- 15.McAuliffe L., Ayling R. D., Ellis R. J., Nicholas R. A.2008. Biofilm-grown Mycoplasma mycoides subsp. mycoides SC exhibit both phenotypic and genotypic variation compared with planktonic cells. Vet. Microbiol. 129: 315–324. doi: 10.1016/j.vetmic.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 16.McAuliffe L., Ellis R. J., Miles K., Ayling R. D., Nicholas R. A.2006. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 152: 913–922. doi: 10.1099/mic.0.28604-0 [DOI] [PubMed] [Google Scholar]
- 17.Messier S., Ross R. F., Paul P. S.1990. Humoral and cellular immune responses of pigs inoculated with Mycoplasma hyopneumoniae. Am. J. Vet. Res. 51: 52–58. [PubMed] [Google Scholar]
- 18.O’Toole G. A., Kolter R.1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30: 295–304. doi: 10.1046/j.1365-2958.1998.01062.x [DOI] [PubMed] [Google Scholar]
- 19.Park S. C., Yibchok-Anun S., Cheng H., Young T. F., Thacker E. L., Minion F. C., Ross R. F., Hsu W. H.2002. Mycoplasma hyopneumoniae increases intracellular calcium release in porcine ciliated tracheal cells. Infect. Immun. 70: 2502–2506. doi: 10.1128/IAI.70.5.2502-2506.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachid S., Ohlsen K., Witte W., Hacker J., Ziebuhr W.2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44: 3357–3363. doi: 10.1128/AAC.44.12.3357-3363.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rottem S.2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83: 417–432. doi: 10.1152/physrev.00030.2002 [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Vargas F. M., Gómez-Duarte O. G.2008. Mycoplasma pneumoniae-an emerging extra-pulmonary pathogen. Clin. Microbiol. Infect. 14: 105–117. doi: 10.1111/j.1469-0691.2007.01834.x [DOI] [PubMed] [Google Scholar]
- 23.Seto S., Kenri T., Tomiyama T., Miyata M.2005. Involvement of P1 adhesin in gliding motility of Mycoplasma pneumoniae as revealed by the inhibitory effects of antibody under optimized gliding conditions. J. Bacteriol. 187: 1875–1877. doi: 10.1128/JB.187.5.1875-1877.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons W. L., Dybvig K.2007. Biofilms protect Mycoplasma pulmonis cells from lytic effects of complement and gramicidin. Infect. Immun. 75: 3696–3699. doi: 10.1128/IAI.00440-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons W. L., Bolland J. R., Daubenspeck J. M., Dybvig K.2007. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J. Bacteriol. 189: 1905–1913. doi: 10.1128/JB.01512-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart P. S.2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292: 107–113. doi: 10.1078/1438-4221-00196 [DOI] [PubMed] [Google Scholar]
- 27.Thacker E. L.2004. Diagnosis of Mycoplasma hyopneumoniae. J. Swine. Health Prog. 12: 252–254. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguti M., Muller E. E., Piffer A. I., Kich J. D., Klein C. S., Kuchiishi S. S.2008. Detection of Mycoplasma hyopneumoniae by polymerase chain reaction in swine presenting respiratory problems. Braz. J. Microbiol. 39: 471–476. doi: 10.1590/S1517-83822008000300011 [DOI] [PMC free article] [PubMed] [Google Scholar]