Summary
Polyhydroxyalkanoates (PHAs) are biopolymers with desirable material properties similar to petrochemically derived plastics. PHAs are naturally produced by a wide range of microorganisms as a carbon storage mechanism and can accumulate to significantly high levels. PHAs are an environmentally friendly alternative to their petroleum counterparts because they can be easily degraded, potentially reducing the burden on municipal waste systems. Nevertheless, widespread use of PHAs is not currently realistic due to a variety of factors. One of the major constraints of large‐scale PHA production is the cost of carbon substrate for PHA‐producing microbes. The cost of production could potentially be reduced with the use of waste carbon from food‐related processes. Food wastage is a global issue and therefore harbours immense potential to create valuable bioproducts. This article's main focus is to examine the state of the art of converting food‐derived waste into carbon substrates for microbial metabolism and subsequent conversion into PHAs.
Introduction
Microbially produced polyhydroxyalkanoates (PHAs) are among the most well‐studied biologically derived plastics. This is due to their suitability as potential replacements for petrochemically derived plastics because they are biodegradable and biocompatible (Bordes et al., 2009; Chanprateep, 2010). PHAs are carbon‐based polymers naturally created to store excess carbon sources and maintain energy balances (Escapa et al., 2012). Under certain conditions, such as nitrogen, phosphorus or oxygen limitation in the presence of excess carbon sources, some microorganisms accumulate high concentrations of PHAs (Anderson and Dawes, 1990; Lee, 1996).
There are over 155 confirmed unique PHA monomer subunits, which demonstrates the diversity of potential PHA polymers that can be produced using microorganisms (Agnew and Pfleger, 2013). The diversity of available monomers could lead to many different applications, as each resulting polymer has different material properties. For example, the melting temperatures of PHAs range from 50 to 180 °C and crystallinities of PHAs range from 30 to 70% (Rehm, 2010). Polyhydroxybutyrate (PHB), a short‐chain‐length (scl) PHA, is by far the most well‐studied PHA polymer and is able to accumulate to high concentrations in cells growing on a variety of carbon substrates (Reddy et al., 2003). For example, Cupriavidus necator has been recorded to have as high as 74% of its cell weight as PHB and recombinant Escherichia coli have been recorded to accumulate up to 85% of their dry cell weight as PHB (Kim et al., 1994; Wang et al., 2009). Some potential applications of PHAs could include commercial packaging (Bhardwaj et al., 2014; Bugnicourt et al., 2014), agricultural purposes (Akaraonye et al., 2010) and medical uses (Wu et al., 2009; Dinjaski and Prieto, 2015; Mozejko‐Ciesielska and Kiewisz, 2016). Although it is hoped that PHAs will be able to replace petrochemical plastics in these areas as production processes improve, the cost of production is currently prohibitive to all applications except higher‐value medical uses (Keshavarz and Roy, 2010; Chen and Patel, 2011; Zhu et al., 2016).
While the properties of PHAs seem suitable as potential petrochemical plastic replacements, there are still bottlenecks for scaling up microbial production systems. One of the major bottlenecks is the cost of carbon substrates, which have been estimated to be 28–50% of the total production process (Choi and Lee, 1999; Obruca et al., 2015; Strong et al., 2016). There are a number of complex waste streams that can potentially act as carbon substrates for microbial PHA manufacture, such as waste streams from biodiesel production (Kenny et al., 2012; Escapa et al., 2013), municipal wastewater (Rahman et al., 2014, 2015), agricultural waste (Linton et al., 2012), syngas production (Drzyzga et al., 2015), traditional plastic waste (Wierckx et al., 2015) and others (Gomez et al., 2012). Food waste is a prime candidate for an inexpensive carbon source, due to its wide spread availability and the potential to solve significant waste problems when used to produce PHAs.
Food wastage is a global problem and occurs at different stages in food production systems, starting from the harvesting of food to storage, packaging and end of life (Parfitt et al., 2010). In Europe, it is estimated that approximately 88 million tons of food is wasted and of this, 57 million tons is from households and food service (Stenmarck et al., 2016). In the United States, the Environmental Protection Agency (EPA) estimated that in 2013 approximately 37 million tons of food ended up in municipal solid waste systems, which was approximately 14% of all waste in the United States (US EPA, 2015). Another factor that is coupled to food waste is the energy that is lost in producing, processing and transporting the waste. This energy has been reported to be upwards of 2000 trillion British thermal units (BTU) in the United States, equivalent to 2.11 × 1012 megajoules (MJ) (Cuéllar and Webber, 2010). If an alternative means of transforming food waste into value‐added products are developed, then energy is essentially being transformed into useful products. Food waste conversion to tangible bioproducts has gathered plenty of attention, with systems being developed to produce a wide range of value‐added products such as biofuels, materials and a variety of additional feedstock chemicals (Lin et al., 2013; Pfaltzgraff et al., 2013; Matharu et al., 2016).
The main objective of this review is to summarize the current state of PHA production from food waste using microbes, as depicted in Fig. 1. More specifically, the purpose of this investigation was to study systems that convert a variety of food wastes into microbially‐derived PHAs. Some considerations taken into account were as follows: food waste pre‐treatment steps, scalability, bioreactor design, microorganisms used and final PHA polymer produced.
Figure 1.
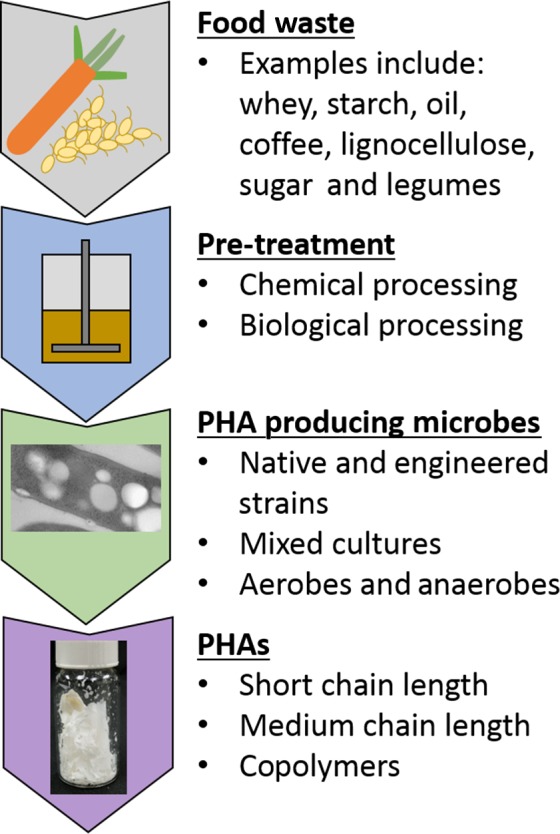
Conversion of food waste into PHAs using PHA‐producing microbes. Conversion of food waste often requires a pre‐treatment step where complex food waste is broken down into subcomponents. PHA‐producing microbes can then metabolize the carbon substrate and accumulate the biopolymer PHA.
PHAs from food waste using pure cultures
There are many different systems that have been proposed to convert food waste into PHAs as there are numerous waste streams generated by food production, processing and use. Each food source has its own complexities and requires different pre‐treatments, bacterial strains, culturing conditions and downstream processing. Often, the organics associated with food wastes are complex compounds that cannot be directly used by PHA‐producing organisms (Anderson and Dawes, 1990). In these cases, a pre‐treatment or processing method is necessary to convert the complex molecules found in food waste into PHA precursors. Precursors include simple sugars like glucose or lactose and fatty acids like acetic or propionic acids. Many of the simpler food wastes are hydrolysed to convert the food waste into suitable precursor molecules and then fed directly to a pure culture of an appropriate microorganism. Whey, starch, oils, lignocellulosic materials, legume and sugar wastes each have methods proposed to create PHAs.
PHAs from whey
One of the food wastes of interest is dairy whey. Whey is a by‐product of the cheese‐making process that consists of lactose, proteins, fats, water‐soluble vitamins, mineral salts and other essential nutrients for microbial growth. Although whey may be used as a source for producing lactose, casein and protein powder, it is estimated that approximately 50% of whey is still disposed of in wastewater treatment plants or used in animal feed (Pescuma et al., 2015). One study indicated that 1.15 × 108 to 1.60 × 108 tons of whey were produced worldwide, surpassing the requirements for whey powder production (Koller et al., 2013). When whey is used to produce proteins like lactoferrin or lactoferricin, lactose‐rich whey retentate remains as a waste material that must be disposed. Furthermore, acid whey is a by‐product of cottage cheese, cream cheese and Greek yogurt manufacturing. While acid whey can be used as animal feed, it is difficult to process into traditional whey protein concentrates due to the high acid content (Chandrapala et al., 2015; Lievore et al., 2015; Pescuma et al., 2015; Ryan and Walsh, 2016). Disposal of whey is currently a notable problem facing the dairy industry, making it a potentially inexpensive carbon source for PHA production (Girotto et al., 2015).
In addition to being a low‐cost carbon source, whey has the advantage of not requiring extensive pre‐treatment for use in fermentation via hydrolysis using enzymes or acid methods (Gomez et al., 2012). A life cycle assessment demonstrated that optimized production of PHAs from whey has a comparable ecological footprint to that of producing petroleum‐based plastics and is superior to producing whey powder (Koller et al., 2013). This life cycle assessment was based on producing PHAs using the archaeal production strain Haloferax mediterranei DSM 1411. Producing whey powder was inferior because it was a low market value product that used high amounts of energy to concentrate whey by evaporation. The main limitations on PHA production were found to be energy requirement for the fermentation process and a low amount of PHA output per kg whey input.
Most studies that used whey to produce PHAs have involved recombinant Escherichia coli, which will be described in a subsequent section. E. coli was selected because many of the traditional PHA‐producing microorganisms cannot directly metabolize whey. Studies have also examined using organisms such as the halophile archaeon H. mediterranei (Koller, 2015; Pais et al., 2016), an unidentified highly osmophilic organism (Koller et al., 2005); Thermus thermophiles HB8 (Pantazaki et al., 2009); and Cupriavidus necator DSM 545 transformed to include the lacZ, lacI and lacO genes of E. coli (becoming C. necator mRePT) (Povolo et al., 2010) (see Table 1). Particularly notable is the fact that H. mediterranei produces the copolymer poly‐(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) (PHBV), while T. thermophiles HB8 produced a unique combination of 3‐hydroxyvalerate (3HV) with medium‐chain‐length (mcl) PHAs. Both of these polymer blends have improved ductility compared to PHB, making them preferable for industrial use (Reddy et al., 2003; Rehm, 2010). Apart from C. necator mRePT, these organisms are capable of utilizing whey to produce PHAs with more desirable properties than PHB.
Table 1.
Production of PHAs from food waste using pure microbial cultures
| Food waste source | Microorganisms(s) | PHA polymer type | Cultivation | Dry cell weight (g l−1) | Maximum PHA production reported (g PHA g−1 dcw) | References |
|---|---|---|---|---|---|---|
| Whey | Highly osmophilic organism | PHBV | Fermenter, batch | NA | 8–10% | Koller et al. (2005) |
| Whey | Haloferax mediterranei | PHBV | Fermenter, fed‐batch | 10.91 | 66% | Koller (2015) |
| Whey | Haloferax mediterranei | PHBV | Fermenter, batch | 7.45 | 53% | Pais et al. (2016) |
| Whey | Thermus thermophiles HB8 | PHV and mcl‐PHAs | Flask, batch | 1.60 | 35.6% | Pantazaki et al. (2009) |
| Whey permeate | Cupriavidus necator mRePT | PHB | Flask, batch | 8 | 25% | Povolo et al. (2010) |
| Cassava starch wastewater | Cupriavidus sp. KKU38 | PHB | Flask, batch | 9.69 | 61.60% | Poomipuk et al. (2014) |
| Starch | Azotobacter chroococcum | PHB | Fermenter, batch | 54 | 46% | Kim (2000) |
| Soy bean and rapeseed oil | Cupriavidus necator H16 | PHB | Fermenter, two‐stage batch | 6.1 | 57% | Taniguchi et al. (2003) |
| Soy bean, rapeseed and corn oil and lard | Cupriavidus necator H16 | PHB | Fermenter, two‐stage batch | 6.5 | 79% | Taniguchi et al. (2003) |
| Palm oil and lard | Cupriavidus necator H16 | PHB | Fermenter, two‐stage batch | 6.8 | 83% | Taniguchi et al. (2003) |
| Tallow | Cupriavidus necator H16 | PHBV | Fermenter, two‐stage batch | 5.8 | 80% | Taniguchi et al. (2003) |
| Waste frying rapeseed oil | Cupriavidus necator H16 | PHB | Flask, batch | 10.8 | 67.9% | Obruca et al. (2014b) |
| Waste frying palm oil | Cupriavidus necator H16 | PHB | Flask, batch | 11.9 | 58.0% | Obruca et al. (2014b) |
| Waste frying sunflower oil | Cupriavidus necator H16 | PHB | Flask, batch | 10.812.53 | 52.4% | Obruca et al. (2014b) |
| Corn oil | Psuedomonas species | mcl‐PHA | Flask, batch | 12.53 | 35.63% | Chaudhry et al. (2011 ) |
| Spent coffee grounds oil | Cupriavidus necator DSM 428 | PHB | Fermenter, fed‐batch | 16.7 | 78.40% | Cruz et al. (2014) |
| Spent coffee Grounds oil | Cupriavidus necator H16 | PHB | Fermenter, fed‐batch | 55.4 | 89.10% | Obruca et al. (2014b) |
| Spent coffee grounds hydrolysate | Burkholderia cepacia | PHBV | Flask, batch | 4.91 | 54.79% | Obruca et al. (2014a) |
| Oil palm empty fruit bunch | Bacillus megaterium R11 | PHB | Flask, batch | 24.29 | 51.60% | Zhang et al. (2013) |
| Wheat straw | Burkholderia sacchari DSM 17165 |
PHB PHB |
Fermenter, fed‐batch | 146 | 72% | Cesário et al. (2014) |
| Rice straw | Bacillus firmus NII 0830 | PHB | Fermenter, batch | 1.9 | 89% | Sindhu et al. (2013) |
| Wheat bran | Halomonas boliviensis LC1 | PHB | Fermenter, batch | 1.08 | 34% | Van‐Thuoc et al. (2008) |
| Tequila bagasse | Saccharophagus degradans | PHA | NA | NA | > 0% | Munoz and Riley (2008) |
| Molasses | Psuedomonas species | PHA | Flask, batch | 10.54 | 20.63% | Chaudhry et al. (2011) |
| Fermented mash | Psuedomonas species | PHA | Flask, batch | 7.02 | 23.56% | Chaudhry et al. (2011) |
| Spent wash | Psuedomonas species | PHA | Flask, batch | 8.56 | 25.46% | Chaudhry et al. (2011) |
| Sugarcane molasses | Bacillus megaterium BA019 | PHB | Fermenter, fed‐batch | 72.2 | 42% | Kulpreecha et al. (2009) |
| Sugar beet juice | Alcaligenus latus | PHB | Flask, two‐ stage batch | 4.01 | 38.66% | Wang et al. (2013) |
| Sugarcane bagasse | Burkholderia sp. | PHB | Fermenter, fed‐batch | 6.8 | 48% | Lopes et al. (2014) |
| Sugarcane bagasse | Cupriavidus necator | PHB | Flask, batch | NA | 57% | Yu and Stahl (2008) |
| Sugarcane vinasse | Haloarcula marismortui | PHB | Flask, batch | 12 | 23% | Pramanik et al. (2012) |
| Sugarcane vinasse | Haloferax mediterrranei | PHBV | Flask, batch | 28.1 | 70% | Bhattacharyya et al. (2012) |
| Rice‐based ethanol stillage | Haloferax mediterrranei | PHBV | Flask, batch | 23 | 71% | Bhattacharyya et al. (2014) |
| Malt waste | Alcaligenus eutrophus DSM1124 | PHB | Fermenter, batch | 32.36 | 70% | Yu et al. (1998) |
| Soy waste | Alcaligenus eutrophus DSM1124 | PHB | Fermenter, batch | 18.42 | 32.57% | Yu et al. (1999) |
| Bean curd waste | Alcaligenus latus | PHB | Flask, batch | 3.73 | 66.56% | Kumalaningsih et al. (2011) |
NA is not available.
PHAs from starch
Starch is another carbon source derived from food wastes that has been studied for production of PHAs. Starch is a glucose polymer produced by plants such as rice, wheat, potatoes, maize and cassava. Although starch is readily consumed by humans, there are some significant starchy waste streams from food production that can be used by PHA‐producing microorganisms. In the work of Poomipuk et al. (2014), Cupriavidus sp. KKU38 isolated from cassava starch wastewater was used to produce PHAs from cassava starch hydrolysate. This study demonstrated that under optimal conditions and nitrogen starvation, Cupriavidus sp. could produce a moderately high biomass concentration of 5.97 g l−1, with a PHA content of 61.6% (Table 1). A study by Kim (2000) avoided using expensive enzymes to hydrolyse starch using Azotobacter croococcum, a type of bacteria that can digest starch directly. In this study, 54 g l−1 dry cell weight with 46% PHB was obtained with oxygen limitation (Table 1). These studies indicate that high concentrations of cells containing PHAs are possible on starchy food wastes, even with minimal pre‐treatments in the case of the Kim (2000) study.
PHAs from waste oil
Waste oils from both household and industrial applications are potential carbon sources for producing PHAs. These oils generally require no pre‐treatment and may be added directly to media as a carbon substrate. A study by Taniguchi et al. (2003) investigated the use of Cupriavidus necator H16 to convert waste oils and tallow to PHAs. The highest amount of PHA produced in this study came from using palm oil and lard as carbon sources, achieving a dry cell weight of 6.8 g l−1 and a PHB accumulation of 83%. Another notable finding is that when tallow was used as a carbon source, the copolymer PHBV was produced instead of pure PHB (Table 1).
Obruca et al. (2014b) also tested waste frying rapeseed oil, waste frying palm oil and waste frying sunflower oil as carbon sources for PHA production via C. necator H16. They demonstrated dry cell weights (g l−1) and PHB contents (%) of 10.8 and 67.9%, 11.9 and 58.0%, and 10.8 and 52.4%, respectively, for the different oil types. Chaudhry et al. (2011) used corn oil and found that a Pseudomonas strain could achieve a dry cell weight of 12.53 g l−1 with a mcl‐PHA content of 35.63% (Table 1). Although lower quantities of PHA were produced by the Pseudomonas species used by the Chaudhry group when compared to the C. necator H16 used by the Obruca group, the mcl‐PHA produced by the Pseudomonas strain when it was fed corn oil is more desirable than PHB. These studies indicate that using waste oils as a carbon source allow cells to produce high concentrations of PHAs relative to dry cell weight in low titres of cells.
PHAs from spent coffee grounds
An alternative oil waste from food is spent coffee grounds (SCG) oil. SCG are produced during coffee processing and consumption. Approximately 9–15% of the grounds is oil that can be extracted for use (Al‐Hamamre et al., 2012). The remaining portion of the spent coffee grounds is primarily lignocellulosic materials that can be combusted for heat or hydrolysed and converted into PHAs by Burkholderia cepacia (Obruca et al., 2014a). Obruca et al. (2014b) directly compared use of SCG oil to other waste oils in C. necator H16 and found that the SCG oil was superior for PHB production. In a shake flask experiment, the SCG oil produced a dry cell weight of 14.2 g l−1 with a PHB content of 70.3% compared to the values for the waste oils discussed previously (Table 1). When scaled up, the SCG oil achieved an impressive dry cell weight of 55 g l−1 with a PHB concentration of 89.1% in fed‐batch mode (Table 1). The main difficulty encountered is that the SCG oil is a natural foaming agent; however, other plant oils, such as waste frying oils, can be added to serve as both carbon sources and as antifoaming agents.
One study by Cruz et al. (2014) used supercritical fluid extraction with CO2 (scCO2) to extract the SCG oil and then fed it directly to C. necator DSM 428 in fed‐batch mode. The culture reached a dry cell weight of 10.7 g l−1 with a PHB content of 78.4% (Table 1). In the same study, batch mode operation was also used to produce PHB from SCG oil, but this approach produced lower amounts of PHB in comparison with the fed‐batch mode. The maximum biomass accumulation observed in batch mode was up to 55% (w/w) of PHB, which yielded a polymer concentration of 6 g l−1, in comparison with a polymer concentration of 13.1 g l−1 observed in the fed‐batch mode. The main difference between the study by the Cruz group and the study by the Obruca group was that the Cruz group used an extraction method that avoided the use of hazardous organic solvents like n‐hexane. As such, the Cruz process may be superior for mass production processes despite achieving lower dry cell weights. Both studies indicate that SCG oils are a carbon source with great potential for PHA production.
Lignocellulosic waste conversion to PHAs
Lignocellulosic materials are tough plant‐based materials that are made of cellulose, pectin, hemicellulose and lignin. Examples of this type of waste from food industry include bagasse, rice straw, wheat straw and bran. Waste streams of lignocellulosic compounds generally require hydrolysis to convert them into fermentable sugars and then detoxification to remove inhibitory compounds produced during hydrolysis, as reviewed in Obruca et al. (2015). A variety of lignocellulosic materials have been investigated for PHA production, including oil palm empty fruit bunch (Zhang et al., 2013), wheat and rice straw (Sindhu et al., 2013; Cesário et al., 2014), wheat bran (Van‐Thuoc et al., 2008), sugarcane bagasse (Yu and Stahl, 2008) and tequila bagasse (Munoz and Riley, 2008) (see Table 1). Despite pre‐treatments, the lignocellulosic materials often resulted in low levels of cell growth. One of the more promising lignocellulosic processes, however, was investigated by Cesário et al. (2014). In this study, an ammonia fibre expansion (AFEX) process was used as pre‐treatment followed by an enzymatic hydrolysis of the cellulose and hemicellulose fractions of ground wheat straw to produce glucose, xylose and arabinose. The hydrolysate was fed to Burkholderia sacchari DSM 17165 in a fed‐batch fermentation process. A biomass concentration of 146 g l−1 with a PHA concentration of 72% was achieved using this method (Table 1). While lignocellulosic materials generally require extensive pre‐treatment, they do offer some potential as carbon substrates.
PHAs from sugar industry waste
Several waste streams from the sugar industry have been investigated for their PHA‐producing potential. One example is low‐grade molasses, which is a residual syrup generated in sugar‐refining mills that is high in sucrose, but not suitable for food (Gomez et al., 2012). Most studies using molasses indicate that cell production and polymer content are not currently cost competitive. For example, Chaudhry et al. (2011) used a Pseudomonas species to convert sugar industry wastes to PHAs, and found that the dry cell weight and PHA contents were 7.02–12.53 g l−1 and 20.63–35.63%, respectively, with molasses functioning the best overall (Table 1). One promising study by Kulpreecha et al. (2009) used sugarcane molasses as the main carbon source for Bacillus megaterium BA‐019 to achieve a dry cell weight of 72.7 g l−1 in 24 h, with a PHB content of 42%. This latter study does indicate that molasses may be used to produce a considerable amount of PHB.
Sugar beet is another industrial waste with high sucrose content. Alcaligenes latus (ATCC 29714) was demonstrated to grow in sugar beet juice with supplemental nutrients to achieve optimal growth of 10.3 g l−1 and a PHB content 38.66% (Table 1) (Wang et al., 2013). The Italian company, Bio‐on, has also developed a range of PHA polymers using local sugar beet juice (Dietrich et al., 2017). The company now uses sugar beet and sugar cane wastes from around the world to produce PHAs for use in cosmetics and pharmaceuticals (Bio‐on, 2017).
Bagasse, the lignocellulosic residue of crushed sugar beets or sugar cane stalks, has been examined as a source of xylose for PHA production (Silva et al., 2014; Sindhu et al., 2016). Bagasse requires a pre‐treatment step to convert it to digestible sugars and to remove inhibitory compounds like formic acid, acetic acid and furfural. In a study by Lopes et al. (2014), for example, acid‐treated sugarcane bagasse at 120 °C produced 3.264 g l−1 PHB in Burkholderia sp. In addition, PHBV was produced when levulinic acid was added. Yu and Stahl also used acid and moderate heat (100–130 °C) to pre‐treat sugarcane bagasse for use as a carbon source for C. necator. The inhibitory effects of the solution were overcome by a large inoculum of a tolerant strain of C. necator and a diluted hydrolysate solution which yielded PHB to 57% dry cell weight (Yu and Stahl, 2008). This demonstrates that bagasse can not only be used as a fuel for boilers and as a raw material for paper, but also as a carbon source for PHA production.
Another significant sugar industry waste is vinasse, an acidic compost with a pH of 3.5–5.0 and high organic content. Recent research into using vinasse as a carbon source for PHA production has focused on using extremely halophilic archaea like Haloarcula marismortui and H. mediterranei (Bhattacharyya et al., 2012; Pramanik et al., 2012). These organisms have the advantage of not requiring a sterile environment due to the high salinity of fermentation broth and are also notable for being able to produce PHBV without the addition of organic acids as precursors. One of the main drawbacks of using halophilic organisms is disposing the saline solution after fermentation, which Bhattacharyya et al. (2014) addressed using a two‐stage desalination of spent stillage medium to reuse medium salts, tested with rice‐based ethanol stillage as a carbon source. In addition, vinasse contains polyphenolic inhibitory compounds that make pre‐treatment such as adsorption on activated carbon necessary to use vinasse as a carbon source in concentrations above 10% (Bhattacharyya et al., 2012; Pramanik et al., 2012). After pre‐treating vinasse, concentrations of up to 50% were used with H. mediterranei to produce 17.4–19.7 g l−1 PHA (Bhattacharyya et al., 2012) and 100% vinasse was used with H. marismortui to generate 4.5 ± 0.2 g l−1 PHA (Pramanik et al., 2012).
PHAs from legume waste
Legumes have also been demonstrated as suitable carbon sources for PHA production. In a study by Kumalaningsih et al. (2011), liquid bean curd waste supplemented with an initial sucrose concentration of 25 g l−1 was fed to A. latus. A dry cell weight of 3.73 g l−1 with a PHA content of 66.56% was observed after 60 h of culturing (Table 1). Soya waste was used by Yu et al. (1998, 1999) to produce PHAs using Alcaligenus eutrophus DSM 1124, but it was found that the organism was more successful at converting malt waste to PHAs than soy, with 32.57% PHA accumulated out of 18.42 g l−1 dcw for soy compared to 70% PHA out of 32.36 g l−1 dcw for malt (Table 1).
Pure cultures grown on food wastes tend to promote high cell growth and accumulation of PHAs. Some substrates, such as starch or SCG oil, are more promising than others, such as molasses or waste oils. While using waste food substrates to feed these cultures reduces costs due to carbon source, pre‐treatments are often necessary. Most of the traditional bacteria used for PHA production produce the PHB polymer, and the cost of sterilization and oxygen supply for cultures are not economically favorable. Scale‐up and optimization of processes may be able improve these difficulties. Using processes that require less energy expenditure may also reduce costs. For example, using the halophilic organism H. mediterranei has the dual benefits of making sterilization unnecessary and producing PHBV polymer instead of PHB alone. Even with these drawbacks, using pure cultures to produce PHAs from food wastes has promise.
Recombinant microbes for PHA production
Natively accumulating microbial strains are most commonly utilized for conversion of food‐based carbon substrates into PHAs. The use of recombinant organisms, however, could be advantageous as the microorganisms can be triggered to produce PHAs without inducing stressed conditions, such as nitrogen or phosphorus starvation, which could potentially lead to cost savings. In addition, recombinant microorganisms are well defined and thus could be further engineered for optimization. Bacteria known to be able to utilize certain substrates that the native PHA producers cannot use may also be transformed with PHA‐producing genes to produce PHA from food wastes like whey, starch or oils. Furthermore, culturing recombinant microbes could allow faster growth and turnaround times for bioreactors.
Escherichia coli is the standard organism used in genetic engineering and has been shown to be advantageous for producing PHAs. Some strains of E. coli are known to be able to utilize lactose, a substrate that many PHA‐producing organisms like C. necator are not able to metabolize. As such, most studies using high lactose containing dairy whey as a carbon source have used E. coli with the PHA‐producing genes (the pha operon) from C. necator. Traditional laboratory strains of E. coli like XL1‐Blue, JM or DH5α often lack the ability to utilize lactose as a nutrient source, which has made developing alternative strains from wild‐type E. coli cells necessary. When nine different strains derived from wild‐type cells were tested for their ability to produce PHA using lactose as a sole carbon source, it was documented that strains GCSC4401 and GCSC6576 transformed with a high‐copy‐number plasmid, pSYL107 containing the A. eutrophus PHA biosynthesis operon, were best able to produce PHAs. The maximum PHB concentration and PHB content obtained were 5.2 g l−1 and 81% of dry cell weight respectively (Table 2) (Lee et al., 1997).
Table 2.
Production of PHAs from food waste using recombinant bacteria
| Food waste source | Strain | Plasmid | PHA operon origin | PHA polymer type | Cultivation | Dry cell Weight (g l−1) | Maximum PHA Production Reported (g PHA g−1 dcw) | References |
|---|---|---|---|---|---|---|---|---|
| Whey |
E. coli
GCSC657 |
pSYL107 | C. necator | PHB | Shake flask | 6.4 | 81% | Lee et al. (1997) |
| Whey |
E. coli
GCSC657 |
pSYL107 | C. necator | PHB | Fermenter, fed‐batch | 87 | 80% | Wong (1998) |
| Whey | E. coli | pSYL107 | C. necator | PHB | Fermenter, fed‐batch | 31 | 80% | Kim (2000) |
| Whey |
E. coli
CML3‐1 |
pMAB26 | C. necator | PHB | Fermenter, fed‐batch | 33.09 | 28.65% | Pais et al. (2014) |
| Whey |
E. coli
CGSC 4401 |
pJC4 | Alcaligenes latus | PHB | Fermenter, fed‐batch | 119.5 | 80.50% | Ahn et al. (2000) |
| Whey |
E. coli
CGSC 4401 |
pJC4 | Alcaligenes latus | PHB | Fermenter, fed‐batch | 194 | 87% | Ahn et al. (2001) |
| Whey | E. coli | pJC4 | Alcaligenes latus | PHB | Fermenter, fed‐batch | 14.5 | 71% | Park et al. (2002) |
| Whey |
E. coli
K24K |
pJP24K | Azotobacter sp. FA8 | PHB | Fermenter, fed‐batch | 70.1 | 72.90% | Nikel et al. (2006) |
| Malt waste | E. coli | pUC19/PHA | C. necator | PHBV | Fermenter, fed‐batch | NA | 16% | Law et al. (2004) |
| Soy waste | E. coli | pUC19/PHA | C. necator | PHBV | Fermenter, fed‐batch | NA | 23% | Law et al. (2004) |
| Soy waste |
E. coli
XL1‐Blue |
pKS | C. necator | PHB | Fermenter, batch | 3.025 | 27.83% | Hong et al. (2000) |
| Organic acids |
E. coli
pnDTM2 |
NA | NA | PHB | Fermenter, batch | 2.9 | 45% | Eshtaya et al. (2013) |
| Starch | Aeromonas Sp. KC007‐R1 |
pRK415 H16 |
C. necator | PHB | Fermenter, batch | 1.83 | 32.70% | Chien and Ho (2008) |
NA is not available.
Initial studies using recombinant E. coli showed potential, and subsequent studies focused on improving production. The same group that developed E. coli strains GCSC4401 and GCSC6576 published another study focusing on scale‐up. E. coli GCSC6576 (pSYL107) was grown on a high concentration of whey in a fed‐batch system, where a dry cell weight of 87 g l−1 and PHB content of 79% was achieved (Table 2) (Wong, 1998). A follow‐up study improved on these methods by controlling the timing of PHB biosynthesis in recombinant E. coli using lactose concentrations. This allowed E. coli strain GCSC6576 (pSYL107) to accumulate PHA concentrations up to 80% of the dry cell weight without removing culture broth (Table 2) (Kim, 2000). As such, E. coli has been successfully used to produce PHAs from whey using the pha operon from C. necator.
Other studies have also used E. coli to produce PHAs on whey using a PHB operon from A. latus rather than C. necator. Several strains of E. coli that were known to be able to utilize lactose were transformed with plasmid pJC4 with A. latus pha genes. It was found that strain CGSC4401 was the ideal strain and dry cell weight and PHB content of 119.5 g l−1 and 80.5% were achieved (Table 2) (Ahn et al., 2000). This study and other early studies had problems with volumetric limitations of fermenter due to the low solubility of lactose in water and low PHB productivity. The former was partially addressed by highly concentrated whey solution. A subsequent study by Ahn et al. (2001) used a cell recycle membrane fed‐batch system to increase PHB productivity, achieving a final cell concentration and PHB content of 194 g l−1 and 87% respectively (Table 2). In a third study, the same recombinant strain grown in whey in a 30‐l fermenter (26 h) and 300‐l fermenter (20 h) produced 70% and 67% PHB, respectively, demonstrating the process of using whey with this strain was scalable. (Park et al., 2002).
Two other studies used recombinant E. coli to produce PHAs from whey. Pais et al. used proton suicide methodology to select for a recombinant strain of E. coli that synthesized a low amount of organic acids after it was transformed with the C. necator pha operon. The results indicated that the lower organic acid production resulted in slower growth, but a higher production of PHB (18.88 g PHB l−1 versus 7.8 g PHB l−1 in the original transformed strain) (Pais et al., 2014). Another study used recombinant E. coli harbouring the PHB biosynthetic genes from Azotobacter sp. strain FA8 to produce PHB from whey and corn steep liquor as the main carbon and nitrogen sources. The maximum cell density and PHB concentrations attained were 70.1 g l−1 and 73% (Table 2) (Nikel et al., 2006). These studies demonstrate the use of metabolic engineering to improve production of PHA and another option for PHA‐producing genes that can be used in E. coli.
While whey is the primary food waste substrate investigated for recombinant E. coli, other nutrient sources have been pursued. A study by Hong et al. (2000) successfully cloned the pha operon from C. necator into E. coli XL1‐Blue and demonstrated PHB production from soya waste. Soya waste was hydrolysed in NaOH for 8 h and then fed into a batch 3‐l fermenter, and 27.83% PHB accumulation was observed after 9 h of culturing (Table 2). The same group later demonstrated recombinant production of PHBV using E. coli HMS174 with a plasmid containing the pha operon. Malt and soya waste were obtained locally and recombinant E. coli accumulated up to 16% and 23% PHAs, respectively (Table 2). As a comparison, when this strain was grown in glucose, it produced approximately 43% dry cell weight PHAs (Law et al., 2004). This demonstrates that soya and malt waste are potential carbon sources for PHA production.
In addition to malt and soya waste, another study used locally procured restaurant waste that was anaerobically digested to produce lactic and acetic acids. Similar to processes mentioned previously, these precursors were fed to recombinant E. coli pnDTM2, which accumulated 44% PHB (Table 2) (Eshtaya et al., 2013). While using restaurant waste required an extra step to convert the complex mixture into organic acids, it provided an opportunity to use an inexpensive and widely available carbon source.
Many studies that use recombinant bacteria to produce PHAs from food waste focus on using E. coli, which is the common workhorse of molecular biology. In addition to E. coli, another Aeromonas sp. (strain KC007‐1) has also been used. The strain was chosen for its ability to directly use starch as a carbon source, and the pha operon from C. necator H16 was added to increase production rates of PHAs. In this case, the bacteria were able to accumulate 32.7% PHA (Chien and Ho, 2008), indicating that E. coli is not the only organism to be successfully modified to produce PHAs from food waste.
The examples mentioned here showed that non‐native microorganisms were able to successfully produce high amounts of PHAs. In all cases, pathways for PHA production were transferred from a native host to the non‐native microorganism. As genetic tools increase, it will be possible to optimize pathways for non‐native hosts to consume substrates and produce PHAs. The idea to genetically engineer microbial strains with a dual purpose of consuming inexpensive substrates and producing valuable bioproducts has been mentioned previously (Gustavsson and Lee, 2016), although relatively few studies in the past 10 years have used recombinant bacteria to produce PHAs from food waste. Future research into scale‐up and maintaining high productivity, cell concentrations and PHA content is still necessary to make PHA production from food wastes using recombinant bacteria feasible on an industrial scale.
Production of PHAs from food waste using anaerobic digestion
One method that has been used to biologically convert complex food wastes to PHA precursors is anaerobic digestion using open systems of mixed microbial cultures (MMCs). The general process that has been followed in most studies using food waste has involved three steps: (i) acidogenic fermentation; (ii) culture selection; and (iii) PHA accumulation. The primary advantage to this approach is that MMCs have lower investment and operating costs, because substrate pre‐treatment processes are not required, sterilization is typically not necessary and less expensive carbon sources can be used (Reis et al., 2003; Gurieff and Lant, 2007). This is important, as analyses have indicated that the high energy expenditure used in sterilization, aeration and agitation to produce PHAs in pure cultures causes these bioplastics to have little advantage over traditional synthetic plastics in environmental impact (Gomez et al., 2012; Koller et al., 2013). In addition, due to the greater variety of organisms working together with complex substrates in MMCs, more diverse PHAs, such as polyhydroxyhexanoate (PHH), polyhydroxyoctanoate (PHO) and polyhydroxydecanoate (PHD), are produced (Chae and Shin, 2007). This indicates that MMCs are a potentially viable option for producing PHAs from food waste.
The first step in producing PHAs from MMCs is acidogenic fermentation. During this step, complex wastes, such as food scraps, spentwash and wastewater, are broken down into simpler and smaller fermentative acids, mainly C2‐C4 acids such as acetic, propionic, butyric and lactic acids (Serafim et al., 2008; Albuquerque et al., 2012). A variety of feedstocks have been used for anaerobic production of volatile fatty acids (VFAs) from food waste, including food scraps from restaurants or kitchens (Zhang et al., 2007; Hafuka et al., 2011; Omar et al., 2011; Eshtaya et al., 2013), whey (Duque et al., 2014; Valentino et al., 2015; Colombo et al., 2016), jowar grain spentwash and rice spentwash (Khardenavis et al., 2007), tomato cannery wastewater (Liu et al., 2008), olive oil mill pomace and wastewater (Waller et al., 2012; Campanari et al., 2017), palm oil mill effluent (Din et al., 2006; Salmiati et al., 2007), sugarcane molasses (Albuquerque et al., 2007, 2011, 2012), pea shell waste (Patel et al., 2012), condensate of food waste (Chae and Shin, 2007) and fermented brewery wastewater (Mato et al., 2008; Ben et al., 2016) (see Table 3 and Table 4). Applying pre‐treatments such as filtering and deproteinization to these waste streams has been examined, with mixed results (Khardenavis et al., 2007; Liu et al., 2008). Another pre‐treatment is buffering the waste solution to keep pH between 5.5 and 7.0, which has been shown to improve VFA production (Zeng et al., 2006; Waller et al., 2012; Eshtaya et al., 2013). A variety of food waste sources have been used to produce VFAs for use by cultures of bacteria that accumulate PHAs.
Table 3.
Production of PHAs from anaerobically digested food waste
| Food waste source | Microorganisms | PHA polymer type | Cultivation | Maximum PHA Production Reported (g PHA g−1 dcw) | References |
|---|---|---|---|---|---|
| Whey | Wastewater microbes | PHBV | Flask, batch | NA | Valentino et al. (2015) |
| Whey | Pre‐selected mixed microbial culture | PHBV | Flask, batch | 81% | Colombo et al. (2016) |
| Whey | Activated sludge consortium | PHBV | Three‐stage reactors system | 65% | Duque et al. (2014) |
| Sugarcane molasses | Activated sludge consortium | PHBV | Three‐stage reactors system | 56% | Duque et al. (2014) |
| Brewery wastewater | Activated sludge consortium | PHBV | SBR | 39% | Ben et al. (2016) |
| Food processing wastewater effluent | Activated sludge consortium | PHB | Flask, batch | 60.70% | Khardenavis et al. (2007) |
| Jowar grain‐based distillery spentwash | Activated sludge consortium | PHB | Flask, batch | 42.30% | Khardenavis et al. (2007) |
| Rice grain‐based distillery spentwash | Activated sludge consortium | PHB | Flask, batch | 40% | Khardenavis et al. (2007) |
| Condensate of food waste | Enriched activated sludge consortium | PHBV and mcl‐PHAs | VSMBR | 1.8% | Chae and Shin (2007) |
| Olive oil mill pomace | Activated sludge consortia | PHBV | SBR | 39% | Waller et al. (2012) |
| Olive oil mill wastewater | Wastewater microbes | PHBV | SBR | 11.3% | Campanari et al. (2017) |
| Tomato wastewater | Activated sludge consortium | PHA | Fermenter, batch | 20% | Liu et al. (2008) |
| Fermented food waste | Wastewater microbes | PHA | Fermenter, anaerobic/aerobic | 51% | Rhu et al. (2003) |
| Fermented molasses | Mixed microbial culture | PHBV | Fermenter, pulse feed | 56% | Albuquerque et al. (2011) |
| Fermented molasses | Mixed microbial culture | PHBV | Fermenter, batch | 60.50% | Albuquerque et al. (2012) |
VSMBR is a vertical submerged membrane bioreactor; SBR is stirred batch reactor.
Table 4.
Production of PHAs from organic acids derived from anaerobically digested food waste
| Food waste source for organic acids | Microorganism | PHA polymer type | Cultivation | Dry cell Weight (g l−1) | Maximum PHA production Reported (g PHA g−1 dcw) | References |
|---|---|---|---|---|---|---|
| Restaurant waste | Recombinant E. coli pnDTM2 | PHB | Fermenter, batch | 2.9 | 45% | Eshtaya et al. (2013) |
| Restaurant waste | C. necator H16 | PHB | Fermenter, continuous feeding | 1.4 | 87% | Hafuka et al. (2011) |
| Food scraps from cafeteria | C. necator | PHBV | Fermenter, batch | 22.7 | 72.60% | Du and Yu (2002) |
| Kitchen waste |
C. necator
CCGUG 52238 |
PHB | Fermenter, batch | 4.6 | 52.79% | Omar et al. (2011) |
| Pea shells |
Bacillus cereus
Strain EGU3 |
PHB | Fermenter, batch | 1.32 | 71% | Patel et al. (2012) |
The second step of producing PHAs from mixed cultures is culture selection. During this step, bacteria are subjected to alternating conditions to obtain a microbial community where almost all microorganisms have a high PHA‐storing capacity and production rate. This is often carried out in sequencing batch reactors (SBRs), compact systems where the full feast and famine cycle is performed in one single reactor, and the length of each phase could be varied. The cycle may be either alternating conditions of external substrate excess (feast) and limitation (famine) in aerobic conditions or alternating anoxic and aerobic microenvironments. In both cases, limiting cell growth (through famine or anoxic conditions) increases PHA production and presents pressures that allow PHA‐producing strains to become predominant (Serafim et al., 2008). Using microautoradiography and fluorescence in situ hybridization (FISH), Albuquerque et al. (2012) found that in a culture grown on molasses, bacteria that their process selected for were primarily from the genera Azoarcus, Thauera and Paracoccus. Each of these populations specialized in digesting specific products of the acidogenic fermentation. Azoarcus and Thauera primarily consumed acetate and butyrate, respectively, while Paracoccus consumed a broader range of substrates. Other studies on culture selection have indicated that PHA‐storing bacteria in MMC are predominantly from the classes of Alphaproteobacteria, Betaproteobacteria and Gammaproteobacteria, as has been reviewed elsewhere (Serafim et al., 2008; Morgan‐Sagastume, 2016).
The third step of producing PHAs from MMCs is PHA accumulation. This step is designed to maximize PHA production in cells harvested from the enrichment bioreactor. The feast–famine cycle (FF) is known to increase synthesis and storage of PHA granules (Villano et al., 2010). Alternating anoxic and aerobic conditions have also been used in SBRs to improve PHA production from food waste. It has been documented that anoxic microenvironments tend to promote higher PHA accumulation due to better access to VFAs and lack of an electron acceptor, while aerobic environments tend to promote PHA degradation, but better nutrient removal (Venkateswar Reddy and Venkata Mohan, 2012). The alternating conditions pressure the cells to uptake nutrients and convert them to PHAs.
Several problems exist with MMCs. First, they generally have lower performance than pure cultures (when measured by volumetric productivity). This is due, in part, to lower cell concentrations that are usually found in MMCs. Often, concentrations are < 10 g l−1 compared to values greater than 100 g l−1 that can be found in pure culture studies. Further, information on the quality of PHAs produced in MMCs is scarce (Albuquerque et al., 2011). Another concern is that many of the organic acids produced during MMC fermentation inhibit bacterial growth, causing decreased productivity. These issues must be overcome to make MMCs a viable approach to producing PHAs from inexpensive carbon sources.
Indirect coupling of MMC to PHA production
One approach to overcoming some of the difficulties associated with MMCs is using an indirect coupling approach where complex food wastes are digested in an MMC to produce VFAs, which are then harvested and fed to a pure culture fermentation (see Table 4). This increases the chance that PHA polymers will be consistent in their quality and produced in high concentrations (Du and Yu, 2002). Like the MMCs, complex food wastes and other inexpensive carbon sources like food scraps from restaurants or kitchens (Du and Yu, 2002; Du et al., 2004; Hafuka et al., 2011; Omar et al., 2011; Eshtaya et al., 2013) or pea shells (Patel et al., 2012) can be used as nutrient sources for PHA production with minimal pre‐treatment steps.
One of the main difficulties of the indirect coupling process is efficiently harvesting the VFAs and transferring them to a pure culture fermenter. This was initially achieved using evaporation and ion exchange, but the processes were costly. In one of the earliest alternative approaches, Du and Yu (2002) and Du et al. (2004) compared using a silicone membrane with a dialysis membrane to diffuse the acids into an air‐bubbling reactor, while preventing solids from mixing. The dialysis membrane worked considerably better, allowing a maximum dry cell weight and PHBV concentration of 22.7 g l−1 and 72.6%, respectively, compared to a maximum of 11.3 g l−1 and 60.2% PHB (as opposed to the PHBV) when a silicon membrane was used. The primary problem was that the process still consumed a high amount of operational energy due to small membrane pore sizes. A subsequent study saw success with harvesting slurry once a week and filtering the VFAs using a 0.45‐μm filter (Hafuka et al., 2011). Another difficulty with indirect coupling is that the VFAs are inhibitory to growth in high concentrations, which means that it is often desirable to use fed‐batch approaches to keep concentrations low, while ensuring that VFAs are available for consumption (Omar et al., 2011). Despite these problems, using MMC to produce VFAs that are utilized by pure cultures is a way to combine many of the best aspects of pure culture and MMC production of PHAs.
Conclusions and outlook
Utilizing food waste to create PHAs has potential for long term applications, but is not without some hurdles. In most cases, many of the food waste sources were locally procured and this is an important consideration as transportation of food waste to the source of the microbial PHA production systems could be cost prohibitive. Furthermore, the contributions of food waste pre‐treatment to overall process costs of microbial PHA synthesis need to be examined in‐depth. Ideally, pre‐treatment of food waste should be kept to a minimal to reduce time and cost. PHA extraction from microbial biomass should also be considered in a technoeconomic analysis. The most common PHA extraction methods are solvent‐based extraction; however, there are a variety of different methods that can be used (Sabirova et al., 2006; Madkour et al., 2013; Rahman et al., 2013). In addition, a biorefinery concept could be realized with food waste being the feedstock to producing PHAs and additional products, as has been proposed by others (Lin et al., 2013; Pfaltzgraff et al., 2013; Dietrich et al., 2017). Biorefineries offer the advantages of not depending on a single product to be produced and thus are flexible and potentially sustainable.
The microbes used to convert food waste to PHAs are diverse, ranging from known, well‐defined microorganisms to mixed microbial consortia. As mentioned, both natively accumulating PHA strains and genetically engineered strains could be used as platforms for PHA production. Strain selection is important aspect of PHA production and bioprospecting could lead to the discovery of additional microbes that can be used as PHA production strains in the future (Prieto, 2016). In addition to engineering microbes to produce PHAs, microbes could also be optimized to use specific food substrates and generate defined chain‐length PHAs.
Food wastage is a global issue; the ability to upgrade complex carbon substrates into a tangible product such as PHAs could help reduce the burden of waste processing by municipalities. As described here, many different routes on food waste conversion to PHAs exist. There is not a single solution to a specific type of food waste, rather there are multiple paths that could be taken, each with different pros and cons. New and innovative methods of food waste processing to PHAs will continue to grow in the future, and these emerging technologies could make the economic production of microbial PHAs a reality in the not‐to‐distant future.
Conflict of interest
None declared.
Acknowledgements
The authors would also like to acknowledge the Department of Biological Engineering at Utah State University. Asad Ur Rehman was supported by a U.S. Fulbright Scholarship.
Microbial Biotechnology (2017) 10(6), 1338–1352
Funding Information
No funding information provided.
References
- Agnew, D.E. , and Pfleger, B.F. (2013) Synthetic biology strategies for synthesizing polyhydroxyalkanoates from unrelated carbon sources. Chem Eng Sci 103: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, W.S. , Park, S.J. , and Lee, S.Y. (2000) Production of poly (3‐Hydroxybutyrate) by fed‐batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol 66: 3624–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, W.S. , Park, S.J. , and Lee, S.Y. (2001) Production of poly (3‐hydroxybutyrate) from whey by cell recycle fed‐batch culture of recombinant Escherichia coli . Biotechnol Lett 23: 235–240. [Google Scholar]
- Akaraonye, E. , Keshavarz, T. , and Roy, I. (2010) Production of polyhydroxyalkanoates: the future green materials of choice. J Chem Technol Biotechnol 85: 732–743. [Google Scholar]
- Albuquerque, M.G.E. , Eiroa, M. , Torres, C. , Nunes, B.R. , and Reis, M.A.M. (2007) Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol 130: 411–421. [DOI] [PubMed] [Google Scholar]
- Albuquerque, M.G.E. , Martino, V. , Pollet, E. , Avérous, L. , and Reis, M.A.M. (2011) Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)‐rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J Biotechnol 151: 66–76. [DOI] [PubMed] [Google Scholar]
- Albuquerque, M.G.E. , Carvalho, G. , Kragelund, C. , Silva, A.F. , Barreto Crespo, M.T. , Reis, M.A.M. and Nielsen, P.H. (2012) Link between microbial composition and carbon substrate‐uptake preferences in a PHA‐storing community. ISME J 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Hamamre, Z. , Foerster, S. , Hartmann, F. , Kröger, M. , and Kaltschmitt, M. (2012) Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 96: 70–76. [Google Scholar]
- Anderson, A.J. , and Dawes, E.A. (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54: 450–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben, M. , Kennes, C. , and Veiga, M.C. (2016) Optimization of polyhydroxyalkanoate storage using mixed cultures and brewery wastewater. J Chem Technol Biotechnol 91: 2817–2826. [Google Scholar]
- Bhardwaj, U. , Dhar, P. , Kumar, A. , and Katiyar, V. (2014) Based Nanobiocomposites for Food Packaging In Food Additives and Packaging American Chemical Society Symposium Series. Komolprasert V., and Turowski P. (eds). Washington, DC: American Chemical Society, pp. 275–314. [Google Scholar]
- Bhattacharyya, A. , Pramanik, A. , Maji, S.K. , Haldar, S. , Mukhopadhyay, U.K. , and Mukherjee, J. (2012) Utilization of vinasse for production of poly‐3‐(hydroxybutyrate‐co‐hydroxyvalerate) by Haloferax mediterranei . AMB Express 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, A. , Saha, J. , Haldar, S. , Bhowmic, A. , Mukhopadhyay, U.K. , and Mukherjee, J. (2014) Production of poly‐3‐(hydroxybutyrate‐co‐hydroxyvalerate) by Haloferax mediterranei using rice‐based ethanol stillage with simultaneous recovery and re‐use of medium salts. Extremophiles 18: 463–470. [DOI] [PubMed] [Google Scholar]
- Bio‐on (2017) Bio‐on | Where. Bio‐on website.
- Bordes, P. , Pollet, E. , and Avérous, L. (2009) Nano‐biocomposites: biodegradable polyester/nanoclay systems. Prog Polym Sci 34: 125–155. [Google Scholar]
- Bugnicourt, E. , Cinelli, P. , Lazzeri, A. , and Alvarez, V. (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym Lett 8: 791–808. [Google Scholar]
- Campanari, S. , Augelletti, F. , Rossetti, S. , Sciubba, F. , Villano, M. , and Majone, M. (2017) Enhancing a multi‐stage process for olive oil mill wastewater valorization towards polyhydroxyalkanoates and biogas production. Chem Eng J 317: 280–289. [Google Scholar]
- Cesário, M.T. , Raposo, R.S. , de Almeida, M.C.M.D. , van Keulen, F. , Ferreira, B.S. , and da Fonseca, M.M.R. (2014) Enhanced bioproduction of poly‐3‐hydroxybutyrate from wheat straw lignocellulosic hydrolysates. N Biotechnol 31: 104–113. [DOI] [PubMed] [Google Scholar]
- Chae, S.R. , and Shin, H.S. (2007) Effect of condensate of food waste (CFW) on nutrient removal and behaviours of intercellular materials in a vertical submerged membrane bioreactor (VSMBR). Bioresour Technol 98: 373–379. [DOI] [PubMed] [Google Scholar]
- Chandrapala, J. , Duke, M.C. , Gray, S.R. , Zisu, B. , Weeks, M. , Palmer, M. , and Vasiljevic, T. (2015) Properties of acid whey as a function of pH and temperature. J Dairy Sci 98: 4352–4363. [DOI] [PubMed] [Google Scholar]
- Chanprateep, S. (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110: 621–632. [DOI] [PubMed] [Google Scholar]
- Chaudhry, W.N. , Jamil, N. , Ali, I. , Ayaz, M.H. , and Hasnain, S. (2011) Screening for polyhydroxyalkanoate (PHA)‐producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol 61: 623–629. [Google Scholar]
- Chen, G.‐Q. , and Patel, M.K. (2011) Plastics derived from biological sources: present and future: a technical and environmental review. Chem Rev 112: 2082–2099. [DOI] [PubMed] [Google Scholar]
- Chien, C.C. , and Ho, L.Y. (2008) Polyhydroxyalkanoates production from carbohydrates by a genetic recombinant Aeromonas sp. Lett Appl Microbiol 47: 587–593. [DOI] [PubMed] [Google Scholar]
- Choi, J. , and Lee, S.Y. (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51: 13–21. [Google Scholar]
- Colombo, B. , Pepè, T. , Reis, M. , Scaglia, B. , and Adani, F. (2016) Bioresource technology polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour Technol 218: 692–699. [DOI] [PubMed] [Google Scholar]
- Cruz, M.V. , Paiva, A. , Lisboa, P. , Freitas, F. , Alves, V.D. , Simões, P. , et al (2014) Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour Technol 157: 360–363. [DOI] [PubMed] [Google Scholar]
- Cuéllar, A.D. , and Webber, M.E. (2010) Wasted food, wasted energy: the embedded energy in food waste in the United States. Environ Sci Technol 44: 6464–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, K. , Dumont, M.J. , Del Rio, L.F. , and Orsat, V. (2017) Producing PHAs in the bioeconomy – Towards a sustainable bioplastic. Sustain Prod Consum 9: 58–70. [Google Scholar]
- Din, M.F.M. , Ujang, Z. , van Loosdrecht, M.C. , Ahmad, A. , and Sairan, M.F. (2006) Optimization of nitrogen and phosphorus limitation for better biodegradable plastic production and organic removal using single fed‐batch mixed cultures and renewable resources. Water Sci Technol 53: 15–20. [DOI] [PubMed] [Google Scholar]
- Dinjaski, N. , and Prieto, M.A. (2015) Smart polyhydroxyalkanoate nanobeads by protein based functionalization. Nanomed Nanotechnol Biol Med 11: 885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzyzga, O. , Revelles, O. , Durante‐Rodríguez, G. , Díaz, E. , García, J.L. , and Prieto, A. (2015) New challenges for syngas fermentation: towards production of biopolymers. J Chem Technol Biotechnol 90: 1735–1751. [Google Scholar]
- Du, G. , and Yu, J. (2002) Green technology for conversion of food scraps to biodegradable thermoplastic polyhydroxyalkanoates. Environ Sci Technol 36: 5511–5516. [DOI] [PubMed] [Google Scholar]
- Du, G. , Chen, L.X.L. , and Yu, J. (2004) High‐efficiency production of bioplastics from biodegradable organic solids. J Polym Environ 12: 89–94. [Google Scholar]
- Duque, A.F. , Oliveira, C.S.S. , Carmo, I.T.D. , Gouveia, A.R. , Pardelha, F. , Ramos, A.M. , et al (2014) Response of a three‐stage process for PHA production by mixed microbial cultures to feedstock shift: impact on polymer composition. N Biotechnol 31: 5–11. [DOI] [PubMed] [Google Scholar]
- Escapa, I.F. , García, J.L. , Bühler, B. , Blank, L.M. , and Prieto, M.A. (2012) The polyhydroxyalkanoate metabolism controls carbon and energy spillage in Pseudomonas putida . Environ Microbiol 14: 1049–1063. [DOI] [PubMed] [Google Scholar]
- Escapa, I.F. , del Cerro, C. , García, J.L. , and Prieto, M.A. (2013) The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol 15: 93–110. [DOI] [PubMed] [Google Scholar]
- Eshtaya, M.K. , Rahman, N.A. , and Hassan, M.A. (2013) Bioconversion of restaurant waste into Polyhydroxybutyrate (PHB) by recombinant E. coli through anaerobic digestion. Int J Environ Waste Manag 11: 27–37. [Google Scholar]
- Girotto, F. , Alibardi, L. , and Cossu, R. (2015) Food waste generation and industrial uses: a review. Waste Manag 45: 32–41. [DOI] [PubMed] [Google Scholar]
- Gomez, J.G.C. , Méndez, B.S. , Nikel, P.I. , Pettinari, M.J. , Prieto, M.a. and Silva, L.F. (2012) Making green polymers even greener : towards sustainable production of polyhydroxyalkanoates from agroindustrial by‐products. In Advances in Applied Biotechnology Petre M. (ed.), pp 41–62. [Google Scholar]
- Gurieff, N. , and Lant, P. (2007) Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresour Technol 98: 3393–3403. [DOI] [PubMed] [Google Scholar]
- Gustavsson, M. , and Lee, S.Y. (2016) Prospects of microbial cell factories developed through systems metabolic engineering. Microb Biotechnol 9: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafuka, A. , Sakaida, K. , Satoh, H. , Takahashi, M. , Watanabe, Y. , and Okabe, S. (2011) Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator . Bioresour Technol 102: 3551–3553. [DOI] [PubMed] [Google Scholar]
- Hong, K. , Leung, Y.C. , Kwok, S.Y. , Law, K.H. , Lo, W.H. , Chua, H. , and Yu, P.H. (2000) Construction of recombinant Escherichia coli strains for polyhydroxybutyrate production using soy waste as nutrient. Appl Biochem Biotechnol 84–86: 381–390. [DOI] [PubMed] [Google Scholar]
- Kenny, S.T. , Runic, J.N. , Kaminsky, W. , Woods, T. , Babu, R.P. , and O'Connor, K.E. (2012) Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl Microbiol Biotechnol 95: 623–633. [DOI] [PubMed] [Google Scholar]
- Keshavarz, T. , and Roy, I. (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol 13: 321–326. [DOI] [PubMed] [Google Scholar]
- Khardenavis, A.A. , Suresh Kumar, M. , Mudliar, S.N. , and Chakrabarti, T. (2007) Biotechnological conversion of agro‐industrial wastewaters into biodegradable plastic, poly β‐hydroxybutyrate. Bioresour Technol 98: 3579–3584. [DOI] [PubMed] [Google Scholar]
- Kim, B.S. (2000) Production of poly(3‐hydroxybutyrate) from inexpensive substrates. Enzyme Microb Technol 27: 774–777. [DOI] [PubMed] [Google Scholar]
- Kim, B.S. , Lee, S.C. , Lee, S.Y. , Chang, H.N. , Chang, Y.K. , and Woo, S.I. (1994) Production of poly(3‐hydroxybutyric‐co‐3‐hydroxyvaleric acid) by fed‐batch culture of Alcaligenes eutrophus with substrate control using on‐line glucose analyzer. Enzyme Microb Technol 16: 556–561. [Google Scholar]
- Koller, M. (2015) Recycling of waste streams of the biotechnological poly (hydroxyalkanoate) production by Haloferax mediterranei on whey. Int J Polym Sci 2015: 1–8. [Google Scholar]
- Koller, M. , Bona, R. , Braunegg, G. , Hermann, C. , Horvat, P. , Kroutil, M. , et al (2005) Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromol 6: 561–565. [DOI] [PubMed] [Google Scholar]
- Koller, M. , Sandholzer, D. , Salerno, A. , Braunegg, G. , and Narodoslawsky, M. (2013) Resources, conservation and recycling biopolymer from industrial residues: life cycle assessment of poly(hydroxyalkanoates) from whey. Resour Conserv Recycl 73: 64–71. [Google Scholar]
- Kulpreecha, S. , Boonruangthavorn, A. , Meksiriporn, B. , and Thongchul, N. (2009) Inexpensive fed‐batch cultivation for high poly(3‐hydroxybutyrate) production by a new isolate of Bacillus megaterium . J Biosci Bioeng 107: 240–245. [DOI] [PubMed] [Google Scholar]
- Kumalaningsih, S. , Hidayat, N. , and Aini, N. (2011) Optimization of polyhydroxyalkanoates (PHA) Production from liquid bean curd waste by Alcaligenes Latus bacteria. J Agric Food Technol 1: 63–67. [Google Scholar]
- Law, K.H. , Chan, P.L. , Lau, W.S. , Cheng, Y.C. , Leung, Y.C. , Lo, W.H. , et al (2004) Construction of recombinant Escherichia coli strains for production of poly‐(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate). Appl Biochem Biotechnol 113–116: 361–372. [PubMed] [Google Scholar]
- Lee, S.Y. (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49: 1–14. [DOI] [PubMed] [Google Scholar]
- Lee, S.Y. , Middelberg, A.P.J. , and Lee, Y.K. (1997) Poly (3‐hydroxybutyrate) production from whey using recombinant Escherichia coli . Biotechnol Lett 19: 1033–1035. [Google Scholar]
- Lievore, P. , Simões, D.R.S. , and Silva, K.M. (2015) Chemical characterisation and application of acid whey in fermented milk. J Food Sci Technol 52: 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.S.K. , Pfaltzgraff, L.A. , Herrero‐Davila, L. , Mubofu, E.B. , Abderrahim, S. , Clark, J.H. , et al (2013) Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci 6: 426–464. [Google Scholar]
- Linton, E. , Rahman, A. , Viamajala, S. , Sims, R.C. , and Miller, C.D. (2012) Polyhydroxyalkanoate quantification in organic wastes and pure cultures using a single‐step extraction and 1H NMR analysis. Water Sci Technol 66: 1000–1006. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Hall, P.V. , Darby, J.L. , Coats, E.R. , Green, P.G. , Thompson, D.E. , and Loge, F.J. (2008) Production of polyhydroxyalkanoate during treatment of tomato cannery wastewater. Water Environ Res 80: 367–372. [DOI] [PubMed] [Google Scholar]
- Lopes, M.S.G. , Gomez, J.G.C. , Taciro, M.K. , Mendonça, T.T. , and Silva, L.F. (2014) Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. J Ind Microbiol Biotechnol 41: 1353–1363. [DOI] [PubMed] [Google Scholar]
- Madkour, M.H. , Heinrich, D. , Alghamadi, M.A. , Shabbaj, I.I. , and Steinbüchel, A. (2013) PHA recovery from biomass. Biomacromol 14: 2963–2972. [DOI] [PubMed] [Google Scholar]
- Matharu, A.S. , de Melo, E.M. and Houghton, J.A. (2016) Opportunity for high value‐added chemicals from food supply chain wastes. Bioresour Technol 215: 123–130. [DOI] [PubMed] [Google Scholar]
- Mato, T. , Ben, M. , Kennes, C. , and Veiga, M. (2008) PHA production using brewery wastewater Proceedings of 4th IWA Specialised Conference on Sequencing Batch Reactor Technology (SBR4)), 7–10 April, Rome, Italy, pp. 59–66. [Google Scholar]
- Morgan‐Sagastume, F. (2016) Characterisation of open, mixed microbial cultures for polyhydroxyalkanoate (PHA) production. Rev Environ Sci Biotechnol 15: 1–33. [Google Scholar]
- Mozejko‐Ciesielska, J. , and Kiewisz, R. (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res 192: 271–282. [DOI] [PubMed] [Google Scholar]
- Munoz, L.E.A. , and Riley, M.R. (2008) Utilization of cellulosic waste from tequila bagasse and production of polyhydroxyalkanoate (pha) bioplastics by Saccharophagus degradans . Biotechnol Bioeng 100: 882–888. [DOI] [PubMed] [Google Scholar]
- Nikel, P.I. , De Almeida, A. , Melillo, E.C. , Galvagno, M.A. , and Pettinari, M.J. (2006) New recombinant Escherichia coli strain tailored for the production of poly(3‐hydroxybutyrate) from agroindustrial by‐products. Appl Environ Microbiol 72: 3949–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obruca, S. , Benesova, P. , Petrik, S. , Oborna, J. , Prikryl, R. , and Marova, I. (2014a) Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem 49: 1409–1414. [Google Scholar]
- Obruca, S. , Petrik, S. , Benesova, P. , Svoboda, Z. , Eremka, L. , and Marova, I. (2014b) Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl Microbiol Biotechnol 98: 5883–5890. [DOI] [PubMed] [Google Scholar]
- Obruca, S. , Benesova, P. , Marsalek, L. , and Marova, I. (2015) Use of lignocellulosic materials for PHA production. Chem Biochem Eng Q 29: 135–144. [Google Scholar]
- Omar, F.N. , Rahman, N.A.A. , Hafid, H.S. , Mumtaz, T. , Yee, P.L. , and Hassan, M.A. (2011) Utilization of kitchen waste for the production of green thermoplastic polyhydroxybutyrate (PHB) by Cupriavidus necator CCGUG 52238. Afr J Microbiol 5: 2873–2879. [Google Scholar]
- Pais, J. , Farinha, I. , Freitas, F. , Serafim, L.S. , Martínez, V. , Martínez, J.C. , et al (2014) Improvement on the yield of polyhydroxyalkanotes production from cheese whey by a recombinant Escherichia coli strain using the proton suicide methodology. Enzyme Microb Technol 55: 151–158. [DOI] [PubMed] [Google Scholar]
- Pais, J. , Serafim, S. , Freitas, F. , and Reis, M.A.M. (2016) Conversion of cheese whey into poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) by Haloferax mediterranei . N Biotechnol 33: 224–230. [DOI] [PubMed] [Google Scholar]
- Pantazaki, A.A. , Papaneophytou, C.P. , Pritsa, A.G. , Liakopoulou‐kyriakides, M. , and Kyriakidis, D.A. (2009) Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem 44: 847–853. [Google Scholar]
- Parfitt, J. , Barthel, M. , and Macnaughton, S. (2010) Food waste within food supply chains: quantification and potential for change to 2050. Philos Trans R Soc Lond B Biol Sci 365: 3065–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.J. , Park, J.P. , and Lee, S.Y. (2002) Production of poly(3‐hydroxybutyrate) from whey by fed‐batch culture of recombinant Escherichia coli in a pilot‐scale fermenter. Biotechnol Lett 24: 185–189. [Google Scholar]
- Patel, S.K.S. , Singh, M. , Kumar, P. , Purohit, H.J. , and Kalia, V.C. (2012) Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea‐shells. Biomass Bioenerg 36: 218–225. [Google Scholar]
- Pescuma, M. , Valdez, G.F.De. , and Mozzi, F. (2015) Whey‐derived valuable products obtained by microbial fermentation. Appl Microbiol Biotechnol 99: 6183–6196. [DOI] [PubMed] [Google Scholar]
- Pfaltzgraff, L.a. , De bruyn, M. , Cooper, E.C. , Budarin, V. and Clark, J.H. (2013) Food waste biomass: a resource for high‐value chemicals. Green Chem 15, 307–314. [Google Scholar]
- Poomipuk, N. , Reungsang, A. , and Plangklang, P. (2014) Poly‐β‐hydroxyalkanoates production from cassava starch hydrolysate by Cupriavidus sp. KKU38. Int J Biol Macromol 65: 51–64. [DOI] [PubMed] [Google Scholar]
- Povolo, S. , Toffano, P. , Basaglia, M. , and Casella, S. (2010) Polyhydroxyalkanoates production by engineered Cupriavidus necator from waste material containing lactose. Bioresour Technol 101: 7902–7907. [DOI] [PubMed] [Google Scholar]
- Pramanik, A. , Mitra, A. , Arumugam, M. , Bhattacharyya, A. , Sadhukhan, S. , Ray, A. , et al (2012) Utilization of vinasse for the production of polyhydroxybutyrate by Haloarcula marismortui . Folia Microbiol (Praha) 57: 71–79. [DOI] [PubMed] [Google Scholar]
- Prieto, A. (2016) To be, or not to be biodegradable… that is the question for the bio‐based plastics. Microb Biotechnol 9: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, A. , Linton, E. , Hatch, A.D. , Sims, R.C. , and Miller, C.D. (2013) Secretion of polyhydroxybutyrate in Escherichia coli using a synthetic biological engineering approach. J Biol Eng 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, A. , Anthony, R.J. , Sathish, A. , Sims, R.C. , and Miller, C.D. (2014) Effects of wastewater microalgae harvesting methods on polyhydroxybutyrate production. Bioresour Technol 156: 364–367. [DOI] [PubMed] [Google Scholar]
- Rahman, A. , Putman, R.J. , Inan, K. , Sal, F.A. , Sathish, A. , Smith, T. , et al (2015) Polyhydroxybutyrate production using a wastewater microalgae based media. Algal Res 8: 95–98. [Google Scholar]
- Reddy, C.S.K. , Ghai, R. , Rashmi, and Kalia, V.C. (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87: 137–146. [DOI] [PubMed] [Google Scholar]
- Rehm, B.H.A. (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8: 578–592. [DOI] [PubMed] [Google Scholar]
- Reis, M.A.M. , Serafim, L.S. , Lemos, P.C. , Ramos, A.M. , Aguiar, F.R. , and Van Loosdrecht, M.C.M. (2003) Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst Eng 25: 377–385. [DOI] [PubMed] [Google Scholar]
- Rhu, D. H. , Lee, W. H. , & Kim, J. Y. and Choi, E. (2003) Polyhydroxyalkanoate (PHA) production from waste. Water Sci Technol 48: 221–228. [PubMed] [Google Scholar]
- Ryan, M.P. , and Walsh, G. (2016) The biotechnological potential of whey. Rev Environ Sci Bio/Technol 15: 479–498. [Google Scholar]
- Sabirova, J.S. , Ferrer, M. , Lünsdorf, H. , Wray, V. , Kalscheuer, R. , Steinbüchel, A. , et al (2006) Mutation in a “tesB‐like” hydroxyacyl‐coenzyme A‐specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J Bacteriol 188: 8452–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmiati, Salmiati Ujang, Z. , Salim, M.R. , Md Din, M.F. and Ahmad, M.A. (2007) Intracellular biopolymer productions using mixed microbial cultures from fermented POME. Water Sci Technol 56: 179–185. [DOI] [PubMed] [Google Scholar]
- Serafim, L.S. , Lemos, P.C. , Albuquerque, M.G.E. , and Reis, M.A.M. (2008) Strategies for PHA production by mixed cultures and renewable waste materials. Appl Microbiol Biotechnol 81: 615–628. [DOI] [PubMed] [Google Scholar]
- Silva, L.F. , Taciro, M.K. , Raicher, G. , Piccoli, R.A.M. , Mendonça, T.T. , Lopes, M.S.G. , and Gomez, J.G.C. (2014) Perspectives on the production of polyhydroxyalkanoates in biorefineries associated with the production of sugar and ethanol. Int J Biol Macromol 71: 2–7. [DOI] [PubMed] [Google Scholar]
- Sindhu, R. , Silviya, N. , Binod, P. , and Pandey, A. (2013) Pentose‐rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly‐3‐hydroxybutyrate. Biochem Eng J 78: 67–72. [Google Scholar]
- Sindhu, R. , Gnansounou, E. , Binod, P. , and Pandey, A. (2016) Bioconversion of sugarcane crop residue for value added products – An overview. Renew Energy 98: 203–215. [Google Scholar]
- Stenmarck, Å. , Jensen, C. , Quested, T. and Moates, G. (2016) Estimates of European Food Waste Levels. IVL Swedish Environmental Research Institute. [Google Scholar]
- Strong, P. , Laycock, B. , Mahamud, S. , Jensen, P. , Lant, P. , Tyson, G. , and Pratt, S. (2016) The opportunity for high‐performance biomaterials from methane. Microorganisms 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, I. , Kagotani, K. , and Kimura, Y. (2003) Microbial production of poly(hydroxyalkanoate)s from waste edible oils. Green Chem 5: 545–548. [Google Scholar]
- US EPA (2015) Advancing sustainable materials management: facts and figures 2013. United States Environ Prot Agency 1–16. [Google Scholar]
- Valentino, F. , Riccardi, C. , Campanari, S. , Pomata, D. , and Majone, M. (2015) Bioresource technology fate of b‐hexachlorocyclohexane in the mixed microbial cultures (MMCs) three‐stage polyhydroxyalkanoates (PHA) production process from cheese whey. Bioresour Technol 192: 304–311. [DOI] [PubMed] [Google Scholar]
- Van‐Thuoc, D. , Quillaguamán, J. , Mamo, G. , and Mattiasson, B. (2008) Utilization of agricultural residues for poly(3‐hydroxybutyrate) production by Halomonas boliviensis LC1. J Appl Microbiol 104: 420–428. [DOI] [PubMed] [Google Scholar]
- Venkateswar Reddy, M. , and Venkata Mohan, S. (2012) Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour Technol 103: 313–321. [DOI] [PubMed] [Google Scholar]
- Villano, M. , Beccari, M. , Dionisi, D. , Lampis, S. , Miccheli, A. , Vallini, G. , and Majone, M. (2010) Effect of pH on the production of bacterial polyhydroxyalkanoates by mixed cultures enriched under periodic feeding. Process Biochem 45: 714–723. [Google Scholar]
- Waller, J.L. , Green, P.G. , and Loge, F.J. (2012) Mixed‐culture polyhydroxyalkanoate production from olive oil mill pomace. Bioresour Technol 120: 285–289. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Yu, H. , Xia, Y. , Kang, Z. , and Qi, Q. (2009) Complete PHB mobilization in Escherichia coli enhances the stress tolerance: a potential biotechnological application. Microb Cell Fact 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Sharma‐Shivappa, R.R. , Olson, J.W. , and Khan, S.A. (2013) Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind Crops Prod 43: 802–811. [Google Scholar]
- Wierckx, N. , Prieto, M.A. , Pomposiello, P. , de Lorenzo, V. , O'Connor, K. , and Blank, L.M. (2015) Plastic waste as a novel substrate for industrial biotechnology. Microb Biotechnol 8: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L. (1998) Poly‐(3‐hydroxybutyrate) production from whey by high‐density cultivation of recombinant Escherichia coli . Appl Microbiol Biotechnol 50: 30–33. [DOI] [PubMed] [Google Scholar]
- Wu, Q. , Wang, Y. , and Chen, G.Q. (2009) Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif Cells Blood Substitutes Immobil Biotechnol 37: 1–12. [DOI] [PubMed] [Google Scholar]
- Yu, J. , and Stahl, H. (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour Technol 99: 8042–8048. [DOI] [PubMed] [Google Scholar]
- Yu, P.H. , Chua, H. , Huang, A.L. , Lo, W. and Chen, G.Q. (1998) Conversion of food industrial wastes into bioplastics. Appl Biochem Biotechnol 70–72, 603–614. [DOI] [PubMed] [Google Scholar]
- Yu, P.H. , Chua, H. , Huang, A.‐L. , and Ho, K.‐P. (1999) Conversion of industrial food wastes by Alcaligenes latus into polyhydroxyalkanoates. Appl Biochem Biotechnol 77–79: 445–454. [DOI] [PubMed] [Google Scholar]
- Zeng, R.J. , Yuan, Z. , and Keller, J. (2006) Effects of solids concentration, pH and carbon addition on the production rate and composition of volatile fatty acids in prefermenters using primary sewage sludge. Water Sci Technol 53: 263–269. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , El‐Mashad, H.M. , Hartman, K. , Wang, F. , Liu, G. , Choate, C. , and Gamble, P. (2007) Characterization of food waste as feedstock for anaerobic digestion. Bioresour Technol 98: 929–935. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Sun, W. , Wang, H. , and Geng, A. (2013) Polyhydroxybutyrate production from oil palm empty fruit bunch using Bacillus megaterium R11. Bioresour Technol 147: 307–314. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Romain, C. , and Williams, C.K. (2016) Sustainable polymers from renewable resources. Nature 540: 354–362. [DOI] [PubMed] [Google Scholar]


