Summary
Due to the rising global environment protection awareness, recycling strategies that comply with the circular economy principles are needed. Polyesters are among the most used materials in the textile industry; therefore, achieving a complete poly(ethylene terephthalate) (PET) hydrolysis in an environmentally friendly way is a current challenge. In this work, a chemo‐enzymatic treatment was developed to recover the PET building blocks, namely terephthalic acid (TA) and ethylene glycol. To monitor the monomer and oligomer content in solid samples, a Fourier‐transformed Raman method was successfully developed. A shift of the free carboxylic groups (1632 cm−1) of TA into the deprotonated state (1604 and 1398 cm−1) was observed and bands at 1728 and 1398 cm−1 were used to assess purity of TA after the chemo‐enzymatic PET hydrolysis. The chemical treatment, performed under neutral conditions (T = 250 °C, P = 40 bar), led to conversion of PET into 85% TA and small oligomers. The latter were hydrolysed in a second step using the Humicola insolens cutinase (HiC) yielding 97% pure TA, therefore comparable with the commercial synthesis‐grade TA (98%).
Introduction
Global population and rising living standards are directly correlated to the continuous increase of textile waste (Allwood et al., 2006). Overproduction of fabrics since 2010 is driven by the increased rate of replacement of products. In 2008, around 14 M tons of textile waste was generated in Europe, but only 5 M tons was recovered (Zamani, 2014). About 75% of the recovered fabrics were reused or recycled mainly in industrial applications (Jorgensen et al., 2008). The remaining collected waste textiles are either landfilled or incinerated. However, the recycling of used textiles would lead to several environmental benefits such as energy saving, as this process requires less energy than the production of the same products from virgin materials and reduction of the carbon footprint of the overall process (Clark et al., 2016).
The textile and clothing industry is a heterogeneous business which covers different types of fibres, with a consistent 54% that is represented by man‐made synthetic materials. The consumption of these synthetic fibres increased by 77% between 2000 and 2012 (Harder et al., 2017). On the other hand, the growth share of synthetic fibres in global consumption results in the rising demand for petroleum‐based chemicals (Pellis et al., 2016a). Among the synthetic textiles, poly(ethylene terephthalate) (PET) is one of the most widely used polymers in the global textile industry (Herrero Acero et al., 2011). PET is a semi‐crystalline thermoplastic polymer which shows excellent tensile strength, chemical resistance and high thermal stability. Two PET grades are dominating the global market: the fibre grade PET, with a Mw of 15–20 Kg mol−1 and intrinsic viscosity between 0.4 and 0.75 dl g−1, and the bottle grade PET, which refers to a higher Mw polymer (> 20 Kg mol−1) with an intrinsic viscosity above 0.95 dl g−1 (Tasca et al., 2010; Al‐Sabagh et al., 2016).
Due to its wide production and utilization (Cavaco‐Paulo and Guebitz, 2008), PET represents a broad disposal inert textile. The non‐toxic nature, durability and crystal clear transparency of PET during use are the principal advantages of this polyester, while its rather slow biodegradability is the major cause of concern to the environmentalists. Recycling the textile waste‐derived polyesters can significantly cut down the energy usage, resource depletion and greenhouse gas emissions. Unfortunately, different factors such as colouring dyes (Giannotta et al., 1994) and other chemicals such as detergents, fuels and pesticides (Demertzis et al., 1997) reduce the quality of recycled PET reducing the number of the possible applications. When compared to mechanical recycling and incineration, chemical hydrolysis could lead to higher value products (Paszun and Spychaj, 1997). The most common chemical‐based PET hydrolysis processes are alkaline hydrolysis using 4–20% NaOH/KOH solutions (Karayannidis et al., 2002), phase transfer catalysts and acidic hydrolysis using concentrated sulphuric acid or other mineral acids (Yoshioka et al., 2001). All these processes are very costly and toxic because the chemicals required and laborious purification steps are needed. In recent years, more environmentally friendly PET recycling strategies based on neutral hydrolysis (carried out using water or steam at 1–4 MPa and temperatures of 200–300 °C) were reported (Launay et al., 1994). In last decade, the interest of biotechnologies towards polyesters biodegradation and recycling is gaining a key role. Yoshida et al. (2016) showed a novel bacterium, Ideonella sakaiensis 201‐F6, able to break down the plastic using two enzymes to hydrolyse PET and assimilate its building block for growth. Earlier, various studies demonstrated that a class of enzymes belonging to the α/β hydrolase family, namely cutinases, are able to hydrolyse the ester bonds of PET and several other polyesters (Pellis et al., 2015, 2016b; Barth et al., 2016; Wei et al., 2016). Among them, cutinase are currently under investigation for the bioprocessing of PET textiles on an industrial scale (Silva et al., 2005). Earlier, it was reported that cutinases from Thermobifida fusca and Humicola insolens were able to hydrolyse low‐crystallinity PET while complete hydrolysis by enzymes only seems to be difficult if not impossible for PET with higher crystallinity (Mueller, 2006; Nimchua et al., 2007; Ronkvist et al., 2009). In this work, we propose an innovative synergistic chemo‐enzymatic hydrolysis of PET for the production of high‐purity TA (97%) avoiding harsh chemical treatments.
Results and discussion
FT‐Raman analysis
In the past, several methods, mainly HPLC‐related, have been established to follow enzymatic hydrolysis of PET in aqueous solutions (Herrero Acero et al., 2011; Pellis et al., 2016c). However, water‐insoluble oligomers would obviously escape quantification of TA in the presence of insoluble oligomers; hence, a novel method based on FT‐Raman and triethylamine was adapted for this study. TA, p‐C6H4(COOH)2, has two carboxylic groups which are detectable using Fourier transform Raman spectroscopy. The analysis of benzene dicarboxylic acids such as isophthalate and terephthalate using FT‐Raman was previously described by Arenas and Tellez (Arenas, 1979; Téllez et al., 2001). According to these reports, the ‐COOH group of solid TA reveals a typic‐centred band at 1631 cm−1, mainly given by the asymmetric stretching (νas) of C=O. As described by Tellez, a coupled vibrational mode is characteristic of the single band at 1286 cm−1. The discrimination of monomeric TA from esterified species was performed converting these functional groups into the corresponding anions. The simplest reaction of carboxylic acid is salification by a base. This reaction causes the shift of (νas) of the C=O group and appearance of new bands due to the in‐phase and out of phase ‐COO(−) stretching vibration (Socrates, 2004). The shift of the acid peak of the carboxylic group from 1720 cm−1 to 1580 cm−1 after alkaline treatment was previously reported for the grafting of cotton with cyclodextrins (Voncina and Majcen, 2005) or for detection of end groups of fluoropolymers (Pianca et al., 1999).
The conversion of the ‐COOH group into the carboxylate species was carried out using triethylamine (TEA) in chloroform solution (Di Robert et al., 2015). The deprotonated anion obtained via incubation with tertiary aliphatic amine caused the shift of νasC=O and the increase of the single band of the symmetric stretching (νs) of C=O at 1400 cm−1 and an ammonium‐related band in the 2700 cm−1 region.
Figure 1 shows the shift of the acid peak of solid TA from 1631 to 1604 cm−1 after deprotonation with TEA. Furthermore, the clear appearance of a peak at 1398 cm−1 was observed due the symmetric stretching mode of the carboxylate moiety. On the other hand, the peak at 1286 cm−1 was strongly reduced. The 1:5 TA/TEA ratio showed the clearest signal for the monomers due to a complete conversion of the desired groups (Fig. S2).
Figure 1.
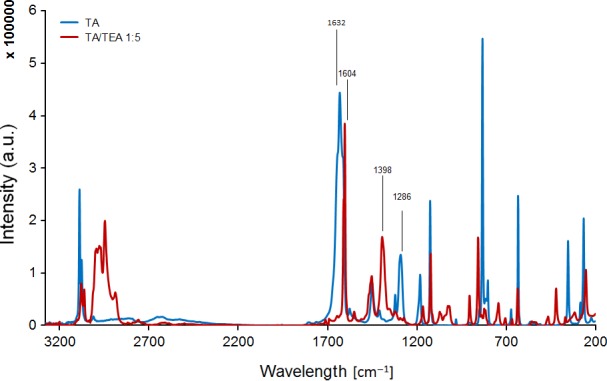
FT‐Raman analysis showing the deprotonation of the TA's carboxylic acid moieties (blue) via incubation with a 1:5 TA/TEA ratio (red). Spectra were normalized between the region 2000 and 2200 cm−1.
To assess the suitability of this method to quantify TA in the presence of oligomers, BHET, DMT and PET were incubated with the tertiary aliphatic amine (1:5 ratio). Expectedly, the shift of 1632 and 1395 cm−1 typical for TA did not occur as all carboxylic groups of these oligomers are esterified (Figs S3–S5).
Water‐based PET hydrolysis
Preliminary experiments showed that water‐based PET depolymerization was not achieved with ratio 1:4 PET/H2O and incubation for 90 min. Therefore, a series of experiments were performed, to define the appropriate depolymerization conditions (Table 1).
Table 1.
Design of experiments strategy used for performing the water‐based hydrolysis of PET
| Experiment | Initial MW (IV) range of PET | T [°C] | PET/H2O ratioa | Steady‐state time [min]b |
|---|---|---|---|---|
| 1 | 0.62 | 180 | 1/4 | 0 |
| 2 | 1/10 | 30 | ||
| 3 | 250 | 1/4 | ||
| 4 | 1/10 | 0 | ||
| 5 | 180 | 1/4 | ||
| 6 | 1/10 | 30 | ||
| 7 | 250 | 1/4 | ||
| 8 | 1/10 | 0 |
All reported conditions were also tested; zinc acetate was added to the reaction mixture.
a. 25 g of PET.
b. After the transient period (to reach the desired T).
The incubation of PET fibre at 180 °C and 12 bar did not lead to any depolymerization of the sample, and hence, there was no difference of spectra. In Fig. S6, it is possible to observe how the spectra of raw PET fibre and Sample 2 are very similar with no remarkable differences that could be spotted, showing that reaction temperature and pressure were not optimal for the hydrolysis of the polymer. Finally, when temperature and pressure were increased to 250 °C and 39 bar, respectively, the polymer was completely reduced in a whitish powder (Fig. S7). In Fig. S8, it is possible to see that spectrum of PET depolymerization product for Sample 4 (blue) was similar to the spectrum of commercial TA. Using these conditions, a powder consisting of 85% TA was obtained from the water‐based PET depolymerization according to HPLC and 1H‐NMR analysis. Unfortunately, further variation of the reaction conditions including addition of zinc acetate did not lead to a higher yield of TA. Hence, enzymatic hydrolysis of the remaining oligomers was assessed in the next step. To note that experiments with various steady‐state lengths were performed but did not lead to an improvement of the TA yield, therefore the enzymatic finishing step was critical in order to obtain high‐purity TA.
Enzymatic PET hydrolysis
The kinetics of the formation of degradation products via enzymatic treatment depends on various factors, including the chemical–physical structure of polyester substrate (Eberl et al., 2008). Already, various authors have previously demonstrated that cutinase preferentially hydrolyze the amorphous region on PET (Donelli et al., 2009; Gamerith et al., 2017). On the other hand, it was shown that enzymatic hydrolysis of PET oligomers is way faster than of a long‐chain polymer (Ribitsch et al., 2011). Therefore, due to the high crystallinity of the PET fibres used in the textile industry, a chemical pre‐hydrolysis was established in this work.
The sample from chemical pre‐treatment with the highest degree of hydrolysis (Sample 4, 85% TA) was further incubated with different concentrations of Humicola insolens cutinase (HiC) to hydrolyse remaining oligomers (Fig. 2). The highest amount of soluble TA (6.5 mM) was obtained after 6 h of incubation both when 1 or 2 mg ml−1 of HiC was applied without any further increase until 24 h of incubation. The lower concentrations of enzyme of 0.1 and 0.5 mg ml−1 led to the release of 0.53 and 1.9 mM of TA respectively.
Figure 2.
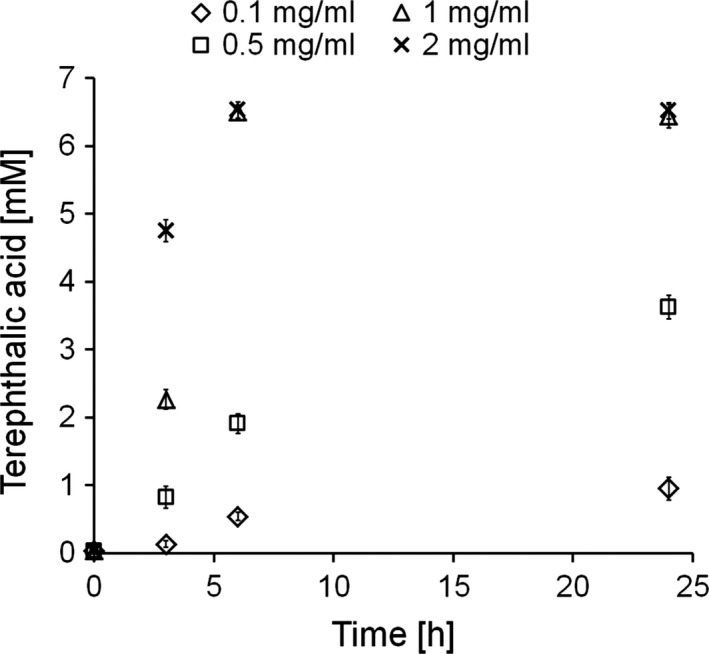
Enzymatic hydrolysis of chemically pre‐treated PET with different concentrations of Humicola insolens cutinase (HiC).
Terephthalic acid with 97% purity was obtained after enzymatic treatment of the chemical‐treated PET which is comparable to synthesis‐grade TA (98% pure). When chemical pre‐hydrolysis of PET was performed in the presence of zinc acetate as a catalyst, a negative influence on enzymatic hydrolysis was observed (Fig. 3). In fact, there was no further increase in the amount of TA seen (Fig. 3).
Figure 3.
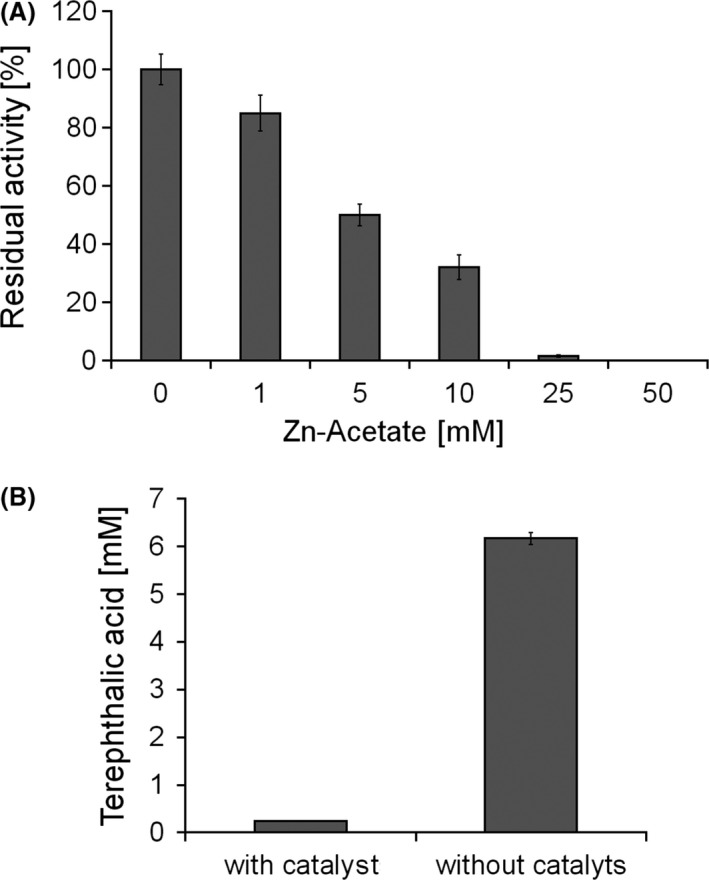
Residual activity of HiC in the presence of Zn acetate (A) and enzymatic hydrolysis of PET chemically pre‐treated in the presence of Zn acetate (B).
Zinc and other metal ions are well known to be potential inhibitors of many enzymes, including cutinases as recently reported by Chen et al. that biochemically characterized Thermobifida fusca cutinases (Chen et al., 2010).
FT‐Raman detection of depolymerized PET
While in the chemically pre‐treated sample oligomers were present, the peak at 1728 cm−1 indicative of ester bond was considerably reduced after enzymatic hydrolysis (Fig. 4). In parallel, the increase in the band at 1398 cm−1 indicated formation of TA. Finally, the signal at 1286 cm−1, as expected, had the opposite trend of the asymmetric stretching of CO, further confirming the reduction in the oligomers to TA (Fig. 5).
Figure 4.
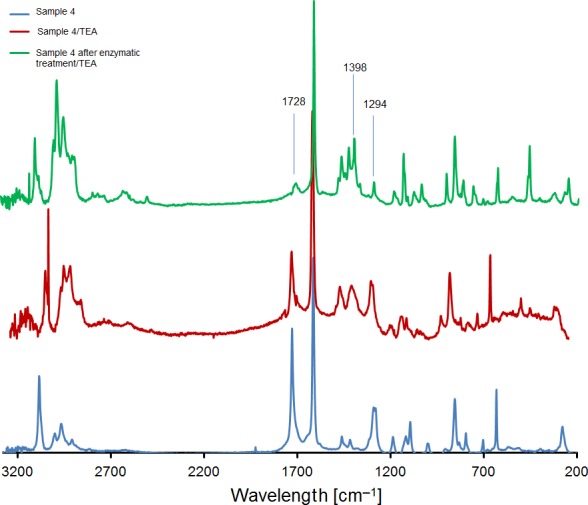
FT‐Raman analysis showing the incubation of Sample 4 with a 1:5 TEA ratio before (red) and after (green) enzymatic hydrolysis. Spectra were normalized between the 2000 and 2200 cm−1 region.
Figure 5.
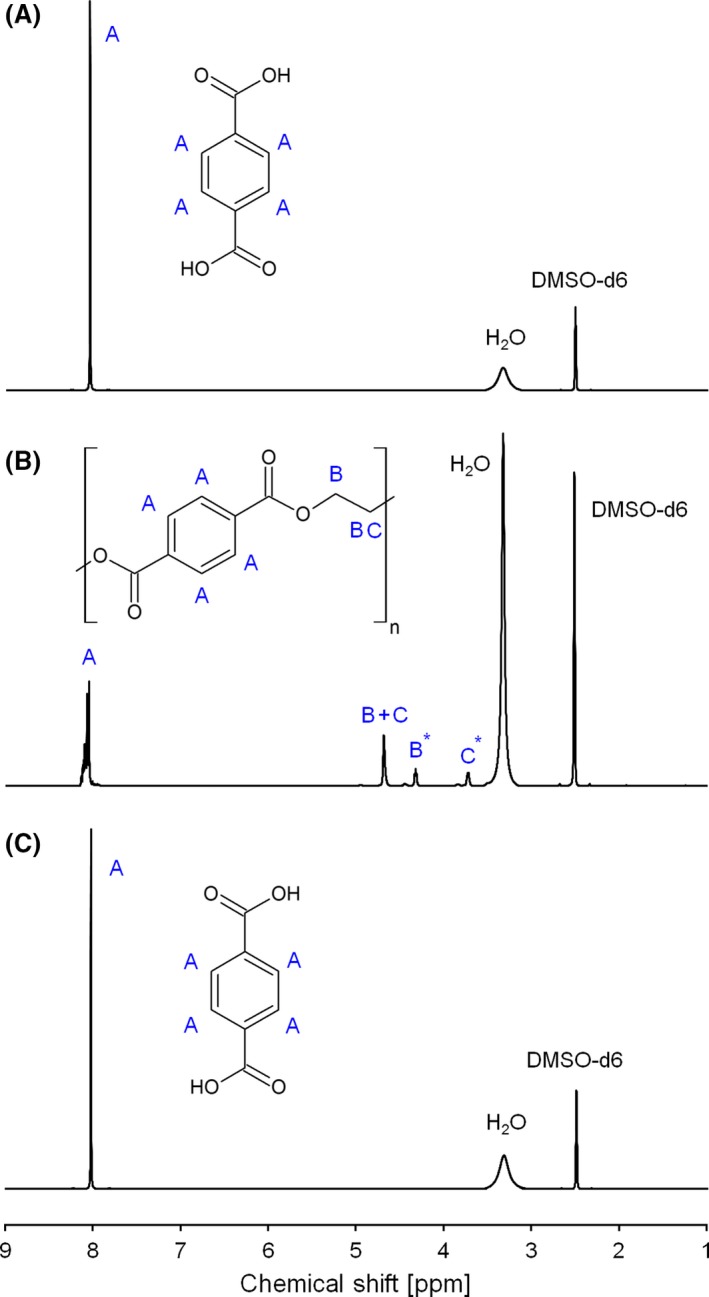
1H‐NMR of pure TA (A), PET degradation after the chemical treatment (Sample 4, B) and PET degradation after enzymatic finishing (C). All spectra were recorded in DMSO‐d6. All samples were fully soluble in the selected solvent. For detailed proton assignments, please see ESI.
In addition to FT‐Raman, 1H‐NMR analysis was performed. Likewise, 1H spectra (recorded in DMSO‐d6) indicate the presence of some longer oligomers in the chemically pre‐treated sample while the spectra of the reaction product after subsequent enzymatic hydrolysis suggest their conversion into TA. Based on the 1H‐NMR‐performed calculations (see ESI for details), the oligomers are mostly comprised of less than four (but nearer two) constitutional repeat units, and TA end groups dominate over EG suggesting the oligomerization is more effective at releasing free EG than free TA, or that the purification steps involved preferentially remove EG.
Materials and Methods
Chemicals, substrates and enzymes
Buffer components, bovine serum albumin (BSA), para‐nitrophenyl‐butyrate (p‐NPB), methanol, zinc acetate, TA and formic acid were purchased from Sigma‐Aldrich (USA). All other chemicals and reagents used in this work were of analytical grade and used without further purification if not otherwise specified. Humicola insolens cutinase (HiC) was a gift from Novozymes (Beijing, China). All hydrolyses were performed using Wellman PET fibre with a viscosity of 0.62 dl g−1.
Water‐based PET hydrolysis
Water‐based PET hydrolysis was performed in 1 l stainless steel high‐pressure and high‐temperature reactor at different temperatures (180 and 250 °C) and with and without the addition of zinc acetate as catalyst. All experiments were carried out with 25 g of virgin PET fibre in 250 ml of deionized water. The reactions were stopped after 60 and 90 min, respectively (30 min after reaching the steady state), and two different ratios PET/H2O were assessed. A temperature and pressure profile of a typical reaction is shown in Fig. S1. At the end of the process, H2O and EG were removed and a white powder was obtained (Fig. S7B). The EG stream can be further processed anaerobically due to the current low cost of the compound.
ATR FT‐IR analysis
ATR FT‐IR spectra were recorded on a Perkin‐Elmer Spectrum GX spectrometer. The ATR accessory (supplied by Specac Ltd., UK) contained a diamond crystal. A total of 16 scans for each sample were taken with a resolution of 4 cm−1. All spectra were recorded at 21 °C over the wavelength interval between 4000 and 650 cm−1.
FT‐Raman analysis
To remove the water, terephthalic acid (TA), bis(2‐hydroxyethyl) terephthalate (BHET), dimethyl terephthalate (DMT) and poly(ethylene terephthalate) PET as standards were lyophilized for 24 h; 50 mg of samples was incubated with different volumes of pure triethylamine in 10 ml of chloroform for 6 h. Afterwards, chloroform was removed by evaporation at 21 °C for 24 h. The virgin material and the powders obtained from the chemical process and after the enzymatic treatment were also incubated as described above. The FT‐Raman spectra were recorded using a Perkin‐Elmer Raman station 400, coupled with a 785 nm laser. Spectra were collected at a resolution of 2 cm−1 for 25 scans and normalized in the region 2200–2400 cm−1 before any data processing. The bands were assigned as follows: 3000–2700 cm−1 ν(Et‐NH4 +), 1728 cm−1 ν(C=O, bended), 1632–1604 cm−1 νas(C=O), single band 1398 cm−1 δ(C‐O), single band 1286 cm−1 coupled mode ν(C=O)+δ(COH).
Protein quantification and SDS‐PAGE analysis
Protein concentration was measured using the Bio‐Rad Protein Assay Kit (Bio‐Rad, Vienna, Austria). BSA was used as protein standard; 10 μl of the sample was added into the wells of a 96‐well plate. Afterwards, 200 μl of the prepared Bio‐Rad reagent solution was added (Bio‐Rad Reagent diluted 1:5 with MQ‐water). The plate was incubated for 5 min at 21 °C and 400 rpm. Buffer (100 mM Tris‐HCl pH 7) was used as blank. The absorption after 5 min was measured at λ = 595 nm in a plate reader (Tecan INFINITE M200) and the concentration calculated from the average of triplicates. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS‐PAGE) was performed according to Laemmli using 4% stacking gels and 15% separating gels and run at 150 V (Laemmli, 1970). Pre‐stained protein marker IV (Peqlab, Germany) was used as a molecular mass marker. Proteins were stained with Coomassie method.
Esterase activity assay
Esterase activity was measured at 25 °C using p‐nitrophenyl‐butyrate (p‐NPB) as a substrate according to Biundo et al. (Biundo et al., 2015). The final assay was carried out by mixing 200 μl of the substrate stock solution, in 100 mM Tris–HCl buffer pH 7, with 20 μl of enzyme solution. The increase in the absorbance at 405 nm due to the release of p‐nitrophenol (ε 405 nm = 10.27 ml (μmol cm)−1) was measured for 5 min, every 18 s with a plate reader. A blank was included using 20 μl of buffer instead of enzyme solution. The activity was calculated in units (U), where 1 unit is defined as the amount of enzyme required to hydrolyse 1 μmol of substrate per minute under the given assay conditions. The activity assay was also performed in the presence of 1, 5, 10, 25 or 50 mM of the chemical catalyst (zinc acetate). All the experiments were performed in triplicates.
Enzymatic hydrolysis of PET oligomers
The enzymatic hydrolysis of the PET powder resulting from the chemical treatments was performed as previously described with some modifications (Herrero Acero et al., 2011; Pellis et al., 2016a). Briefly, 10 mg of pre‐treated PET powder and 2.0 ml of enzyme solution (diluted in 100 mM Tris‐HCl buffer pH 7) were added in a test tube. The mixture was incubated for 24 h at 50 °C and 600 rpm in an orbital shaker. Different concentrations 0.1, 0.5, 1 and 2 mg ml−1 of enzyme were used to understand the optimal concentration of HiC. All the experiments were performed in triplicates.
High‐Performance Liquid Chromatography
Proteins were removed using a 1:1 (v v−1) ice‐cold methanol precipitation. Therefore, samples were centrifuged at 14 000 rpm (Centrifuge Beckman JU‐MI) at 0 °C for 15 min. The supernatant was acidified by adding 8 μl of 6 M HCl and then transferred into HPLC vials. The released products were analysed by high‐performance liquid chromatography (HPLC) (Agilent Technologies, Palo Alto, CA) coupled with a UV detector, at 241 nm, using a water/methanol linear gradient (Table S1). To determine the TA percentage in the mixture, 10 mg of PET powder resulting from before and after the enzymatic hydrolysis was diluted in 100 ml of 100 mM Tris‐HCl buffer pH 7 and analysed as described before.
1H‐NMR analysis
1H nuclear magnetic resonance was performed on a Bruker Avance II 400 spectrometer (resonance frequency of 400.13 MHz for 1H) equipped with a 5 mm observe broadband probe head (BBFO) with z‐gradients. DMSO‐d 6 was used as NMR solvent for all samples.
Conclusions
In this study, the synergism of chemical and enzymatic hydrolysis of PET was demonstrated on a multigram scale. To monitor the PET hydrolysis and formation of TA, a FT‐Raman method based on the deprotonation of the –COOH group was established. The chemical pre‐treatment performed in an environmentally friendly way under neutral conditions, therefore avoiding harsh chemicals, leads to depolymerization of the polyester‐composed waste textiles yielding about 85% TA. The enzymatic hydrolysis performed in a second reaction step leads to further hydrolysis of the remaining oligomers yielding TA with a purity of 97%. Future studies should consider the chemo‐enzymatic treatment of different PET containing textiles wastes as well as studies on synthesis of PET based on the recovered TA.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflict of interest
None declared.
Supporting information
Fig. S1. Temperature (blue, Y‐axis left) and pressure (red, Y‐axis right) increase according to the reaction time (X‐axis).
Fig. S2. FT‐Raman analysis showing the deprotonation of the TA's carboxylic acid moieties via incubation with different TA/TEA ratio.
Fig. S3. FT‐Raman analysis showing the deprotonation of the BHET via incubation with TEA 1:5.
Fig. S4. FT‐Raman analysis showing the deprotonation of the DMT via incubation with TEA 1:5.
Fig. S5. FT‐Raman analysis showing the deprotonation of the PET via incubation with TEA 1:5.
Fig. S6. FT‐IR spectrum of Sample 1 (black) and spectrum of untreated virgin PET.
Fig. S7. Samples obtained by three different depolymerisation operational conditions.
Fig. S8. FT‐IR spectra of Sample 4 (blue), untreated virgin PET (black) and pure TA (red).
Fig. S9. SDS‐PAGE of HiC. Lane 1 Protein Marker IV (bands 10‐170 KDa). Lane 2 HiC (dilution 1:10), MW ~24 KDa.
Table S1. Mobile phase gradient used for the HPLC‐DAD analysis of the PET degradation release products.
Acknowledgements
This project received funding from the European Union's Horizon 2020 research and innovation programme under the grant agreement 641942 (Resyntex project).
Microbial Biotechnology (2017) 10(6), 1376–1383
Funding Information
No funding information provided.
References
- Allwood, J.M. , Laursen, S.E. , deRodriguez, C.M. and Bocken, N.M.P. (2006) Well dressed? The present and future sustainability of clothing and textiles in the United Kingdom. Technical annex. University of Cambridge.
- Al‐Sabagh, A.M. , Yehia, F.Z. , Eshaq, Gh. , Rabie, A.M. and ElMetwally, A.E. (2016) Greener routes for recycling of polyethylene terephthalate. Egypt J Petrol 25: 53–64. [Google Scholar]
- Arenas, J.F. (1979) Infrared and Raman spectra of phthalate, isophthalate and terephthalate ions. Spectrochim Acta, Part A 35: 355–363. [Google Scholar]
- Barth, M. , Honak, A. , Oeser, T. , Wei, R. , et al (2016) A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol J 11: 1082–1087. [DOI] [PubMed] [Google Scholar]
- Biundo, A. , Hromic, A. , Pavkov‐Keller, T. , Gruber, K. , et al (2015) Characterization of a poly(butylene adipate‐co‐terephthalate)‐hydrolyzing lipase from Pelosinus fermentans. Appl Microbiol Biotechnol 100: 1753–1764. [DOI] [PubMed] [Google Scholar]
- Cavaco‐Paulo, A. , and Guebitz, G.M. (2008) Enzymes go big: surface hydrolysis and functionalisation of synthetic polymers. Trends Biotechnol 26: 32–38. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Su, L. , Billig, S. , Zimmermann, W. , Chen, J. , and Wu, J. (2010) Biochemical characterization of the cutinases from Thermobifida fusca . J Mol Catal B: Enzym 63: 121–127. [Google Scholar]
- Clark, J.H. , Farmer, T.J. , Herrero‐Davila, L. , and Sherwood, J. (2016) Circular economy design considerations for research and process development in the chemical sciences. Green Chem 14: 3914–3934. [Google Scholar]
- Demertzis, P.G. , Johansson, F. , Lievens, C. , and Franz, R. (1997) Studies on the development of a quick inertness test procedure for multi‐use PET containers‐sorption behaviour of bottle wall strips. Packag Technol Sci 10: 45–58. [Google Scholar]
- Di Robert, M. , Silverstein, F.W. , David, J. and Bryce, D.L. (2015). Spectrometric Identification of Organic Compounds.
- Donelli, I. , Taddei, P. , Smet, P.F. , Poelman, D. , Nierstrasz, V.A. , et al (2009) Enzymatic surface modification and functionalization of PET: a water contact angle, FTIR, and fluorescence spectroscopy study. Biotechnol Bioeng 103: 845–856. [DOI] [PubMed] [Google Scholar]
- Eberl, A. , Heumann, S. , Kotek, R. , Kaufmann, F. , et al (2008) Enzymatic hydrolysis of PTT polymers and oligomers. J Biotechnol 135: 45–51. [DOI] [PubMed] [Google Scholar]
- Gamerith, C. , Zartl, B. , Pellis, A. , Guillamot, F. , et al (2017) Enzymatic recovery of polyester building blocks from polymer blends. Proces Biochem [In press] https://doi.org/10.1016/j.procbio.2017.01.004. [Google Scholar]
- Giannotta, G. , Po, R. , Cardi, N. , Tampellini, E. , et al (1994) Processing effects on poly(ethylene terephthalate) from bottle scraps. Polym Eng Sci 34: 1219–1223. [Google Scholar]
- Harder, R. , Kalmykova, Y. , Morrison, G.M. , Feng, F. , et al (2017) Quantification of goods purchases and waste generation at the level of individual households. J Ind Ecol 18: 227–241. [Google Scholar]
- Herrero Acero, E. , Ribitsch, D. , Steinkellner, G. , Gruber, K. , et al (2011) Enzymatic surface hydrolysis of pet: effect of structural diversity on kinetic properties of cutinases from thermobifida. Macromolecules 44: 4640. [Google Scholar]
- Jorgensen, A. , Le Bocq, A. , Nazarkina, L. , et al (2008) Methodologies for social life cycle assessment. Int J LCA 13: 96–103. [Google Scholar]
- Karayannidis, G.P. , Chatziavgoustis, F. , Achilias, A.P. , et al (2002) Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv Polym Technol 21: 250–259. [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Launay, A. , Thominette, F. , and Verdu, J. (1994) Hydrolysis of poly(ethylene terephthalate): a kinetic study. Polym Degrad Stabil 46: 319–324. [Google Scholar]
- Mueller, R.‐J. (2006) Biological degradation of synthetic polyesters‐Enzymes as potential catalysts for polyester recycling. Process Biochem 41: 2124–2128. [Google Scholar]
- Nimchua, T. , Punnapayak, H. , and Zimmermann, W. (2007) Comparison of the hydrolysis of polyethylene terephthalate fibers by a hydrolase from Fusarium oxysporum LCH I and Fusarium solani f. sp. pisi. Biotechnol J 2: 361–364. [DOI] [PubMed] [Google Scholar]
- Paszun, D. , and Spychaj, T. (1997) Chemical recycling of Poly(ethylene terephthalate). Ind Eng Chem Res 36: 1373–1383. [Google Scholar]
- Pellis, A. , Herrero Acero, E. , Weber, H. , and Obersriebnig, M. (2015) Biocatalyzed approach for the surface functionalization of poly(L‐lactic acid) films using hydrolytic enzyme. Biotechnol J 10: 1739–1749. [DOI] [PubMed] [Google Scholar]
- Pellis, A. , Herrero Acero, E. , Ferrario, V. , Ribitsch, D. , et al (2016a) The closure of the cycle: enzymatic synthesis and functionalization of bio‐based polyesters. Trends Biotechnol 34: 316–328. [DOI] [PubMed] [Google Scholar]
- Pellis, A. , Gamerith, C. , Ghazaryan, G. , Ortner, A. , et al (2016b) Ultrasound‐enhanced enzymatic hydrolysis of poly(ethylene terephthalate). Biores Technol 218: 1298–1302. [DOI] [PubMed] [Google Scholar]
- Pellis, A. , Haernvall, K. , Pichler, C.M. , Ghazaryan, G. , et al (2016c) Enzymatic hydrolysis of poly(ethylene furanoate). J Biotechnol 235: 47–53. [DOI] [PubMed] [Google Scholar]
- Pianca, M. , Esposto, G. , and Radice, S. (1999) End groups in fluoropolymers. J Fluorine Chem 95: 71–84. [Google Scholar]
- Ribitsch, D. , Heumann, S. , Trotscha, E. , Herrero Acero, E. , et al (2011) Hydrolysis of polyethyleneterephthalate by p‐nitrobenzylesterase from Bacillus subtilis. Biotechnol Prog 27: 951–960. [DOI] [PubMed] [Google Scholar]
- Ronkvist, A.M. , Xie, W. , Lu, W. , and Gross, R.A. (2009) Cutinase‐catalyzed hydrolysis of Poly(ethylene terephthalate). Macromolecules 42: 5128–5138. [Google Scholar]
- Silva, C. , Carneiro, F. , O`Neill, A. , Fonseca, L.P. , Cabral, J.M.S. , Guebitz, G. and Cavaco‐Paulo, A. (2005) Cutinase‐ a new tool for biomodification of synthetic fibers. J Polym Chem 43, 2448–2450. [Google Scholar]
- Socrates, G. (2004) Infrared characteristic group frequencies tables and charts, 3rd edn John Wiley & Sons. [Google Scholar]
- Tasca, F. , Gorton, L. , Kujawa, M. , Patel, I. , et al (2010) Increasing the coulombic efficiency of glucose biofuel cell anodes by combination of redox enzymes. Biosens Bioelectron 25: 1710–1716. [DOI] [PubMed] [Google Scholar]
- Téllez, A.C. , Hollauer, E. , Mondragon, M.A. and Castaño, V.M. (2001) Fourier transform infrared and Raman spectra, vibrational assignment and ab initio calculations of terephthalic acid and related compounds. Spectrochim Acta Part A: Mol Biomol Spectrosc 57, 993–1007. [DOI] [PubMed] [Google Scholar]
- Voncina, B. and Majcen, L. M.A. (2005) Grafting of cotton with β‐cyclodextrin via poly(carboxylic acid). J Appl Polym Sci 96, 1323–1328. [Google Scholar]
- Wei, R. , Oeser, T. , Schmidt, J. , Meier, R. , et al (2016) Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng 113: 1658–1665. [DOI] [PubMed] [Google Scholar]
- Yoshida, S. , Hiraga, K. , Takehana, T. , Taniguch, I. , Yamaji, H. , Maeda, Y. , et al (2016) A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351: 1196–1199. [DOI] [PubMed] [Google Scholar]
- Yoshioka, T. , Motoki, T. , and Okuwaki, A. (2001) Kinetics of hydrolysis of Poly(ethylene terephthalate) powder in sulfuric acid by a modified shrinking‐core model. Ind Eng Chem Res 40: 75–79. [Google Scholar]
- Zamani, B. (2014) Towards Understanding Sustainable Textile Waste Management: Environmental impacts and social indicators. Thesis for the degree of licentiate of engineering: Chalmers University of Technology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Temperature (blue, Y‐axis left) and pressure (red, Y‐axis right) increase according to the reaction time (X‐axis).
Fig. S2. FT‐Raman analysis showing the deprotonation of the TA's carboxylic acid moieties via incubation with different TA/TEA ratio.
Fig. S3. FT‐Raman analysis showing the deprotonation of the BHET via incubation with TEA 1:5.
Fig. S4. FT‐Raman analysis showing the deprotonation of the DMT via incubation with TEA 1:5.
Fig. S5. FT‐Raman analysis showing the deprotonation of the PET via incubation with TEA 1:5.
Fig. S6. FT‐IR spectrum of Sample 1 (black) and spectrum of untreated virgin PET.
Fig. S7. Samples obtained by three different depolymerisation operational conditions.
Fig. S8. FT‐IR spectra of Sample 4 (blue), untreated virgin PET (black) and pure TA (red).
Fig. S9. SDS‐PAGE of HiC. Lane 1 Protein Marker IV (bands 10‐170 KDa). Lane 2 HiC (dilution 1:10), MW ~24 KDa.
Table S1. Mobile phase gradient used for the HPLC‐DAD analysis of the PET degradation release products.


