Summary
A number of clinical studies have shown protective effects of lactobacilli against Candida species in the gastrointestinal tract, the urogenital tract and the oral cavity, while others did not show clear effects. Evidence on the mode of action of lactobacilli against Candida is also still lacking. In this study, the anti‐Candida activity of the model probiotic strain Lactobacillus rhamnosus GG was explored in different assays to determine molecular interactions. We found that L. rhamnosus GG was able to interfere with Candida growth, morphogenesis and adhesion. These three aspects of Candida's physiology are all crucial to its opportunistic pathogenesis. In follow‐up assays, we compared the activity of L. rhamnosus GG wild‐type with its exopolysaccharide (EPS)‐deficient mutant and purified EPS to evaluate the involvement of this outer carbohydrate layer. Our data demonstrate that purified EPS can both interfere with hyphal formation and adhesion to epithelial cells, which indicates that EPS is part of a combined molecular mechanism underlying the antihyphal and anti‐adhesion mechanisms of L. rhamnosus GG.
Introduction
Under normal circumstances, members of the Candida genus are non‐pathogenic, commensal microorganisms inhabiting the gastrointestinal tract, oral cavity and female reproductive tract (Sardi et al., 2013). A few species are known to shift to opportunistic pathogens and cause mucosal infections at these sites which in turn can lead to invasive candidiasis. The gastrointestinal tract in particular acts as a frequent source from which Candida can disseminate to other tissues and organs (Manzoni et al., 2006). For example in critically ill patients, Candida species are also regularly cultured from the respiratory tract (Ibàñez‐Nolla et al., 2004). The gastrointestinal tract is not the only site where Candida can shift to a pathogenic state. Affecting almost 50% of women of reproductive age, vulvovaginal candidiasis (VVC) belongs to the most common forms of Candida infections (Foxman et al., 2013). The main cause of all these infections is C. albicans, but C. glabrata has recently gained in importance as a human pathogen (Silva et al., 2012). Together, they are responsible for approximately 65%‐75% of all systemic candidiasis infections, which places C. albicans and C. glabrata currently among the most common fungal pathogens in humans (Brunke and Hube, 2013).
The pathogenesis of Candida starts with fungal overgrowth, followed by adhesion, tissue invasion and mucosal infection (Höfs et al., 2016). This process is facilitated by several virulence factors including host recognition biomolecules and hyphal morphogenesis. In C. albicans, adhesion to epithelial cells is mediated by the agglutinin‐like sequence (ALS) gene family proteins, which mainly recognize certain peptides on the host cell surface, but the binding of the key adhesin Als1 to laminin can be inhibited by galactose (Klotz et al., 2004; Donohue et al., 2011). In C. glabrata, three members of the epithelial adhesin (EPA) gene family were also found to bind to ligands containing a terminal galactose residue (Zupancic et al., 2008). Besides lectin‐like proteins, adhesion of C. albicans and C. glabrata also seems to involve the oligomannosides on their cell wall surface (Dalle et al., 2003; de Groot et al., 2008). Hyphal morphogenesis implies the reversible transition between unicellular yeast cells and filamentous growth forms, which is typical for some yeasts such as C. albicans, but not C. glabrata (Calderone and Fonzi, 2001). The hyphal form of C. albicans is associated with alterations in antigen expression and tissue affinities. In addition, hyphae are more potent in penetrating epithelial tissues and causing damage than the unicellular yeast cells (Gow et al., 2011).
The human microbiota appears to contribute to the prevention of Candida species shifting from a harmless commensal to a disease‐causing pathogen, especially in the gut (Mason et al., 2012). In women with VVC, the protective role of the vaginal microbiota in this disease is less clear (Zhou et al., 2009; Liu et al., 2013). Some studies indicate that lactobacilli, important members of a healthy vaginal microbiota, can protect against vaginal C. albicans infections (Martinez et al., 2009; Ehrström et al., 2010; Kovachev and Vatcheva‐Dobrevska, 2015; Parolin et al., 2015), but in another study, the administration of lactobacilli was insufficient to prevent post‐antibiotic vulvovaginitis (Pirotta, 2004). A few studies have also shown protective effects of some lactobacilli, such as Lactobacillus rhamnosus, in the oral cavity of elderly (Hatakka et al., 2007) and in the gastrointestinal tract of preterm neonates and children (Manzoni et al., 2006; Romeo et al., 2011; Kumar et al., 2013). Proposed modes of action of lactobacilli against Candida include immunomodulation of the host epithelial barrier and reduction of Candida adhesion by co‐aggregation and through competition for binding sites (Lebeer et al., 2010; Rizzo et al., 2013; Parolin et al., 2015). These beneficial effects of lactobacilli are often considered species‐ and even strain‐specific and have been proposed to involve cell surface components, including peptidoglycan, teichoic acids, (glyco)proteins and polysaccharides (Kleerebezem et al., 2010; Lebeer et al., 2010), but without further substantiation. One of the best‐documented probiotic strains is Lactobacillus rhamnosus GG ATCC 53103 (L. rhamnosus GG or LGG). This strain, isolated from a healthy human intestinal microbiota, is one of the probiotic strains with the largest number of documented health benefits, mainly regarding the prevention and recovery of intestinal tract infections (Gorbach, 1996; Doron et al., 2005; Segers and Lebeer, 2014). Here, we first explored the inhibitory effect of L. rhamnosus GG on Candida growth and two important virulence factors, hyphae morphogenesis and adhesion to target epithelial cells. As in both C. albicans and C. glabrata, lectin‐like adhesins recognizing glycans containing galactose residues have been described (Zupancic et al., 2008; Donohue et al., 2011), we investigated the role of the galactose‐rich exopolysaccharides (EPS), the major macromolecules of the outer cell wall later of L. rhamnosus GG (Lebeer et al., 2009), in the anti‐Candida activity by comparing wild‐type L. rhamnosus GG with its isogenic EPS mutant CMPG5351 and purified EPS. We hypothesize that the presence of galactose‐rich EPS on the surface of LGG can play an important role in its antipathogenic properties against Candida species, as it is a strain‐specific, carbohydrate polymer forming a dominant layer on the surface of L. rhamnosus GG (Lebeer et al., 2009).
Results
Lactobacillus rhamnosus GG inhibits the growth of Candida albicans
As fungal overgrowth is the initial step in the pathogenesis of Candida, we first explored whether the presence of live L. rhamnosus GG could control the growth of C. albicans. Growth inhibition was investigated using well‐diffusion and spot assays. In well‐diffusion assays, we added overnight cultures of L. rhamnosus GG (107 or 108 CFU/ml) or cell‐free supernatant (CFS) of the overnight cultures to wells in agar inoculated with C. albicans. In comparison, in spot assays, we added an C. albicans inoculated overlay of soft agar on L. rhamnosus GG spots. Both L. rhamnosus GG added in the wells at 108 CFU/ml and the L. rhamnosus GG spots were able to inhibit C. albicans growth (Figure 1A). A ten times lower concentration (107 CFU) and supernatant were insufficient to inhibit C. albicans growth. To check how specific for C. albicans this inhibition by L. rhamnosus GG is, we performed the same assays with C. glabrata. This species seemed less sensitive to L. rhamnosus GG, as only the spots resulted in growth inhibition (Fig. S1).
Figure 1.
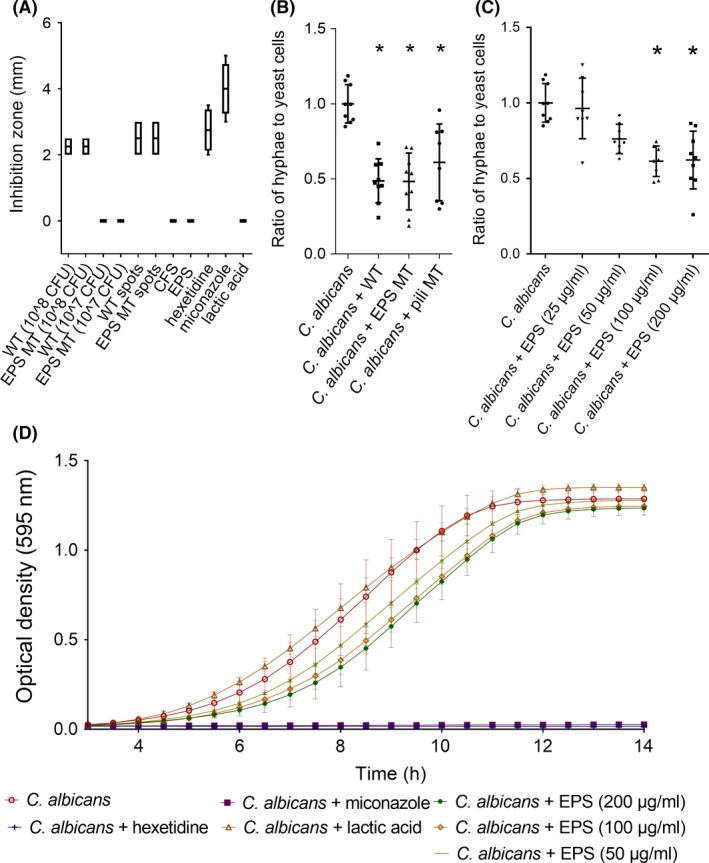
Inhibition of growth and hyphal induction of Candida albicans. (A) Well‐diffusion and spot assays of C. albicans in the presence of wild‐type L. rhamnosus GG (108 CFU ml−1), EPS mutant (EPS MT) (108 CFU ml−1), CFS, isolated EPS (2 mg ml−1) and a 2% L‐lactic acid solution (n=4). Hexetidine (0.1%), miconazole (8 μg ml−1) were used as positive controls and water as negative control. (B‐C) Hyphal induction of C. albicans (106 cells ml−1) during co‐incubation with LGG (108 CFU ml−1), its EPS mutant (EPS MT) (108 CFU ml−1) or isolated EPS. Asterisks indicate p‐values below 0.05 compared to C. albicans solely. (D) Evolution of the optical density of C. albicans and C. glabrata cultures in presence of EPS in different concentrations and a 2% L‐lactic acid solution (n=3). Hexetidine (0.1%), miconazole (8 μg ml−1) were used as positive controls and water as negative control.
To determine possible molecular mechanisms for this growth inhibition, we first tested the effect of lactic acid, as this is one of the major effector molecules of the antimicrobial activity of lactobacilli (De Keersmaecker et al., 2006; van den Broek et al. submitted). However, lactic acid did not inhibit the growth of C. albicans or C. glabrata at concentrations comparable to cell‐free culture supernatant of L. rhamnosus GG after 24‐h growth, namely 2% L‐lactic acid, as determined with the Roche Yellow line kit (Figure 1A). Subsequently, the influence of EPS from L. rhamnosus GG was investigated. The inhibitory capacity of EPS‐deficient strain CMPG5351 in the well‐diffusions and spot assays was comparable to activity of the wild‐type. However, when extracted, purified EPS was able to extend the lag phase in a concentration‐dependent manner. When added to growing Candida cultures in a time‐course experiment, we found that isolated EPS (200 μg/ml) prolonged the lag phase of C. albicans with approximately 68 minutes (Figure 1D). The EPS concentration range applied in these experiments corresponded with approximately 1010 − 4 × 1010 CFU/mL Lactobacillus cells, which is ca. 100‐fold more than the Lactobacillus cultures that interfered with C. albicans growth in the well‐diffusion assays. This indicates that other components of L. rhamnosus GG are also involved in the direct antimicrobial activity against C. albicans activity.
Lactobacillus rhamnosus GG and its isolated EPS molecules inhibit hyphal formation of Candida albicans
A crucial virulence factor in the pathogenesis of C. albicans is hyphal formation. We examined the effect of L. rhamnosus GG on the formation of hyphae by co‐incubating the C. albicans yeast cells (106 CFU/ml) with lactobacilli (108 CFU/ml) during hyphal induction with fetal bovine serum (FBS). The presence of L. rhamnosus GG was able to reduce the hyphae to yeast cells ratio approximately with 50% (Figure 1B). Furthermore, we could not observe differences in the capacity to reduce C. albicans hyphal formation between L. rhamnosus GG and its isogenic EPS mutant CMPG5351 (Figure 1B), but remarkably, the isolated EPS was able to reduce the hyphal induction in a concentration‐dependent manner, with a 40% reduction at 100 μg/ml, showing an important role for the EPS molecules in repressing hyphal formation (Figure 1C).
As we observed these effects only for the purified EPS and not for the welE mutant of L. rhamnosus GG in which specifically galactose‐rich EPS expression was disrupted, we also investigated whether other glycosylated surface components of L. rhamnosus GG (Lebeer et al., 2012; Tytgat et al., 2016) play a role in the antihyphal activity. Hereto, we included the mutant lacking the spaCBA‐encoded pili in our experiments (Figure 1B). This mutant was still capable of significantly decreasing hyphal formation of C. albicans, to the same extent as L. rhamnosus GG wild‐type and the EPS‐deficient welE mutant. Elucidating the role of a certain surface component by investigating mutants herein is often complicated by the fact that not only the expression of the target gene is affected, but other surface components can show an altered expression and exposure, as is the case for the welE mutant (Lebeer et al., 2009, 2012). Therefore, we repeated the antihyphal experiments with EPS isolated from two other Lactobacillus strains by the same procedures. These Lactobacillus strains, L. plantarum CMPG5300 and L. rhamnosus GR‐1, have EPS molecules with a different ratio of galactose and glucose in their monomer composition (see Table S1). EPS molecules from these strains were able to inhibit hyphal formation of C. albicans to the same extent as EPS from L. rhamnosus GG, namely between 45% and 52% at 200 μg/ml (Fig. S2).
Lactobacillus rhamnosus GG and its EPS inhibit adherence of Candida to epithelial cells
The following key step in the pathogenesis of Candida is its adhesion to epithelial cells. We studied the adhesion of C. albicans and C. glabrata to both stratified squamous epithelial cells (represented by vaginal cell line VK2/E6E7) and mucous epithelial cells (represented by bronchial cell line Calu‐3). C. albicans and C. glabrata adhered to both VK2/E6E7 and Calu‐3, although in different amounts. Adherence of both species to VK2/E6E7 cells was approximately 30%, while C. glabrata adhered much better to the Calu‐3 cells than C. albicans (25% compared with 5%) (Figure 2A and 2B).
Figure 2.
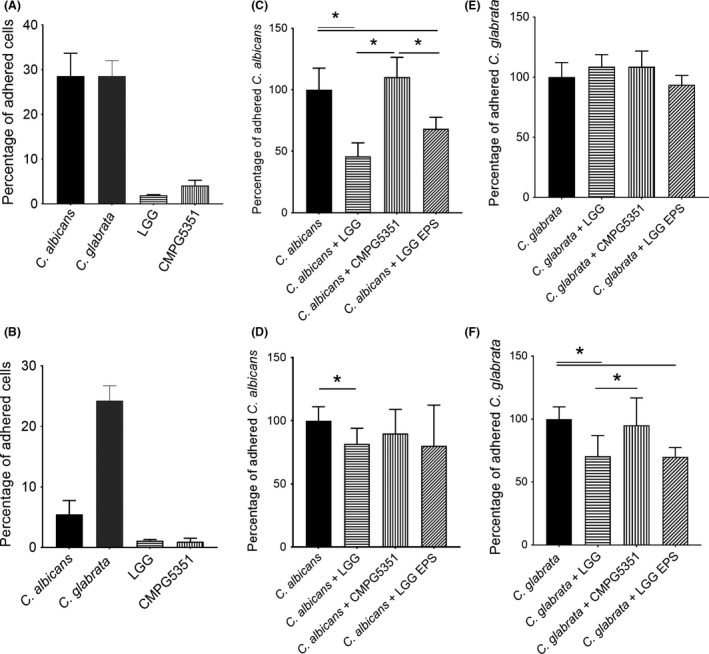
Inhibition of Candida adherence to epithelial cells. (A) Percentage of Candida albicans, C. glabrata, Lactobacillus rhamnosus GG WT and EPS mutant (EPS MT) adhered to VK2/E6E7 monolayers after 1 h of incubation. (B) Percentage of C. albicans, L. rhamnosus GG and EPS mutant adhered to Calu‐3 monolayers after 1 h of incubation. (C, E) Percentage of C. albicans or C. glabrata adhered to VK2/E6E7 monolayers when added in competition with L. rhamnosus GG WT, EPS mutant or L. rhamnosus GG EPS. (D, F) Percentage of C. albicans or C. glabrata adhered to Calu‐3 monolayers when added in competition with L. rhamnosus GG WT, EPS mutant or L. rhamnosus GG EPS. The results were normalized to adherence of C. albicans solely. In all tests, C. albicans and C. glabrata were used at an initial concentration of 106 CFU, L. rhamnosus GG WT and EPS mutant at 108 CFU, and purified EPS was added to a final concentration of 200 μg ml−1. The percentages represent mean values of at least three biological repeats. Asterisks indicate p‐values below 0.05 when conditions were compared to each other.
To determine the main adhesion inhibition mechanism of L. rhamnosus GG, we performed competition, displacement and exclusion assays (Figure 2 and Fig. S3). For the stratified epithelial cells (VK2/E6E7), competition appears to be the main mechanism for C. albicans adhesion inhibition by L. rhamnosus GG. Upon co‐addition of pathogen and probiotic, we observed a 55% reduction of the adhesion of C. albicans. This correlates with a reduction of multiplicity of infection of approximately 2:1 to 1:1 (ration yeast cells: epithelial cells). In contrast, L. rhamnosus GG was not able to significantly reduce C. glabrata adhesion to these cells. For the mucous epithelial cells (Calu‐3), L. rhamnosus GG was able to inhibit C. albicans and C. glabrata adhesion in both competition and displacement assays.
Next, we investigated whether the EPS layer is of importance for L. rhamnosus GG to inhibit Candida adhesion. Hereto, we compared the effect of L. rhamnosus GG wild‐type on the adhesion of C. albicans and C. glabrata with its EPS‐deficient mutant strain. For the displacement and exclusion assays with C. albicans and C. glabrata, no major differences were observed between L. rhamnosus GG and the EPS mutant (Fig. S2). However, the EPS mutant CMPG5351 differed remarkably from L. rhamnosus GG wild‐type in competition assays, as it was not able to inhibit C. albicans nor C. glabrata adhesion (Figure 2). We repeated these assays with isolated EPS from L. rhamnosus GG instead of the live lactobacilli. Isolated L. rhamnosus GG EPS molecules (at 200 μg ml−1) were able to reduce the adhesion of C. albicans to VK2/E6E7, and to Calu‐3, and of C. glabrata to Calu‐3 with approximately 30%, 27% and 25% respectively (Figure 2).
Discussion
In the present study, we showed that L. rhamnosus GG can be used to interfere with growth of Candida in in vitro experiments. In addition, some important pathogenesis characteristics including adhesion, tissue invasion and mucosal infection were also affected. Our comparison of the activity of wild‐type L. rhamnosus GG to its EPS‐deficient mutant CMPG5351 and its purified EPS molecules indicates that its outer EPS layer is – at least partially – involved in its anti‐Candida adhesion activity.
L. rhamnosus GG was able to interfere with the growth of C. albicans, which corresponds with the study of Hasslöf and colleagues, in which they also found inhibitory effects of L. rhamnosus GG (from 107 CFU ml−1) on C. albicans growth in one other growth interference test (Hasslöf et al., 2010). The inhibition of C. albicans by L. rhamnosus GG observed in this study was comparable with inhibition by other lactobacilli, but – in contrast to what we expected – the key metabolite lactic acid could not inhibit C. albicans growth when added in concentrations comparable to those in L. rhamnosus GG supernatant. This is in contrast to a previous study concluding that lactic acid at low pH reduces the metabolic activity of C. albicans and eventually kills the cells (Köhler et al., 2012). Our other control, miconazole, was more efficient at inhibiting C. albicans and C. glabrata growth than L. rhamnosus GG live cells, but use of this antifungal agent is linked to the development of resistance (Sanglard and Odds, 2002), signifying the need for alternative antifungal strategies. Previously, EPS molecules from L. plantarum R315 and kefiran, a branched glucogalactan surrounding kefir, were shown to exhibit anti‐C. albicans activity in agar‐diffusion assays at 300 μg ml−1 and 450 μg ml−1 respectively (Rodrigues et al., 2005; Li et al., 2014). Our data do not indicate an important role for EPS in the anti‐Candida growth activity of L. rhamnosus GG, as adding isolated EPS only resulted in prolonging the lag phase. Results of agglutination assays, as described by (Malik et al., 2016), indicate that this effect is not caused by Candida aggregation by EPS (data not shown). Additional mechanisms in the anti‐Candida growth activity of L. rhamnosus GG thus seem highly probable. For instance, H2O2 production has been suggested to have antagonistic properties against C. albicans (Strus et al., 2005), but this molecule is not highly produced by Lactobacillus rhamnosus strains (Felten et al., 1999).
More striking than the growth reduction by L. rhamnosus GG was its ability to inhibit hyphal formation in C. albicans, which is probably the most important virulence step of C. albicans as this cell type enables epithelial tissue penetration (Gow et al., 2011; Höfs et al., 2016). We found that the isolated EPS molecules from L. rhamnosus GG on themselves can act as effective inhibitors of hyphal formation, as they reduce the number of hyphae with almost 50%. This effect was also found for the EPS from other Lactobacillus strains, having different relative abundances of galactose in their monomer composition, indicating that the activity of EPS from L. rhamnosus GG is probably due to its long, complex, galactose‐containing polymer structure, but not solely to its exact strain‐specific sugar composition and configuration.
Intriguingly, we could not observe differences between L. rhamnosus GG wild‐type and the galactose‐rich EPS‐deficient mutant CMPG5351, which suggests that other cellular factors could also play a significant role. This could be the glucose‐rich cell wall polysaccharides that are overexposed in the welE mutant (Francius et al., 2008). Another glycosylated surface factor we investigated were the spaCBA‐encoded pili, important for certain molecular interactions of L. rhamnosus GG (Lebeer et al., 2012; Tytgat et al., 2016). The pilus‐deficient mutant also inhibited hyphal formation to same extent as L. rhamnosus GG wild‐type and EPS‐deficient mutant, indicating that the pili are not key for this activity. Previously, live lactobacilli, their supernatant and butyric acids have been suggested to interfere with the hyphal induction in C. albicans (Noverr and Huffnagle, 2004), but to our knowledge no Lactobacillus exopolysaccharides with antihyphal activity have yet been described. The exact mechanism by which EPS reduces hyphal formation forms an interesting topic for further investigation, as this EPS is a potent candidate drug to reduce hyphae and thus virulence of C. albicans.
The next key step in the establishment of Candida infection is adhesion to the epithelial cells of mucosal surfaces (Höfs et al., 2016). It has been suggested that lactobacilli can interfere with adhesion of Candida through competition for binding sites or by co‐aggregating with the Candida species, involving peptides on the bacterial surface and carbohydrates on the yeast surface as co‐aggregating factors (Ocaña and Nader‐Macías, 2002; Parolin et al., 2015; Malik et al., 2016). In our results, we observed clear differences in adhesion levels between the two epithelial cell lines and the two tested Candida species. This is probably due to different receptor expression on the different epithelial cell lines. Despite the fact that only the major adhesins of C. glabrata have galactose‐binding properties, we found that L. rhamnosus GG was more efficient in inhibiting adhesion of C. albicans than C. glabrata, mainly by competition and displacement. Exclusion seems the least important anti‐adhesion mechanism of L. rhamnosus GG against Candida, which can be explained by the rather low adhesion of L. rhamnosus GG itself to these epithelial types.
When we used the isogenic EPS mutant CMPG5351 to evaluate the in situ effect of the EPS on anti‐Candida adhesion capacity of L. rhamnosus GG, we found the intriguing result that this mutant inhibited adhesion far less than L. rhamnosus GG in competition assays. Next, we observed that the purified EPS itself was also able to inhibit adhesion in competition assays. To our knowledge, an anti‐Candida adhesion capacity for Lactobacillus EPS molecules has only been described once before. This study showed that EPS from L. crispatus L1 was able to reduce C. albicans adhesion to VK2/E6E7 cells, but mainly by exclusion and at ten times higher concentrations (1 mg ml−1) (Donnarumma et al., 2014) than used in our study. This indicates that the surface polysaccharides of L. rhamnosus GG might serve as one of the key molecules for interfering with the binding between the fungal lectin‐like adhesins and host sugars or between the fungal cell wall carbohydrates and their epithelial adhesion receptor, at least in case of direct competition for adhesion.
In conclusion, our data indicate that L. rhamnosus GG has potential as adjuvant in antifungal strategies, especially against the major pathogen C. albicans, as this probiotic strain inhibits growth, hyphal formation and adhesion. Our reductionist mechanistic approach should, however, be substantiated by future studies considering the role of this probiotic against Candida in a microbiota setting, preferentially in clinical trials. In these follow‐up trials, the clinical relevance of the observed decreased hyphal formation and yeast adherence could then be determined. In our pursuit to unravel the molecular mechanisms involved, we found that the EPS layer of L. rhamnosus GG might be part of a combined mode of action for the reduction of hyphal formation and for the direct competition with Candida during adhesion. Identification of such key effector molecules could substantiate the selection of novel Lactobacillus strains with even more effective anti‐Candida activity and help explain observed biological effects in vivo in clinical trials such as Hatakka et al. (2007); Manzoni (2007); Martinez et al. (2009); Romeo et al. (2011), as well as promote the application of isolated biomolecules.
Experimental procedures
Microbial strains and culture conditions
Lactobacillus strains (Table 1) were grown at 37 °C without agitation in de Man, Rogosa and Sharpe (MRS) broth (Difco, Erembodegem, Belgium). Candida strains (Table 1) were grown while shaking at 37 °C in yeast extract peptone dextrose (YPD) broth (Carl Roth, Karlsruhe, Germany). Hyphal growth of C. albicans was induced by supplementing YPD broth with 10% heat inactivated fetal bovine serum (FBS) (Thermo Fischer, Asse, Belgium).
Table 1.
Bacterial and yeast strains used in this study
| Strain | Reference | Description |
|---|---|---|
| Lactobacillus rhamnosus GG ATCC 53103 | ATCC | |
| L. rhamnosus CMPG5351 | (Lebeer et al., 2009) | Priming enzyme of the production of long galactose‐rich EPS molecules is inactivated |
| L. rhamnosus CMPG5357 | (Lebeer et al., 2012) | spaCBA‐encoded pili are inactivated |
| L. rhamnosus GR‐1 ATCC 55826 | ATCC | |
| L. plantarum CMPG5300 | (Malik et al., 2013) | |
| Candida albicans SC5314 | (Fonzi and Irwin, 1993) | |
| C. glabrata ATCC 2001 | ATCC |
Cell culture
The vaginal epithelial cell line VK2/E6E7 ATCC® CRL‐26216™ (purchased from ATCC, Molsheim, France) was maintained at 37 °C with 5% CO2 and 90% relative humidity in 75 cm2 tissue culture flasks containing serum‐free keratinocyte medium (Life Technologies, Ghent, Belgium) supplemented with CaCl2, human epidermal growth factor and bovine pituitary extract. Every 3 days, when the VK2/E6E7 monolayers reached 70%–80% confluency, cells were reseeded with a 1:7 split ratio in fresh culture medium using a 0.25% trypsin‐EDTA solution (Life Technologies). For adhesion and immunomodulation experiments, VK2/E6E7 cells were seeded in 12‐well culture plates (Cellstar, Diegem, Belgium) at a density of 1.6 × 105 cells ml−1. Within 3–4 days after seeding, confluent monolayers were obtained. The human bronchial epithelial cell line Calu‐3 ATCC® HTB‐55™ (purchased from ATCC) was cultured in 75‐cm² flasks containing 20 ml Minimum Essential Medium (Life Technologies) supplemented with heat inactivated FBS and penicillin‐streptomycin (100 U ml−1) (Life Technologies) and maintained in a humidified 5% CO2 incubator at 37 °C. The culture medium was changed every 3–4 days, and the cells were passaged weekly at a 1:2 split ratio using a trypsin‐EDTA solution. For adhesion and immunomodulation experiments, Calu‐3 cells were seeded in 12‐well culture plates at a density of 1.85 × 106 cells ml−1. Approximately a week after seeding, confluent monolayers were obtained.
Isolation and characterization of EPS
The EPS of the Lactobacillus strains was isolated with the extraction protocol described previously (Lebeer et al., 2007). Briefly, the lactobacilli were grown to an optical density of 0.6 and washed with phosphate‐buffered saline. EPS was then extracted by incubation in 0.05 M EDTA (Sigma‐Aldrich, Diegem, Belgium) (shaking, on ice), followed by ethanol precipitation and dialysis against distilled water [Spectra/Por® dialysis membrane (Spectrum Laboratories, Breda, the Netherlands)]. Afterwards, samples were treated with trichloroacetic acid (Sigma‐Aldrich) to remove proteins, dialysed against water and filter sterilized [pore size 0.2 μm (VWR, Haasrode, Belgium)]. The total amount of carbohydrate was estimated by the phenol‐sulfuric acid method (DuBois et al., 1956). Samples were freeze‐dried in a FreeZone 1 Liter Benchtop Freeze Dry System (Model 7740030) (Labconco, MO, USA) and stored at 4°C until use. Before use, the EPS was dissolved in pure water. The purity of the EPS samples after extraction was checked with 1H NMR. NMR Spectra were recorded in D2O (Sigma‐Aldrich) on a Bruker DRX‐400 instrument, operating at 400 MHz for 1H using standard Bruker software. These spectra (shown in Fig. S4) were compared with the spectra described in (Landersjö et al., 2002) and indicated in the beginning the presence of some unexpected components, originating from the filters and membranes used during extraction. The isolation protocol was subsequently adapted by rinsing all filters and membranes before use, which resulted in elimination of these compounds. The sugar monomer composition of purified EPS from L. rhamnosus GR‐1 and L. plantarum CMPG5300 was determined by gas chromatography after hydrolysis and derivatization to alditol acetates (Englyst and Cummings, 1984). Β‐D‐Allose was used as an internal standard, and calibration samples containing the expected monosaccharides were included with each set of samples (Lebeer et al., 2009).
Preparation and determination of lactic acid concentration in supernatant
After overnight incubation, cell‐free culture supernatant was obtained by centrifugation (15 min, 4000 rpm) and filter sterilization (pore size 0.2 μm). The concentration of lactic acid was measured with the commercially available Roche Yellow line kit (Roche, Basel, Swiss).
Antimicrobial spot‐ and well diffusion‐based agar assays of lactobacilli against Candida
Dedicated agar interaction assays for lactobacilli against Candida were optimized based on (Schillinger and Lücke, 1989) and (Coconnier et al., 1997). Briefly, 2 μl of overnight Lactobacillus cultures (approximately 3 × 109 CFU ml−1) were spotted on MRS agar (40 ml) and incubated for 48 h. After incubation, YPD soft agar (20 ml) was inoculated with 200 μl of an overnight culture of C. albicans or C. glabrata, was poured over the agar. After incubation for 24 h, the resulting inhibition zones around the spots were measured. Alternatively, 60 mL YPD agar was inoculated with 2% of an overnight culture of C. albicans or C. glabrata. After the agar dried, holes (or wells) with a 0.4 cm diameter were made in the agar. Then, 30 μl of L. rhamnosus GG cells (107–108 CFU ml−1), a 2(m V−1)% L‐lactic acid (Carl Roth) solution or purified exopolysaccharides (2 mg ml−1), was added to the holes. The plates were incubated for 24 h, and the resulting inhibition zones around the holes were measured. Hexetidine (0.1%) (Johnson & Johnson, Beerse, Belgium), miconazole (8 μg ml−1) (Sigma‐Aldrich) and sterile water were used as positive and negative controls respectively.
Time‐course analysis of the antimicrobial activity of Lactobacillus EPS for Candida growth in suspension
A time‐course analysis was performed as described previously (De Keersmaecker et al., 2006) with minor modifications. Briefly, an overnight culture of C. albicans or C. glabrata (± 5 × 107 CFU ml−1) was added to the wells of a microtiterplate in a 100‐fold dilution, supplemented with Lactobacillus EPS (50 μg ml−1, 100 μg ml−1 and 200 μg ml−1). Hexetidine (0.1%), miconazole (8 μg ml−1), a 2(m V−1)% L‐lactic acid solution and demineralized H2O (10%) were used as two positive and the negative control respectively. Candida cultures were allowed to grow for 24 h, and the optical density was measured each 30 min at 595 nm using a Synergy HTX multi‐mode reader (Biotek, Drogenbos, Belgium). Each condition was measured at least in triplicate, and the average OD was calculated.
Inhibition of hyphal formation by Candida albicans
C. albicans hyphae (106 CFU ml−1) were induced by FBS, while incubated with or without lactobacilli (108 CFU ml−1) or Lactobacillus EPS (50 μg ml−1, 100 μg ml−1 and 200 μg ml−1). After incubation, at least one hundred yeast cells and/or hyphae in at least three biological repeats were counted, and the ratio of hyphae to yeast cells was calculated.
Inhibition of Candida albicans and C. glabrata adherence to epithelial cells by Lactobacillus species
The influence of L. rhamnosus GG and CMPG5351 on the adherence of Candida species to vaginal epithelial VK2/E6E7 cells and bronchial epithelial Calu‐3 cells was investigated as described previously with minor modifications (Malik et al., 2013; Rizzo et al., 2013). Three different procedures were used to differentiate between competition between Candida and L. rhamnosus GG or CMPG5351, exclusion and displacement of Candida by L. rhamnosus GG or CMPG5351. Competition tests were carried out by adding a volume of 1 ml containing Candida cells (106 CFU) and lactobacilli (108 CFU) to tissue culture plate wells containing confluent monolayers of epithelial cells, which were allowed to incubate at 37 °C for 1 h to mediate adherence. For exclusion tests, the monolayers were first incubated with lactobacilli (108 CFU) for 1 h. After incubation, non‐adhered lactobacilli were removed by washing with Dulbecco's PBS (Life Technologies), C. albicans cells (106 CFU) were added and incubated for 1 h. For displacement tests, the monolayers were first incubated with Candida cells (106 CFU). After incubation of 1 h, the non‐adhered cells were removed by washing with PBS. Afterwards, the lactobacilli were added (108 CFU) to the epithelial cells, and the plates were incubated for another hour. After final incubation, the cells were washed three times with Dulbecco's PBS to remove all non‐adhered cells, and the number of adhered Candida and Lactobacillus cells to the VK2/E6E7 and Calu‐3 cells was determined by the macrodilution method on Sabouraud agar (Carl Roth), which is selective for fungal species, and MRS agar with cycloheximide (10 mg L−1) (Sigma‐Aldrich), which is selective for the lactobacilli. Each condition was carried out at least in triplicate.
Statistics
Data are presented as mean values ± standard deviation. Shapiro–Wilk normality test (GraphPad Prism 7.00, CA, USA) was used to determine whether the data are normally distributed. Each condition was compared to their negative control with the unpaired Student's t‐test (GraphPad Prism 7.00). P‐values below 0.05 were considered to be significant, and significant differences are indicated with asterisks.
Conflict of Interest
None declared.
Supporting information
Fig. S1. Growth inhibition of C. glabrata.
Fig. S2. Inhibition of C. albicans hyphal formation by Lactobacillus EPS.
Fig. S3. Inhibition of Candida adherence to epithelial cells by displacement and exclusion.
Fig. S4. 400‐MHz 1H NMR spectra of EPS from L. rhamnosus GG before (A) and after (B) protocol optimization, recorded in D2O.
Table S1. Monomer composition of Lactobacillus EPS.
Acknowledgements
We would like to thank the members of the Lebeer Lab of Applied Microbiology and Biotechnology (UAntwerp), especially Shweta Malik for EPS isolation of L. rhamnosus GR‐1 and L. plantarum CMPG5300, for their technical help and contribution to the manuscript.
Microbial Biotechnology (2017) 10(6), 1753–1763
Funding Information
Fonds Wetenschappelijk Onderzoek, (Grant/Award Number: ‘ProjectID 32913′). Flanders Innovation and Entrepreneurship Agency, (Grant / Award Number: ‘IWT‐SBO ProCure project (IWT/50052)’)
References
- Brunke, S. , and Hube, B. (2013) Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol 15: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone, R. A. , and Fonzi, W. A. (2001) Virulence factors of Candida albicans. Trends Microbiol 9: 327–335. [DOI] [PubMed] [Google Scholar]
- Coconnier, M.‐H. , Liévin, V. , Bernet‐Camard, M.‐F. , Hudault, S. , and Servin, A. L. (1997) Antibacterial Effect of the Adhering Human Lactobacillus acidophilus Strain LB. Antimicrob Agents Chemother 41: 1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle, F. , Jouault, T. , Trinel, P. A. , Esnault, J. , Mallet, J. M. , D'Athis, P. , et al (2003) 1,2‐ and ‐1,2‐Linked Oligomannosides Mediate Adherence of Candida albicans Blastospores to Human Enterocytes In Vitro. Infect Immun 71: 7061–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keersmaecker, S. C. J. , Verhoeven, T. L. , Desair, J. , Marchal, K. , Vanderleyden, J. , and Nagy, I. (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259: 89–96. [DOI] [PubMed] [Google Scholar]
- Donnarumma, G. , Molinaro, A. , Cimini, D. , De Castro, C. , Valli, V. , De Gregorio, V. , et al (2014) Lactobacillus crispatus L1: high cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol 14: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue, D. S. , Ielasi, F. S. , Goossens, K. V. Y. , and Willaert, R. G. (2011) The N‐terminal part of Als1 protein from Candida albicans specifically binds fucose‐containing glycans. Mol Microbiol 80: 1667–1679. [DOI] [PubMed] [Google Scholar]
- Doron, S. , Snydman, D. R. , and Gorbach, S. L. (2005) Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am 34: 483–498, ix. [DOI] [PubMed] [Google Scholar]
- DuBois, M. , Gilles, K. A. , Hamilton, J. K. , Rebers, P. A. , and Smith, F. (1956) Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem 28: 350–356. [Google Scholar]
- Ehrström, S. , Daroczy, K. , Rylander, E. , Samuelsson, C. , Johannesson, U. , Anzén, B. , and Påhlson, C. (2010) Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect 12: 691–699. [DOI] [PubMed] [Google Scholar]
- Englyst, H. N. , and Cummings, J. H. (1984) Simplified method for the measurement of total non‐starch polysaccharides by gas‐liquid chromatography of constituent sugars as alditol acetates. Analyst 109: 937. [DOI] [PubMed] [Google Scholar]
- Felten, A. , Barreau, C. , Bizet, C. , Lagrange, P. H. , and Philippon, A. (1999) Lactobacillus Species Identification, H2O2 Production, and Antibiotic Resistance and Correlation with Human Clinical Status. J Clin Microbiol 37: 72–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman, B. , Muraglia, R. , Dietz, J.‐P. , Sobel, J. D. , and Wagner, J. (2013) Prevalence of Recurrent Vulvovaginal Candidiasis in 5 European Countries and the United States. J Low Genit Tract Dis 17: 340–345. [DOI] [PubMed] [Google Scholar]
- Francius, G. , Lebeer, S. , Alsteens, D. , Wildling, L. , Gruber, H. J. , Hols, P. , et al (2008) Detection, Localization, and Conformational Analysis of Single Polysaccharide Molecules on Live Bacteria. ACS Nano 2: 1921–1929. [DOI] [PubMed] [Google Scholar]
- Gorbach, S. L. (1996) The Discovery of Lactobacillus GG. Nutr Today 31: 5S. [Google Scholar]
- Gow, N. A. R. , van de Veerdonk, F. L. , Brown, A. J. P. , and Netea, M. G. (2011) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, P. W. J. , Kraneveld, E. A. , Yin, Q. Y. , Dekker, H. L. , Gross, U. , Crielaard, W. , et al (2008) The Cell Wall of the Human Pathogen Candida glabrata: Differential Incorporation of Novel Adhesin‐Like Wall Proteins. Eukaryot Cell 7: 1951–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasslöf, P. , Hedberg, M. , Twetman, S. , and Stecksén‐Blicks, C. (2010) Growth inhibition of oral mutans streptococci and candida by commercial probiotic lactobacilli ‐ an in vitro study. BMC Oral Health 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakka, K. , Ahola, A. J. , Yli‐Knuuttila, H. , Richardson, M. , Poussa, T. , Meurman, J. H. , and Korpela, R. (2007) Probiotics reduce the prevalence of oral candida in the elderly–a randomized controlled trial. J Dent Res 86: 125–130. [DOI] [PubMed] [Google Scholar]
- Höfs, S. , Mogavero, S. , and Hube, B. (2016) Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol 54: 149–169. [DOI] [PubMed] [Google Scholar]
- Ibàñez‐Nolla, J. , Nolla‐Salas, M. , León, M. , García, F. , Marrugat, J. , Soria, G. , et al (2004) Early diagnosis of candidiasis in non‐neutropenic critically ill patients. J Infect 48, 181–192. [DOI] [PubMed] [Google Scholar]
- Kleerebezem, M. , Hols, P. , Bernard, E. , Rolain, T. , Zhou, M. , Siezen, R. J. , and Bron, P. A. (2010) The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34: 199–230. [DOI] [PubMed] [Google Scholar]
- Klotz, S. A. , Gaur, N. K. , Lake, D. F. , Chan, V. , Rauceo, J. , and Lipke, P. N. (2004) Degenerate Peptide Recognition by Candida albicans Adhesins Als5p and Als1p. Infect Immun 72: 2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, G. A. , Assefa, S. , and Reid, G. (2012) Probiotic interference of lactobacillus rhamnosus GR‐1 and lactobacillus reuteri RC‐14 with the opportunistic fungal pathogen candida albicans. Infect Dis Obstet Gynecol 2012: 636474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovachev, S. M. , and Vatcheva‐Dobrevska, R. S. (2015) Local Probiotic Therapy for Vaginal Candida albicans Infections. Probiotics Antimicrob Proteins 7: 38–44. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Bansal, A. , Chakrabarti, A. , and Singhi, S. (2013) Evaluation of Efficacy of Probiotics in Prevention of Candida Colonization in a PICU—A Randomized Controlled Trial*. Crit Care Med 41: 565–572. [DOI] [PubMed] [Google Scholar]
- Landersjö, C. , Zhennai, Y. , Eine, H. , and Göran, W. (2002) Structural studies of the exopolysaccharide produced by lactobacillus rhamnosus strain GG (ATCC 53103). Biomacromolecules 3: 880–884. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Verhoeven, T. L. A. , Perea Vélez, M. , Vanderleyden, J. , and De Keersmaecker, S. C. J. (2007) Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73: 6768–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Verhoeven, T. L. A. , Francius, G. , Schoofs, G. , Lambrichts, I. , Dufrêne, Y. , et al (2009) Identification of a Gene Cluster for the Biosynthesis of a Long, Galactose‐Rich Exopolysaccharide in Lactobacillus rhamnosus GG and Functional Analysis of the Priming Glycosyltransferase. Appl Environ Microbiol 75: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Vanderleyden, J. , and De Keersmaecker, S. C. J. (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171–184. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Claes, I. , Tytgat, H. L. P. , Verhoeven, T. L. , Marien, E. , von Ossowski, I. , et al (2012) Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Huang, R. , Shah, N. P. , Tao, X. , Xiong, Y. , and Wei, H. (2014) Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J Dairy Sci 97: 7334–7343. [DOI] [PubMed] [Google Scholar]
- Liu, M.‐B. , Xu, S.‐R. , He, Y. , Deng, G.‐H. , Sheng, H.‐F. , Huang, X.‐M. , et al (2013) Diverse Vaginal Microbiomes in Reproductive‐Age Women with Vulvovaginal Candidiasis. PLoS ONE 8: e79812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, S. , Petrova, M. I. , Claes, I. J. J. , Verhoeven, T. L. , Busschaert, P. , Vaneechoutte, M. , et al (2013) The highly autoaggregative and adhesive phenotype of the vaginal Lactobacillus plantarum strain CMPG5300 is sortase dependent. Appl Environ Microbiol 79: 4576–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, S. , Petrova, M. I. , Imholz, N. C. E. , Verhoeven, T. L. A. , Noppen, S. , Van Damme, E. J. M. , et al (2016) High mannose‐specific lectin Msl mediates key interactions of the vaginal Lactobacillus plantarum isolate CMPG5300. Sci Rep 6: 37339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni, P. (2007) Use of Lactobacillus casei subspecies Rhamnosus GG and gastrointestinal colonization by Candida species in preterm neonates. J Pediatr Gastroenterol Nutr 45(Suppl 3): S190–S194. [DOI] [PubMed] [Google Scholar]
- Manzoni, P. , Mostert, M. , Leonessa, M. L. , Priolo, C. , Farina, D. , Monetti, C. , et al (2006) Oral Supplementation with Lactobacillus casei Subspecies rhamnosus Prevents Enteric Colonization by Candida Species in Preterm Neonates: A Randomized Study. Clin Infect Dis 42: 1735–1742. [DOI] [PubMed] [Google Scholar]
- Martinez, R. C. R. , Franceschini, S. A. , Patta, M. C. , Quintana, S. M. , Candido, R. C. , Ferreira, J. C. , et al (2009) Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14. Lett Appl Microbiol 48: 269–274. [DOI] [PubMed] [Google Scholar]
- Mason, K. L. , Erb Downward, J. R. , Falkowski, N. R. , Young, V. B. , Kao, J. Y. , and Huffnagle, G. B. (2012) Interplay between the Gastric Bacterial Microbiota and Candida albicans during Postantibiotic Recolonization and Gastritis. Infect Immun 80: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr, M. C. , and Huffnagle, G. B. (2004) Regulation of Candida albicans Morphogenesis by Fatty Acid Metabolites. Infect Immun 72: 6206–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña, V. S. , and Nader‐Macías, M. E. (2002) Vaginal lactobacilli: self‐ and co‐aggregating ability. Br J Biomed Sci 59: 183–190. [DOI] [PubMed] [Google Scholar]
- Parolin, C. , Marangoni, A. , Laghi, L. , Foschi, C. , Ñahui Palomino, R. A. , Calonghi, N. , et al (2015) Isolation of Vaginal Lactobacilli and Characterization of Anti‐Candida Activity. PLoS ONE 10: e0131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta, M. (2004) Effect of lactobacillus in preventing post‐antibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 329: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, A. , Losacco, A. , and Carratelli, C. R. (2013) Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll‐like receptors 2 and 4, interleukin 8 and human β‐defensins 2 and 3. Immunol Lett 156: 102–109. [DOI] [PubMed] [Google Scholar]
- Rodrigues, K. L. , Gaudino Caputo, L. R. , Tavares Carvalho, J. C. , Evangelista, J. , and Schneedorf, J. M. (2005) Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents 25: 404–408. [DOI] [PubMed] [Google Scholar]
- Romeo, M. G. , Romeo, D. M. , Trovato, L. , Oliveri, S. , Palermo, F. , Cota, F. , and Betta, P. (2011) Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late‐onset sepsis and neurological outcome. J Perinatol 31: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard, D. , and Odds, F. C. (2002) Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis 2: 73–85. [DOI] [PubMed] [Google Scholar]
- Sardi, J. C. O. , Scorzoni, L. , Bernardi, T. , Fusco‐Almeida, A. M. , and Mendes Giannini, M. J. S. (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62: 10–24. [DOI] [PubMed] [Google Scholar]
- Schillinger, U. , and Lücke, F.‐K. (1989) Antibacterial Activity of Lactobacillus sake Isolated from Meat. Appl Environ Microbiol 55: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers, M. E. , and Lebeer, S. (2014) Towards a better understanding of Lactobacillus rhamnosus GG ‐ host interactions. Microb Cell Fact 13: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, S. , Negri, M. , Henriques, M. , Oliveira, R. , Williams, D. W. , and Azeredo, J. (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36: 288–305. [DOI] [PubMed] [Google Scholar]
- Strus, M. , Kucharska, A. , Kukla, G. , Brzychczy‐Włoch, M. , Maresz, K. , and Heczko, P. B. (2005) The in vitro Activity of Vaginal Lactobacillus With Probiotic Properties Against Candida. Infect Dis Obstet Gynecol 13: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat, H. L. P. , van Teijlingen, N. H. , Sullan, R. M. A. , Douillard, F. P. , Rasinkangas, P. , Messing, M. , et al (2016) Probiotic Gut Microbiota Isolate Interacts with Dendritic Cells via Glycosylated Heterotrimeric Pili. PLoS ONE 11: e0151824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Westman, R. , Hickey, R. , Hansmann, M. A. , Kennedy, C. , Osborn, T. W. , and Forney, L. J. (2009) Vaginal Microbiota of Women with Frequent Vulvovaginal Candidiasis. Infect Immun 77: 4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic, M. L. , Frieman, M. , Smith, D. , Alvarez, R. A. , Cummings, R. D. , and Cormack, B. P. (2008) Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol Microbiol 68: 547–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Growth inhibition of C. glabrata.
Fig. S2. Inhibition of C. albicans hyphal formation by Lactobacillus EPS.
Fig. S3. Inhibition of Candida adherence to epithelial cells by displacement and exclusion.
Fig. S4. 400‐MHz 1H NMR spectra of EPS from L. rhamnosus GG before (A) and after (B) protocol optimization, recorded in D2O.
Table S1. Monomer composition of Lactobacillus EPS.


