Abstract
Gammaherpesviruses (GHVs) are members of an emerging subfamily of the family Herpesviridae. A recent study identified a novel GHV in domestic cats (Felis catus GHV1, FcaGHV1), and epidemiological surveys have found that FcaGHV1 is distributed worldwide. In this study, we investigated the prevalence of GHVs in domestic cats in Japan with a molecular epidemiological survey. Blood samples were collected from 1,738 domestic cats and GHV-derived DNA was detected with PCR in 1.3% (23/1,738) of the Japanese domestic cats. The FcaGHV1 detected in this study was very similar to FcaGHV1 detected in a domestic cat in North America. Older age (>5 years old) and Feline immunodeficiency virus infection were identified as risk factors for GHV infection.
Keywords: cat, gammaherpesvirus, Japan
Gammaherpesviruses (GHVs) are members of an emerging subfamily of the family Herpesviridae and are classified into four genera: Lymphocryptovirus, Rhandivirus, Macavirus, and Percavirus [4, 10]. These viruses infect a diverse range of vertebrates, including humans and other mammals, in a host-specific manner [10]. The two major human GHVs, Human herpesvirus 4 and Human herpesvirus 8, which are also called Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated virus (KSHV), respectively, are clinically important pathogens in human practice because they have tumorigenic potential, especially in immunocompromised patients [9, 22]. It has been reported that latent membrane protein 1 (LMP1) of EBV is histopathologically detectable in the tumor tissues of almost all patients with Human immunodeficiency virus (HIV)-associated Hodgkin lymphoma [22].
Lymphoma is one of the commonest tumors encountered in feline medicine [8, 12, 19]. Feline leukemia virus (FeLV) has been considered the major cause of lymphoma in the past, but a recent study showed that most cases of feline lymphoma were FeLV-negative [12]. Feline immunodeficiency virus (FIV)-infected cats are also known to be at high risk of lymphoma [8, 14]. However, FIV is believed to play an indirect role in the development of lymphoma because there is no evidence of the clonal proliferation of tumor cells containing FIV proviral DNA [2, 14]. It has been suggested that a specific infectious agent, such as human EBV or KSHV, contributes to the development of lymphoma in FIV-infected cats, although the mechanism of its development in domestic cats is not fully understood [2, 14]. In this context, several studies have looked for infectious agents other than FeLV that are associated with the development of lymphoma in cats [13, 23]. A recent study identified a novel GHV, Felis catus gammaherpesvirus 1 (FcaGHV1), in domestic cats in the United States, and epidemiological surveys have shown that FcaGHV1 is distributed in several countries, including the United States, European countries, Australia, and Singapore [3, 6, 15, 23]. However, no epidemiological survey of GHV infection has yet been conducted in Japanese domestic cats. Therefore, in the present study, we conducted a nationwide molecular epidemiological survey to investigate the prevalence of GHVs in Japan and to clarify the risk factors for GHV infection in domestic cats.
Blood samples were collected from 1,770 cats admitted to 47 private veterinary hospitals located in each of the 47 prefectures in Japan, between March and October 2008. Cats accessing the outdoors at least once a week were included, but cats kept strictly indoors were excluded. The age, sex, major ailments, and other clinical information about each cat were recorded at each hospital. FIV and FeLV infections were screened by the detection of serum anti-FIV antibodies and FeLV p27 antigen, respectively, using a commercially available test kit (SNAP FeLV/FIV Combo Test; IDEXX Laboratories Inc., Westbrook, ME, U.S.A.), in our previous study [18]. The profiles of the examined cats are shown in Table 1.
Table 1. Clinical profiles, GHV frequencies, and risk factors for GHV infection in 1,738 cats.
| Number of cats | GHV positive | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||||
| Total | 1,738 | 23 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Age | >5 years | 826 | 16 | 4.93 | 1.43–16.99 | <0.01 | 3.84 | 1.06–13.86 | 0.04 |
| <5 years | 752 | 3 | |||||||
| Unknown | 160 | 4 | |||||||
| Sex | Male | 923 | 17 | 2.51 | 0.98–6.40 | 0.05 | 1.64 | 0.56–4.79 | 0.36 |
| Female | 809 | 6 | |||||||
| Unknown | 6 | 0 | |||||||
| Breed | Pure | 34 | 1 | 2.31 | 0.30–17.70 | 0.36 | N/A | N/A | N/A |
| Mix | 1,704 | 22 | |||||||
| Bite wound history | Yes | 699 | 12 | 1.62 | 0.69–3.78 | 0.28 | N/A | N/A | N/A |
| No | 941 | 10 | |||||||
| Unknown | 98 | 1 | |||||||
| FeLV infection | Positive | 212 | 5 | 2.02 | 0.74–5.50 | 0.18 | 1.7 | 0.53–5.42 | 0.36 |
| Negative | 1,526 | 18 | |||||||
| FIV infection | Positive | 400 | 14 | 5.35 | 2.30–12.46 | <0.01 | 4.11 | 1.52–11.11 | <0.01 |
| Negative | 1,338 | 9 | |||||||
| Health status | Sick | 1,156 | 19 | 2.37 | 0.80–7.02 | 0.12 | 1.26 | 0.34–4.60 | 0.71 |
| Healthy | 573 | 4 | |||||||
| Unknown | 9 | 0 | |||||||
Total DNA was extracted from the blood samples and was used as the template for the amplification of GHV-derived DNA. As an internal control, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was also amplified from each sample using the primers cGS (5′-CTCATGACCACAGTCCATGC-3′, nucleotides [nt] 514–533, GenBank/EMBL/DDBJ accession no. AB038240) and cGR (5′-TGAGCTTGACAAAGTGGTCA-3′, nt 925–906) [21]. The PCRs involved 25 cycles of denaturation (94°C, 1 min), annealing (60°C, 1 min), and polymerization (72°C, 3 min). The GAPDH gene was successfully amplified from the samples from 1,738 of the 1,770 cats that presented at animal hospitals located in the 47 Japanese prefectures. The blood samples collected from those cats were subjected to a PCR analysis to detect GHV-derived DNA and the cats’ clinical data were subjected to a risk factor analysis.
GHV-derived DNA was detected in the blood samples with nested degenerative PCR targeting the glycoprotein B gene, as previously reported [5, 23]. Primers 2759s (5′-CCTCCCAGGTTCARTWYGCMTAYGA-3′) and 2762as (5′-CCGTTGAGGTTCTGAGTGTARTARTTRTAYTC-3′) and primers 2760s (5′-AAGATCAACCCCAC (N/I) AG (N/I) GT (N/I) ATG-3′) and 2761as (5′-GTGTAGTAGTTGTACTCCCTRAACAT (N/I) GTYTC-3′) were used for the first- and second-round PCRs, respectively [5]. These primers are known to amplify a broad range of mammalian GHVs and the size of the amplicon in this PCR system is approximately 500 bp [5]. The reaction mixture (30 µl) for the first-round PCR contained primers 2759s and 2762as (0.5 µM each), 0.2 mM dNTPs, DNA polymerase (2.0 units, Takara, Kyoto, Japan), 5 µl of template DNA, and the reagents recommended by the manufacturer. The DNA extracted from B95a cells, which are known to be transformed with EBV, was used as a positive control, and was added to the first-round PCR as the template DNA instead of sample DNA [11, 17]. The first-round PCR product (2 µl) was used as the template for the second-round PCR. The amplification steps for the first- and second-round PCRs consisted of initial heating at 94°C for 2 min, and 45 cycles of denaturation (94°C for 30 sec), annealing (45°C for 30 sec), and polymerization (72°C for 30 sec), followed by a final extension at 72°C for 7 min. The sensitivity of this PCR was evaluated by determining its detection limit using a serially diluted plasmid template. A DNA fragment of the glycoprotein B gene derived from EBV was inserted into the plasmid pCR2.1 (Invitrogen, Calsbad, CA, U.S.A.). Serial 10-fold dilutions corresponding to 103–10−2 copies of the plasmid were prepared and used as templates. The genomic DNA from healthy cats was added to the all-PCR reaction mixture to bring the analysis closer to the conditions in clinical surveys. Triplicate analyses were performed for each dilution. The PCR products were then separated electrophoretically and the minimum detection limit was determined. This analysis showed that the PCR detected EBV in one reaction mixture containing 100 copies of the EBV DNA (data not shown).
The nucleotide sequences of the amplified DNA fragments were inserted into the pCR2.1 plasmid vector (Invitrogen), and the nucleotide sequence of each inserted DNA fragment was determined with the dideoxy chain termination method (ABI Prism BigDye Primer Cycle Sequencing Ready Reaction Kit; Applied Biosystems, Foster City, CA, U.S.A.). Homology searches based on the nucleotide sequences of the PCR products were performed with the Basic Local Alignment Search Tool (BLAST) in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp/Welcome-j.html) [1]. We performed the distance matrix calculations and constructed a phylogenetic tree with ClustalW version 1.8 in DDBJ. The Kimura two-parameter method was used to calculate the distance matrix of the aligned sequences, with all gaps ignored, and the neighbor-joining method in the DNADIST program from the PHYLIP software package (http://evolution.genetics.washington.edu/phylip.html) was used to construct the phylogenetic tree, as previously described [7]. The stability of the phylogenetic tree was estimated with a bootstrap analysis of 1,000 replications using the same program. The tree figure was generated with TreeView version 1.6.6.
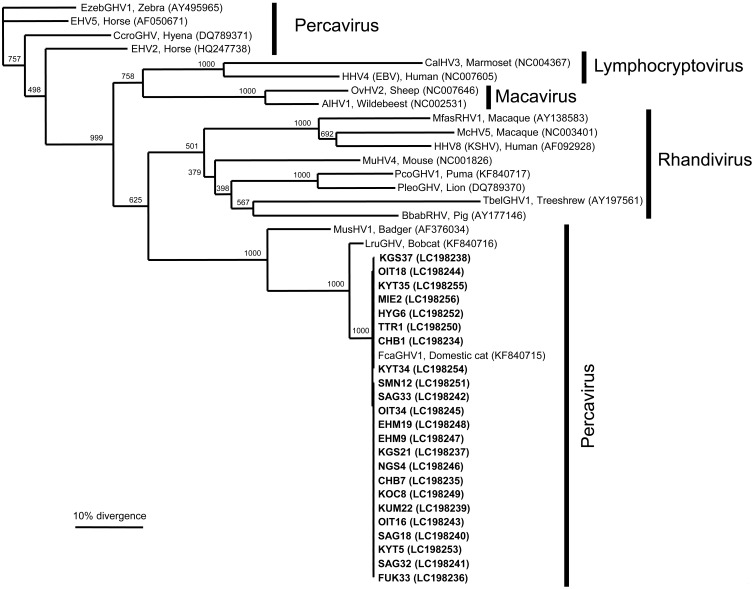
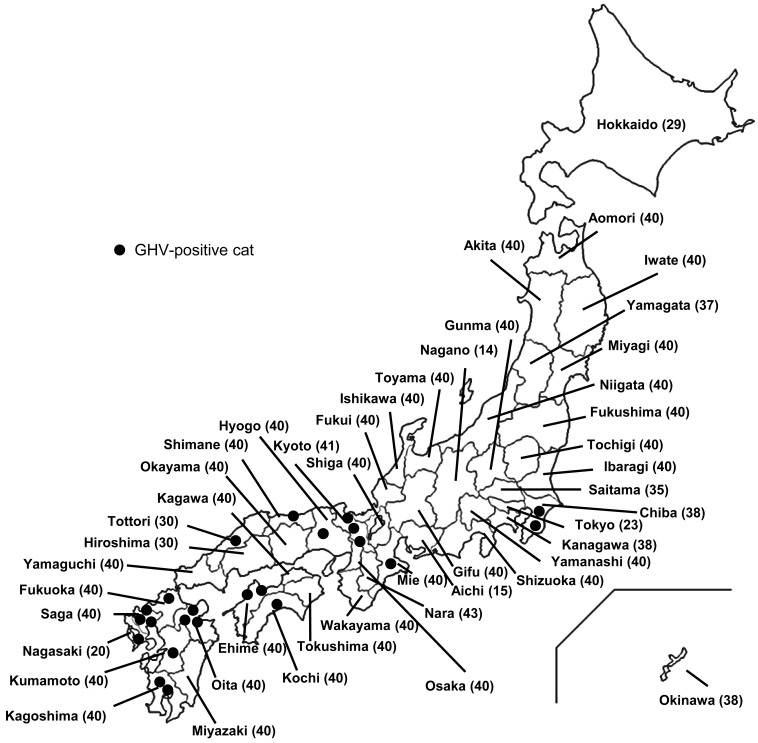
The GHV-derived glycoprotein B gene was detected in 23 of the 1,738 cats, with an overall prevalence of 1.3%. DNA sequencing and a BLAST analysis revealed that all the sequences were strongly similar (99.9%) to FcaGHV1 from the United States (KF840715) [23]. In a phylogenetic analysis of the sequence data, all the GHVs detected in the present study (LC198234-LC198256) formed one cluster and formed a single group with the GHVs from domestic cats and bobcats (Fig. 1). The detected GHVs belonged to the genus Percavirus. The distribution of the FcaGHV1-infected cats was also evaluated by plotting the locations of the cats positive for GHV-derived DNA on a map of Japan. The FcaGHV1-infected cats were mainly distributed in the western part of Japan, as shown in Fig. 2.
Fig. 1.
Phylogenetic relationships of various gammaherpesviruses based on the nucleotide sequences of the glycoprotein B gene. Scale bar indicates the genetic distance (0.1 substitutions/site). The 23 clones of FcaGHV1 detected in this study are shown in bold (LC198234–LC198256). Pathogen names, host species, and GenBank accession numbers (in parentheses) of the sequences are shown on the phylogenetic tree: Human herpesvirus 4 (HHV4, Epstein-Barr virus, NC007605); Callitrichine herpesvirus 3 (CalHV3, NC004367); Alcelaphine herpesvirus 1 (AlHV1, NC002531); Ovine herpesvirus 2 (OvHV2, NC007646); Mustelid herpesvirus 1 (MusHV1, AF376034); Felis catus gammaherpesvirus 1 (FcaGHV1, KF840715); Lynx rufus gammaherpesvirus 1 (LruGHV1, KF840716); Equid herpesvirus 2 (EHV2, HQ247738); Equid herpesvirus 5 (EHV5, AF050671); Crocuta crocuta gammaherpesvirus 1 (CcroGHV1, DQ789371); Equus zebra gammaherpesvirus 1 (EzebGHV1, AY495965); Tupaia belangeri gammaherpesvirus 1 (TbelGHV1, AY197561); Macacine herpesvirus 5 (McHV5, NC003401); Human herpesvirus 8 (HHV8, Kaposi’s sarcoma-associated herpesvirus, NC009333); Macaca fascicularis rhadinovirus 1 (MfasRHV1, AY138583); Murid herpesvirus 4 (MuHV4, NC001826); Puma concolor gammaherpesvirus 1 (PcoGHV1, KF840717); Panthera leo gammaherpesvirus 1 (PleoGHV1, DQ789370); and Babyrousa babyrussa rhadinovirus 1 (BbabRHV1, AY177146). The number under each internal nodes indicates the percentage of 1,000 bootstrap replicates that supported the branch.
Fig. 2.
Map of Japan and the distribution of GHV-positive cats. Dots represent GHV-infected cats. The number in parentheses after the prefecture name indicates the number of samples collected in each prefecture.
The risk factors for GHV infection were determined from various clinical parameters, including age, breed, sex, retroviral infection status, bite wound history, and health status. These parameters were used as variables in the following statistical analyses. Fisher’s exact test was used for the univariable analysis, and the variables with P values <0.2 were included in a logistic regression model for the multivariable analysis. The odds ratio (OR) was calculated with the 95% confidential interval (CI), and variables with P<0.05 were considered significant risk factors for GHV infection.
In the univariable analysis, older age (>5 years old, OR, 4.93; 95% CI, 1.43–16.99; P<0.01), male sex (OR, 2.51; 95% CI, 0.98–6.40; P=0.05), FIV infection (OR, 5.35; 95% CI, 2.30–12.46; P<0.01), FeLV infection (OR, 2.02; 95% CI, 0.74–5.50; P=0.18), and ill health (OR, 2.37; 95% CI, 0.80–7.02; P=0.12) were identified as risk factors for GHV infection (Table 1). In the subsequent multivariable analysis, older age (OR, 3.84; 95% CI, 1.06–13.86; P=0.04) and FIV infection (OR, 4.11; 95% CI, 1.52–11.11; P<0.01) were significant and independent risk factors for GHV infection (Table 1).
In this study, the prevalence of GHVs and the risk factors for GHV infection were evaluated in Japanese domestic cats. This is the first reported detection of GHV in Japanese domestic cats. FcaGHV1 has been identified in domestic cats in North America, central Europe, Southeast Asia, and Australia, with prevalences ranging from 9.6 to 16.2% [3, 6, 23]. These reports suggest that FcaGHV1 is distributed worldwide, including in Asia, and our results support these findings. However, the prevalence of FcaGHV1 in Japanese domestic cats (1.3%) is lower than the prevalence reported in other countries and areas [3, 6, 23], suggesting that the prevalence of FcaGHV1 varies among countries or areas, although the virus is distributed worldwide. However, it is unclear why Japanese domestic cats show a lower prevalence of FcaGHV1 than domestic cats in other countries. We applied the same PCR system which was used in previous studies for our purpose, therefore, the sensitivity of the PCR and the accuracy of obtained prevalence in this study should be same as those in previous studies [3, 6, 23].
GHVs are generally known to establish latent infections and the viral loads of FcaGHV1 in the blood are considered to depend on the immune status of the host and/or infection with other infectious agents, such as FIV [6]. In this study, we knew the FIV infection status of the samples analyzed, but no information was available on the clinical stages of the FIV infections or the immunological status of the cats. Identifying the mechanisms underlying viral activation and the establishment of viremia in the host may clarify the pathophysiology of FcaGHV1 infection and explain the lower prevalence of FcaGHV1 in Japanese domestic cats than in other countries and areas. A recent study reported that the seroprevalence of FcaGHV1 was much higher than that determined with a molecular survey [20]. Serum samples were not collected in the present study, so we could not evaluate the seroprevalence of FcaGHV1. A serological survey might also more accurately determine the prevalence of FcaGHV1 in Japanese domestic cats. The FcaGHV1 detected in the present study were almost identical at the glycoprotein B gene to those in the United States [23], which suggests that the virus is highly conserved in domestic cat species and that GHVs are carried in a host-specific manner, like other herpesviruses [10].
Interestingly, the FcaGHV1-infected cats were predominantly found in the western part of Japan. Other studies have also reported geographic differences in the distribution of FcaGHV1-infected animals [20, 23]. However, FcaGHV1-infected cats displaying the risk factors identified in this study (FIV-positive cats and older cats) were equally distributed in the eastern and western parts of Japan [18]. A recent study revealed that hemoplasma infection increases the risk of GHV infection, but the distribution of hemoplasma-positive cats in Japan did not differ significantly between the eastern and western areas [16, 21]. The risk factors for FcaGHV1 infection in Japanese domestic cats are being an older cat ( >5 years old) or an FIV-infected cat. These factors have also been identified as risk factors for FcaGHV1 infection in previous studies [3, 6]. The high prevalence of the virus in adult cats suggests that FcaGHV1 infection occurs postnatally and is transmitted horizontally. Because FIV infection is a significant risk factor for FcaGHV1 infection, it is possible that the immune suppression caused by FIV infection is an important factor in establishing the viremia of FcaGHV1 and that the transmission route of FcaGHV1 is direct, like that of FIV. Direct contact, such as fighting, might be a major transmission route, although a bite wound history was not identified as a significant risk factor for FcaGHV1 infection in this study (Table 1). If direct transmission is a major route of FcaGHV1 infection, the behavior of cats, such as grooming and sharing feeding dishes, might be important. However, if direct contact is a major transmission route for FcaGHV1, the uneven distribution pattern of FcaGHV1 in Japan cannot be explained.
This study failed to identify any statistical relationship between FcaGHV1 infection and the clinical symptoms in a risk factor analysis. A previous epidemiological study in central Europe also showed no statistical relationship between FcaGHV1 infection and chronic illness [3]. However, previous surveys in Singapore and Australia reported that FcaGHV1-positive cats were more likely to be sick [6]. Although the pathogenicity of FcaGHV1 is not fully understood, including its contribution to lymphomagenesis, the pathogenicity of GHV should be evaluated, keeping in mind that GHV diseases usually develop in immunocompromised human patients [9, 22]. The pathogenicity of FcaGHV1 was not fully evaluated in the present study because no information was available on the immunological status of the cats examined, and the clinical significance of FcaGHV1 infection in feline medicine is still unknown. Further research is required, especially concerning whether viruses of the genus Percavirus are tumorigenic, to determine the pathogenicity of FcaGHV1 in domestic cats.
Acknowledgments
This work was performed with the cooperation of the Japanese Advisory Board of Feline Infectious Diseases.
REFERENCES
- 1.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J.1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 2.Beatty J. A., Lawrence C. E., Callanan J. J., Grant C. K., Gault E. A., Neil J. C., Jarrett O.1998. Feline immunodeficiency virus (FIV)-associated lymphoma: a potential role for immune dysfunction in tumourigenesis. Vet. Immunol. Immunopathol. 65: 309–322. doi: 10.1016/S0165-2427(98)00164-0 [DOI] [PubMed] [Google Scholar]
- 3.Beatty J. A., Troyer R. M., Carver S., Barrs V. R., Espinasse F., Conradi O., Stutzman-Rodriguez K., Chan C. C., Tasker S., Lappin M. R., VandeWoude S.2014. Felis catus gammaherpesvirus 1; a widely endemic potential pathogen of domestic cats. Virology 460-461: 100–107. doi: 10.1016/j.virol.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Davison A. J., Eberle R., Ehlers B., Hayward G. S., McGeoch D. J., Minson A. C., Pellett P. E., Roizman B., Studdert M. J., Thiry E.2009. The order Herpesvirales. Arch. Virol. 154: 171–177. doi: 10.1007/s00705-008-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers B., Dural G., Yasmum N., Lembo T., de Thoisy B., Ryser-Degiorgis M. P., Ulrich R. G., McGeoch D. J.2008. Novel mammalian herpesviruses and lineages within the Gammaherpesvirinae: cospeciation and interspecies transfer. J. Virol. 82: 3509–3516. doi: 10.1128/JVI.02646-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertl R., Korb M., Langbein-Detsch I., Klein D.2015. Prevalence and risk factors of gammaherpesvirus infection in domestic cats in Central Europe. Virol. J. 12: 146. doi: 10.1186/s12985-015-0381-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein J.1991. PHYLIP Manual Version3.69. University Herbarium, University of California, Berkeley. [Google Scholar]
- 8.Gabor L. J., Love D. N., Malik R., Canfield P. J.2001. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Aust. Vet. J. 79: 540–545. doi: 10.1111/j.1751-0813.2001.tb10742.x [DOI] [PubMed] [Google Scholar]
- 9.Gloghini A., Dolcetti R., Carbone A.2013. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin. Cancer Biol. 23: 457–467. doi: 10.1016/j.semcancer.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 10.King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J.2012. Virus Taxonomy: Ninth Report of the International Committeeon Taxonomy of Viruses. Elsevier, London. [Google Scholar]
- 11.Kobune F., Sakata H., Sugiura A.1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64: 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louwerens M., London C. A., Pedersen N. C., Lyons L. A.2005. Feline lymphoma in the post-feline leukemia virus era. J. Vet. Intern. Med. 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 13.Lozano C. C., Sweanor L. L., Wilson-Henjum G., Kays R. W., Moreno R., VandeWoude S., Troyer R. M.2015. Identification of Novel Gammaherpesviruses in Ocelots (Leopardus pardalis) and Bobcats (Lynx rufus) in Panama and Colorado, U.S.A. J. Wildl. Dis. 51: 911–915. doi: 10.7589/2015-01-027 [DOI] [PubMed] [Google Scholar]
- 14.Magden E., Miller C., MacMillan M., Bielefeldt-Ohmann H., Avery A., Quackenbush S. L., Vandewoude S.2013. Acute virulent infection with feline immunodeficiency virus (FIV) results in lymphomagenesis via an indirect mechanism. Virology 436: 284–294. doi: 10.1016/j.virol.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 15.McLuckie A. J., Barrs V. R., Smith A. L., Beatty J. A.2016. Detection of Felis catus gammaherpesvirus 1 (FcaGHV1) in peripheral blood B- and T-lymphocytes in asymptomatic, naturally-infected domestic cats. Virology 497: 211–216. doi: 10.1016/j.virol.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 16.McLuckie A., Tasker S., Dhand N. K., Spencer S., Beatty J. A.2016. High prevalence of Felis catus gammaherpesvirus 1 infection in haemoplasma-infected cats supports co-transmission. Vet. J. 214: 117–121. doi: 10.1016/j.tvjl.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Miller G., Shope T., Lisco H., Stitt D., Lipman M.1972. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc. Natl. Acad. Sci. U.S.A. 69: 383–387. doi: 10.1073/pnas.69.2.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura Y., Nakamura Y., Ura A., Hirata M., Sakuma M., Sakata Y., Nishigaki K., Tsujimoto H., Setoguchi A., Endo Y.2010. An updated nation-wide epidemiological survey of feline immunodeficiency virus (FIV) infection in Japan. J. Vet. Med. Sci. 72: 1051–1056. doi: 10.1292/jvms.09-0574 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J. M., North S. M., Freeman K. P., Ramiro-Ibañez F.2010. Feline paediatric oncology: retrospective assessment of 233 tumours from cats up to one year (1993 to 2008). J. Small Anim. Pract. 51: 306–311. doi: 10.1111/j.1748-5827.2010.00915.x [DOI] [PubMed] [Google Scholar]
- 20.Stutzman-Rodriguez K., Rovnak J., VandeWoude S., Troyer R. M.2016. Domestic cats seropositive for Felis catus gammaherpesvirus 1 are often qPCR negative. Virology 498: 23–30. doi: 10.1016/j.virol.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanahara M., Miyamoto S., Nishio T., Yoshii Y., Sakuma M., Sakata Y., Nishigaki K., Tsujimoto H., Setoguchi A., Endo Y.2010. An epidemiological survey of feline hemoplasma infection in Japan. J. Vet. Med. Sci. 72: 1575–1581. doi: 10.1292/jvms.10-0143 [DOI] [PubMed] [Google Scholar]
- 22.Thompson L. D., Fisher S. I., Chu W. S., Nelson A., Abbondanzo S. L.2004. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am. J. Clin. Pathol. 121: 727–738. doi: 10.1309/PNVQ0PQGXHVY6L7G [DOI] [PubMed] [Google Scholar]
- 23.Troyer R. M., Beatty J. A., Stutzman-Rodriguez K. R., Carver S., Lozano C. C., Lee J. S., Lappin M. R., Riley S. P., Serieys L. E., Logan K. A., Sweanor L. L., Boyce W. M., Vickers T. W., McBride R., Crooks K. R., Lewis J. S., Cunningham M. W., Rovnak J., Quackenbush S. L., VandeWoude S.2014. Novel gammaherpesviruses in North American domestic cats, bobcats, and pumas: identification, prevalence, and risk factors. J. Virol. 88: 3914–3924. doi: 10.1128/JVI.03405-13 [DOI] [PMC free article] [PubMed] [Google Scholar]