Abstract
Progressive rod-cone degeneration (PRCD) is an autosomal recessive disease caused by c.5G>A mutation of the PRCD exon 2. This mutation has been identified in various breeds, including Labrador Retriever. The present study aimed to examine the allelic frequency of PRCD in Labrador Retrievers in Japan. A domestic and a guide dog population were genotyped for PRCD using polymerase chain reaction-restriction fragment length polymorphism. The allelic frequency of c.5G>A in domestic and guide dog populations (0.114 and 0.026, respectively) differed significantly. The allele with c.5G>A mutation appeared to spread widely in the domestic population as compared to that in the guide dog population. This might be the result of mating control for PRCD in the guide dog population.
Keywords: allelic frequency, Labrador Retriever, mutation, progressive rod-cone degeneration
Canine progressive rod cone degeneration (PRCD), a type of progressive retinal atrophy, is an autosomal recessive disease [1, 5, 6]. The responsible gene mutation, c.5G>A in PRCD exon 2, has been identified [8], and it has spread widely in more than 20 dog breeds [1]. However, the frequency of this mutant allele in each dog breed is almost unknown. In Japan, the allelic frequency in Toy poodle and Chihuahua was found to be 0.088 and 0.019, respectively [4].
Labrador Retriever is a popular dog breed used as domestic and guide dogs. Since domestic and guide dog populations have independent breeding stocks, it is expected that the incidence of genetic diseases differs between the two populations. PRCD develops in middle age [1, 5], and thus may develop in the period of contribution as an active guide dog. Once it develops, it is a great loss for the visually impaired user. Therefore, all guide dog associations in Japan have performed mating control for PRCD based on genotype (personal communication). However, mating control for domestic Labrador Retrievers might not be performed. Thus, allelic frequencies might differ in populations that differ in the process of breeding control [7].
In the present study, the genotype of PRCD in domestic and guide dog populations of Labrador Retriever in Japan was examined by polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP). In addition, an allele-specific PCR was conducted as a simple method of genotyping for PRCD. Thus, a significant difference in allelic frequency of PRCD between domestic and guide dog populations in Japan was revealed.
Whole blood or buccal swab was randomly collected from 114 domestic and 170 guide Labrador Retrievers, based on informed consent. None of these dogs was tested earlier for retinal function. Remainder of blood samples from domestic Labrador Retrievers that had visited animal clinics for medical examination was used for genomic DNA extraction. For guide dogs, buccal swabs were smeared on FTA elute cards (Whatman International Ltd., Piscataway, NJ, U.S.A.) and stored with silica gel at room temperature before use. Whole blood was stored in EDTA tubes at −20°C and genomic DNA was extracted with High-pure PCR template kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol. For each of the FTA elute cards, a piece of diameter 1 mm was washed three times with distilled water and used directly as a template for PCR. PCR was carried out in reaction volumes of 10 µl containing 1 × PCR buffer, 1.5 mM Mg++, 2.5 mM deoxyribonucleotide triphosphates, 0.2 µM of each forward and reverse primer, and 0.25 units of Taq polymerase (TaKaRa TaqTM Hotstart version, Takara Bio Inc., Otsu, Japan). Thermal cycling conditions were initial denaturation at 95°C for 1 min, followed by 35 cycles of denaturation at 94°C for 30 sec, annealing at 61°C for 20 sec, and extension at 72°C for 20 sec. Novel primers for PCR-RFLP were designed for this study (forward primer: 5′-GGCTGACCCCACTAATCAGCTTG-3′, reverse primer; 5′-TCAGCTTCTCACGGTTGGACCC-3′). This primer set amplifies a 232-bp product containing c.5G>A in PRCD exon 2. The PCR product was digested with 5 units of ApaLI (New England Biolab, MA, U.S.A.) at 37°C for 90 min to yield 151 and 81-bp fragments in the wild genotype (GG), but not in the mutant genotype (AA). After digestion, the PCR products were electrophoresed on a 2% agarose gel and visualized by UltraPowerTM DNA Dye solution (Gelex International, Tokyo, Japan).
Allele-specific PCR was performed in five dogs (two wild-type dogs, two heterozygous and one homozygous mutant-type), whose genotypes were identified by PCR-RFLP. Reverse primers specific for the wild-type allele (G) and the mutant allele (A) were designed. The primer for the allele G corresponded to the wild-type sequence, except for an artificial artifact introduced at the third codon from 3ʹ end, indicated in lower-case (PRCD-G: 5′-GCTGAGTAGGAAGAGGGTGGTaCA-3′). The primer specific for the mutant allele (A) corresponded to the mutant sequence, except for an artificial artifact introduced at the third codon from 3ʹ end (PRCD-A: 5′-GCTGAGTAGGAAGAGGGTGGTaTA-3′). The second codon from 3ʹ end in each primer was matched to the site where the point mutation occurred. These primers were used with the forward primer used for PCR-RFLP to amplify 173-bp products. Two reactions were set up for each sample, one with the primer specific to allele G, and the other specific to allele A; these primers were used in combination with the common forward primer. PCR was carried out as described above. The following thermocycling conditions were used: initial denaturation at 95°C for 1 min, followed by 35 cycles of denaturation at 94°C for 30 sec, annealing at 65°C for 20 sec, and extension at 72°C for 20 sec, and the amplicons were visualized as described above.
Allelic frequencies in each dog population were calculated based on genotype frequencies. Genotype data were analyzed by chi-square test for deviation of genotype and allele frequencies from the predictions of Hardy-Weinberg equilibrium [8]. Fisher’s exact test was used to compare the allelic frequencies of the domestic and guide dog populations [3].
The genotype frequencies of PRCD obtained with PCR-RFLP agreed with Hardy-Weinberg equilibrium in both the domestic and guide dog populations (Table 1). The frequency of allele A in the domestic population was significantly higher than that in the guide dog population (P<0.01). Based on these results, expected incidence rate of PRCD in domestic and guide dog populations was estimated to be 1.3 and 0.07%, respectively.
Table 1. Genotypic and allelic frequencies of Labrador Retrievers.
| N | GG (%) | GA (%) | AAa) (%) | A allele frequency | |
|---|---|---|---|---|---|
| Domestic | 114 | 89 (78.1) | 24 (21.0) | 1 (0.9) | 0.114 |
| Guide dog | 170 | 161 (94.7) | 9 (5.3) | 0 (0) | 0.026 |
a) AA, homozyhous for c.5G >A.
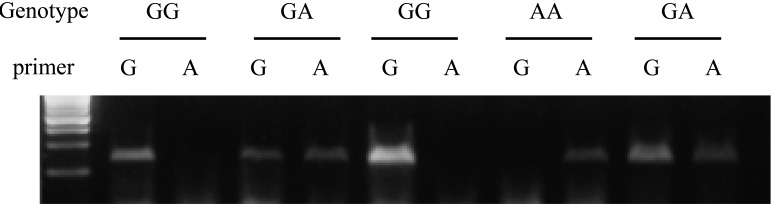
In allele specific PCR, the G allele-specific primer yielded an amplicon in dogs with GG or GA genotypes, but not in AA genotype (Fig. 1). However, the A allele-specific primer yielded an amplicon in dogs with GA or AA genotypes, but not in the GG genotype. The results of an allele-specific PCR were consistent with those of PCR-RFLP.
Fig. 1.
Allele-specific PCR of PRCD. Genotype of each individual using specific primer for G and A alleles are shown. The G allele-specific primer yields an amplicon in the genotypes GG and GA, but not in AA. The primer specific to the A allele yields an amplicon in the genotypes GA and AA, but not GG.
Labrador Retrievers were divided into domestic and guide dog populations. As breeding stocks of these two populations are different, incidence of genetic diseases may differ [7]. In the present study, the genotypic frequency of PRCD and allelic frequency of c.5G>A were examined in each population. The allelic frequency of c.5G>A in the domestic population was significantly higher than that in the guide dog population. Mating control to prevent the spread of PRCD has been introduced in the guide dog population several years ago. This might be the reason for low frequency of c.5G>A in this population (0.07%), indicating that only one affected dog is present in 1,400. Since there are 2,000 individuals in the guide dog population in Japan, no dogs affected by PRCD might be present, suggesting almost complete exclusion of c.5G>A from this population.
The allelic frequency of c.5G>A in domestic population is 0.114 and incidence of PRCD is predicted to be 1.3%. The allelic frequency in Toy poodle and Chihuahua in Japan has been reported to be 0.088 and 0.019, respectively [4], indicating that PRCD incidence is 0.77 and 0.036%, respectively. Incidence of PRCD in domestic Labrador Retrievers is higher than that in Toy poodle or Chihuahua, suggesting that c.5G>A mutation is spreading widely in this population before people realize this disease. As evident from the guide dog population, the frequency of the mutant allele can be decreased by controlled mating. Therefore, if genotype-based mating is performed in the domestic dog population, incidence of PRCD might decrease. Since PRCD develops after middle age of dogs, sometimes the disease is not detected by general eye examination in the age group used for mating. Therefore, genotyping might be a superior method for the diagnosis of PRCD-affected dogs than ophthalmic examination.
In the allele-specific PCR analysis of five dogs, which had been genotyped by PCR-RFLP, the GG, GA and AA genotypes could be clearly identified. Real-time PCR has been developed for genotyping of PRCD previously [2, 4, 9]. However, real-time PCR is more expensive than conventional PCR, despite being an excellent method. Nevertheless, allele-specific PCR described in the present study is a cost-effective method and thus may be another option for genotyping PRCD.
The present study revealed significant difference in the allelic frequency of c.5G>A of PRCD exon 2 in domestic and guide dog populations of Labrador Retriever in Japan. Incidence of this disease in the domestic population is relatively high, whereas that in the guide dog population is low. There results suggested that controlled breeding based on genotyping could reduce the incidence of PRCD in Labrador Retrievers.
Acknowledgments
I am grateful to veterinary clinicians who cooperated in blood sampling, and staff of guide dog associations in Japan for buccal swab sampling. This study was supported by JSPS KAKENHI (Grant Number 24925027).
REFERENCES
- 1.Downs L. M., Hitti R., Pregnolato S., Mellersh C. S.2014. Genetic screening for PRA-associated mutations in multiple dog breeds shows that PRA is heterogeneous within and between breeds. Vet. Ophthalmol. 17: 126–130. doi: 10.1111/vop.12122 [DOI] [PubMed] [Google Scholar]
- 2.Gentilini F., Rovesti G. L., Turba M. E.2009. Real-time detection of the mutation responsible for progressive rod-cone degeneration in Labrador Retriever dogs using locked nucleic acid TaqMan probes. J. Vet. Diagn. Invest. 21: 689–692. doi: 10.1177/104063870902100515 [DOI] [PubMed] [Google Scholar]
- 3.Kanda Y.2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohyama M., Tada N., Mitsui H., Tomioka H., Tsutsui T., Yabuki A., Rahman M. M., Kushida K., Mizukami K., Yamato O.2016. Real-time PCR genotyping assay for canine progressive rod-cone degeneration and mutant allele frequency in Toy Poodles, Chihuahuas and Miniature Dachshunds in Japan. J. Vet. Med. Sci. 78: 481–484. doi: 10.1292/jvms.15-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellersh C. S.2014. The genetics of eye disorders in the dog. Canine Genet. Epidemiol. 1: 3. doi: 10.1186/2052-6687-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyadera K., Acland G. M., Aguirre G. D.2012. Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies. Mamm. Genome 23: 40–61. doi: 10.1007/s00335-011-9361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholas F. W.2010. Introduction of Veterinary Genetics, 3rd ed., Wiley-Blackwell Ltd., Oxford. [Google Scholar]
- 8.Takanosu M., Mori H., Suzuki H., Suzuki K.2012. Genotyping of exercise-induced collapse in Labrador retrievers using an allele-specific PCR. Vet. J. 193: 293–295. doi: 10.1016/j.tvjl.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 9.Zangerl B., Goldstein O., Philp A. R., Lindauer S. J., Pearce-Kelling S. E., Mullins R. F., Graphodatsky A. S., Ripoll D., Felix J. S., Stone E. M., Acland G. M., Aguirre G. D.2006. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 88: 551–563. doi: 10.1016/j.ygeno.2006.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]