Abstract
AIM
To determine the significance and need for investigation of incidental prostatic uptake in men undergoing 18F-labelled fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) for other indications.
METHODS
Hospital databases were searched over a 5-year period for patients undergoing both PET/CT and prostate magnetic resonance imaging (MRI). For the initial analysis, the prostate was divided into six sectors and suspicious or malignant sectors were identified using MRI and histopathology reports respectively. Maximum and mean 18F-FDG standardised uptake values were measured in each sector by an investigator blinded to the MRI and histopathology findings. Two age-matched controls were selected per case. Results were analysed using a paired t-test and one-way ANOVA. For the second analysis, PET/CT reports were searched for prostatic uptake reported incidentally and these patients were followed up.
RESULTS
Over a 5-year period, 15 patients underwent both PET/CT and MRI and had biopsy-proven prostate cancer. Malignant prostatic sectors had a trend to higher 18F-FDG uptake than benign sectors, however this was neither clinically nor statistically significant (3.13 ± 0.58 vs 2.86 ± 0.68, P > 0.05). 18F-FDG uptake showed no correlation with the presence or histopathological grade of tumour. 18F-FDG uptake in cases with prostate cancer was comparable to that from age-matched controls. Forty-six (1.6%) of 2846 PET/CTs over a 5-year period reported incidental prostatic uptake. Of these, 18 (0.6%) were investigated by PSA, 9 (0.3%) were referred to urology, with 3 (0.1%) undergoing MRI and/or biopsy. No cases of prostate cancer were diagnosed in patients with incidental 18F-FDG uptake in our institute over a 5-year period.
CONCLUSION
18F-FDG uptake overlaps significantly between malignant and benign prostatic conditions. Subsequent patient management was not affected by the reporting of incidental focal prostatic uptake in this cohort.
Keywords: 18F-labelled fluorodeoxyglucose, Positron emission tomography reporting, Positron emission tomography/computed tomography, Prostate cancer, Magnetic resonance imaging
Core tip: 18F-labelled fluorodeoxyglucose (18F-FDG) uptake overlaps significantly between malignant and benign prostatic conditions. In a cohort of nearly 3000 patients over a 5-year period, the reporting of incidental elevated prostatic 18F-FDG uptake did not affect subsequent clinical management or patient outcomes.
INTRODUCTION
Positron emission tomography of 18F-labelled fluorodeoxyglucose uptake combined with computed tomography (18F-FDG PET/CT) is a mainstay of oncologic imaging. PET/CT imaging is well-tolerated and therefore has become a powerful tool for the diagnosis, staging and monitoring of many metabolically-active cancers. However, 18F-FDG PET/CT imaging is not routinely used for detecting prostate cancer for both biological and technical reasons. Firstly, glucose uptake in well-differentiated prostatic adenocarcinoma is less avid than in many other cancers due to low glycolytic activity[1]. Secondly, urinary excretion of 18F-FDG in the bladder and urethra can mask pathological uptake in the adjacent prostate. Thirdly, there is a large overlap in 18F-FDG uptake between malignant disease, benign hyperplasia and inflammation of the prostate[1].
In men undergoing 18F-FDG PET/CT for unrelated reasons, incidental prostatic uptake is found in 0.6%-2.8% of studies[1-5]. Although this is a small percentage of cases, it affects a large number of men given the growing number of PET/CT studies performed per year: 50000 annually in the UK and 2 million annually in the United States[6,7]. The significance of such incidental uptake, together with the need for further investigation, is both uncertain and controversial.
A previous meta-analysis of prostatic uptake on 18F-FDG PET/CT imaging showed that PET/CT cannot reliably differentiate benign from malignant disease, although only a small percentage of these patients underwent a definitive biopsy[8]. The published positive predictive value of 18F-FDG uptake for detecting prostate cancer ranges between 30% (in a low-risk population of men with bladder cancer undergoing radical prostatectomy) to 65% [in a high-risk population of men undergoing prostate magnetic resonance imaging (MRI)][9,10]. Some studies argue that the positive predictive value is increased if 18F-FDG uptake shows a high SUVmax, the lesion is in a peripheral location and the CT demonstrates a lack of calcification[11-13]. However, these features all show considerable overlap between malignant and benign disease.
Serum prostate-specific antigen (PSA), multiparametric prostate magnetic resonance imaging (mpMRI) and prostate biopsy can be used to investigate incidental prostatic 18F-FDG uptake to determine if the patient has significant prostate cancer[5,9]. However, there is no consensus on the management of patients with incidental prostatic 18F-FDG uptake[9].
In order to better understand the significance of incidental prostatic 18F-FDG uptake, we investigated both the correlation of prostatic 18F-FDG uptake with findings from MRI and histopathology, and the impact on patient management of reporting increased 18F-FDG uptake in the prostate.
MATERIALS AND METHODS
Study design and patient population
This single-institution retrospective study was approved locally, with the need for informed consent for data analysis waived. The hospital radiology database was searched to identify a total of 2846 18F-FDG PET/CT studies performed on male patients in the period January 2010 to September 2015. For the first part of the study, 23 eligible men were identified who had both a prostate MRI and an 18F-FDG PET/CT study. 15 of these men had prostate adenocarcinoma on ultrasound-guided biopsy or MRI/ultrasound fusion biopsy. Five men were excluded (the prostate cancer was treated prior to undergoing PET/CT in 4 patients, and one patient had > 4 years between MRI and PET/CT). For the second part of the study, the 18F-FDG PET/CT reports were searched to identify patients with incidentally reported focal prostatic 18F-FDG uptake. Patient records were examined for details of follow-up investigations and management. Two cases were included in both the first and second parts of the study.
MRI and 18F-FDG PET/CT analysis
A proprietary workstation and software (Volume Viewer, Advantage Workstation, GE Healthcare, Milwaukee, WI, United States) were used to review the 18F-FDG PET/CT images. The prostate was divided into six sectors: Left and right sides at the apex, mid-zone and base of the gland. Standardised uptake values (SUV) in each sector were measured by an investigator who was blinded to the MRI and histopathological findings. A threshold of 75% of the SUVmax was used to calculate the SUVmean[14].
MRI reports were used to identify the prostatic sectors that were suspicious for tumour. Histopathology reports were used to identify the prostatic lobe(s) in which cancer had been detected. Sectorial analysis could not be performed for patients with no tumour focus on MRI, or bilateral tumour on histopathology (Figure 1).
Figure 1.
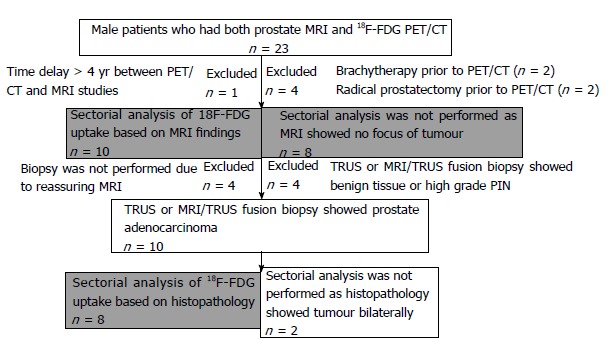
Flowchart showing inclusion and exclusion criteria for selection of cases for sector-based analysis.
Age-matched controls undergoing 18F-FDG PET/CT but without prostate cancer were randomly selected for each case from PET/CT studies recently undertaken in the department; two controls for each case were acquired. Age matching within 18 mo was used as the criterion, and patients with a known tumour close to the prostate were excluded.
Statistical analysis
A paired two-tailed student’s t-test was used to compare the 18F-FDG uptake within suspicious or malignant sectors, with that in the remaining prostate for each individual patient. A paired two-tailed student’s t-test was also used to compare prostatic 18F-FDG uptake in patients with that from the controls. A one-way ANOVA was used to compare prostatic 18F-FDG uptake between histopathological subgroups. Statistical analyses were performed using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA, United States).
RESULTS
Eighteen patients who had both 18F-FDG PET/CT and prostate MRI studies were included in the first part of the study. The median age was 72 years, median PSA was 7.30 ng/mL and median time difference between the 18F-FDG PET/CT and the prostate MRI was 11.5 mo. See Table 1 for patient characteristics.
Table 1.
Characteristics of patients who had both 18F-labelled fluorodeoxyglucose positron emission tomography/computed tomography and prostate magnetic resonance imaging studies
| Age (yr) | MRI before or after PET/CT? | 18F-FDG PET/CT indication | Prostate SUVmax | Prostate MRI indication | MRI result | PSA (ng/mL) | Biopsy result |
| 73 | 2 mo before | Bone metastases (prostate primary) | 3.4 | Negative TRUS biopsy | T2aNxMx | 20.8 | Gleason 4 + 5 = 9 |
| 72 | 11 mo after | Non-Hodgkin lymphoma | 2.7 | Elevated PSA, negative TRUS biopsy | T2aNxMx | 8.8 | Gleason 5 + 3 = 8 |
| 62 | 3 mo after | Cancer of unknown primary | 3.9 | Prostate cancer staging | T3bNxMx | 37 | Gleason 4 + 3 = 7 |
| 75 | 46 mo after | Head and neck cancer | 3.4 | Active surveillance | T1NxMx | 2.28 | Gleason 4 + 3 = 7 |
| 76 | 6 mo before | Gastrointestinal stromal tumour | 3 | Elevated PSA, negative TRUS biopsy | T2bNxMx | 150 | Gleason 3 + 4 = 7 |
| 79 | 22 mo after | Non-Hodgkin lymphoma | 2.9 | Elevated PSA | T2aNxMx | 5.4 | Gleason 3 + 4 = 7 |
| 66 | 30 mo after | Non-Hodgkin lymphoma | 3.1 | Active surveillance | T2cNxMx | 4.8 | Gleason 3 + 4 = 7 |
| 73 | 26 mo after | Oesophageal cancer | 2.4 | Active surveillance | T2cNxMx | 7.8 | Gleason 3 + 3 = 6 |
| 68 | 18 mo after | Cancer of unknown primary | 3.9 | Elevated PSA | T2aNxMx | 6.1 | Gleason 3 + 3 = 6 |
| 74 | 5 mo before | Oesophageal cancer | 3.9 | Elevated PSA | T1NxMx | 7.3 | Gleason 3 + 3 = 6 |
| 68 | 5 mo before | Hodgkin lymphoma | 9.9 | Elevated PSA, negative biopsy | No focus of tumour | 4.7 | High-grade PIN |
| 65 | 39 mo before | Colorectal cancer | 5.2 | Elevated PSA, negative TRUS biopsy | No focus of tumour | 8.6 | High-grade PIN |
| 76 | 34 mo before | Non-Hodgkin lymphoma | 4.2 | Elevated PSA | Suspicious foci bilaterally | 15 | Benign |
| 67 | 4 mo before | Non-Hodgkin lymphoma | 4.1 | Incidental prostatic 18F-FDG uptake | Suspicious foci bilaterally | 5.5 | Benign |
| 72 | 16 mo after | Pyrexia of unknown origin | 2.7 | Chronic urinary infection | Likely prostatitis | Not done | Biopsy not performed |
| 78 | 1 mo after | Non-Hodgkin lymphoma | 3.1 | Elevated PSA | No focus of tumour | 11.4 | Biopsy not performed |
| 61 | 12 mo after | Lung nodule | 5.2 | Elevated PSA, positive family history | No focus of tumour | 4.5 | Biopsy not performed |
| 68 | 7 mo after | Colorectal cancer | 8.8 | Incidental prostatic 18F-FDG uptake | No focus of tumour | 3 | Biopsy not performed |
SUV: Standardised uptake value, PSA: Prostate specific antigen, TRUS: Transrectal ultrasound.
There was a trend for a higher 18F-FDG uptake in prostatic sectors shown to be suspicious on MRI or malignant on histopathology, compared to those in the remainder of the prostate, but this was not statistically significant (Figure 2). There was no significant difference in 18F-FDG uptake between cases with prostate cancer and age-matched controls undergoing PET/CT who did not have prostate cancer. Patients were classified into the following subgroups according to histopathology findings: biopsy not performed (n = 4), benign biopsy or high-grade prostatic intraepithelial neoplasia (PIN) (n = 4), low-grade prostate cancer with Gleason score ≤ 3 + 4 (n = 6), and high-grade prostate cancer with Gleason score ≥ 4 + 3 (n = 4). 18F-FDG uptake was not significantly different between subgroups; we therefore found no correlation between prostatic 18F-FDG uptake and the presence or grade of tumour confirmed on histopathology. Figure 3 illustrates a representative case of a 70-year-old man with high-grade prostate cancer that showed no uptake on PET/CT. See Table 2 for mean values of SUVmax and SUVmean derived from sectorial, case-control and subgroup analysis.
Figure 2.
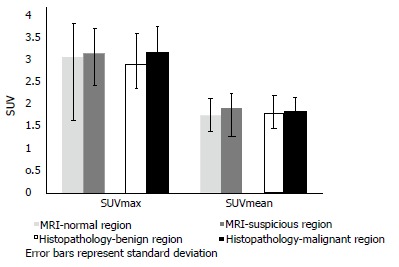
Sectorial analysis comparing 18F-labelled fluorodeoxyglucose uptake in sectors found to be suspicious on magnetic resonance imaging or malignant on histopathology with 18F-labelled fluorodeoxyglucose uptake in the remaining sectors. Mean values and standard deviations have been shown.
Figure 3.
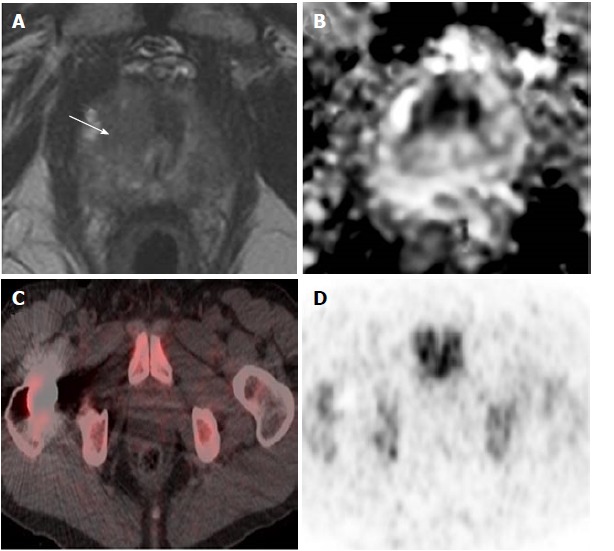
High-grade prostate cancer showing no increased uptake on positron emission tomography/computed tomography in a 73-year-old man. A, B: Prostate MRI performed for raised PSA (19 ng/mL) showed a high probability lesion in the right apex transition zone (arrow in A) with matching restricted diffusion on the ADC map (B). Subsequent targeted transperineal biopsy confirmed Gleason 4 + 5 disease in 40% of cores; C, D: PET/CT performed after a two-month interval and no intervening treatment showed no focal uptake in this region shown as both fused PET/CT imaging (C) and PET alone (D). PET/CT: Positron emission tomography/computed tomography; MRI: Magnetic resonance imaging; ADC: Apparent diffusion co-efficient; PSA: Prostate specific antigen.
Table 2.
Sectorial analysis, case-control analysis and subgroup analysis showed no significant difference in 18F-labelled fluorodeoxyglucose uptake
| Mean SUVmax | Mean SUVmean | |
| Sectorial analysis | ||
| MRI - normal prostatic sectors | 3.02 | 1.74 |
| MRI - suspicious prostatic sectors | 3.1 | 1.89 |
| Histopathology - benign prostatic lobe | 2.86 | 1.79 |
| Histopathology - malignant prostatic lobe | 3.13 | 1.82 |
| Case-control analysis | ||
| Age-matched controls | 3.09 | 1.83 |
| Cases with prostate cancer | 3.26 | 1.81 |
| Subgroup analysis | ||
| Biopsy not performed | 4.95 | 1.91 |
| Benign disease and high-grade PIN | 5.85 | 2.86 |
| Low-grade prostate cancer (Gleason ≤ 3 + 4) | 3.2 | 1.83 |
| High-grade prostate cancer (Gleason score ≥ 4 + 3) | 3.35 | 1.78 |
SUV: Standardised uptake value, PIN: Prostatic intraepithelial neoplasia.
For the second part of the study, 2846 male patients undergoing 18F-FDG PET/CT over a 5-year period were followed-up. 46 men (1.6%) had an incidental and unexplained finding of elevated prostatic 18F-FDG uptake. 18 (0.6%) of these patients underwent further investigation. They had a median age of 68 years, median prostatic SUVmax of 7.80 and median PSA of 3.04 ng/mL. See Table 3 for patient characteristics.
Table 3.
Characteristics of patients in whom elevated prostatic 18F-labelled fluorodeoxyglucose uptake was investigated
| Age (yr) | 18F-FDG PET/CT indication | Prostate SUVmax | PSA (ng/mL) | Urology referral made | Urology outcome |
| 68 | Adrenal nodule | 10.4 | 3 | Yes | MRI - no suspicious foci |
| 77 | Lung nodule | 4.5 | 2.78 | Yes | Biopsy - high-grade PIN |
| 67 | Non-Hodgkin lymphoma | 4.5 | 5.5 | Yes | MRI - suspicious foci |
| Biopsy - benign | |||||
| 68 | Colorectal cancer | 5.9 | 3.04 | Yes | PSA monitoring |
| 58 | Colorectal cancer | 7.6 | 1.38 | Yes | PSA monitoring |
| 64 | Non-Hodgkin lymphoma | 5.4 | 1.84 | Yes | PSA monitoring |
| 58 | Non-Hodgkin lymphoma | 19.9 | 7.44 | Yes | PSA monitoring |
| 81 | Cholangiocarcinoma | 10.3 | 18 | Yes | Lost to follow up |
| 75 | Hepatic metastases (colorectal primary) | 8 | - | Yes | Lost to follow up |
| 61 | Colorectal cancer | 14 | 1.47 | No - PSA normal | |
| 55 | Paraneoplastic syndrome | 4.8 | 0.62 | No - PSA normal | |
| 61 | Non-Hodgkin lymphoma | 5.8 | 2.85 | No - PSA normal | |
| 68 | Gastrointestinal stromal tumour | 13.2 | 1.48 | No - PSA normal | |
| 71 | Hepatic metastases (colorectal primary) | 9.2 | 4.9 | No - palliative care | |
| 87 | Oesophageal cancer | 5.3 | 11.86 | No - palliative care | |
| 82 | Colorectal cancer | 15.4 | 3.85 | No - palliative care | |
| 35 | Hodgkin lymphoma | 11.8 | 3.04 | No - suspected prostatitis | |
| 71 | Oesophageal cancer | 7.3 | 4.58 | No - likely urethral uptake |
SUV: Standardised uptake value; PSA: Prostate specific antigen; PIN: Prostatic intraepithelial neoplasia.
Of these 18 men, 9 (0.3%) were referred to urology. Two men had a prostate biopsy, which showed benign disease and high-grade PIN respectively (Figure 4). No cases of prostate cancer were diagnosed in the 5-year period. See Figure 5 for more detailed clinical outcomes.
Figure 4.
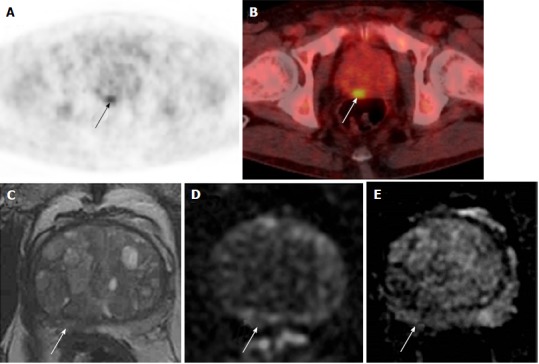
Incidental prostatic 18F-labelled fluorodeoxyglucose uptake in a 67-year-old patient with Stage IV diffuse large B-cell lymphoma. A, B: Focal uptake in the posterior right peripheral zone of the prostate at the level of the mid-gland as demonstrated on PET (A) and fused PET/CT (B); SUVmax = 4.5; C-E: Prostate MRI shows non-specific geographical intermediate signal on T2-weighted imaging (C), but with no matching restricted diffusion on b-1400 diffusion-weighted images (D) or ADC maps (E). The MRI findings are low probability for tumour. Subsequent transrectal ultrasound-guided biopsy showed no cancer. PET/CT: Positron emission tomography/computed tomography; MRI: Magnetic resonance imaging; ADC: Apparent diffusion co-efficient.
Figure 5.
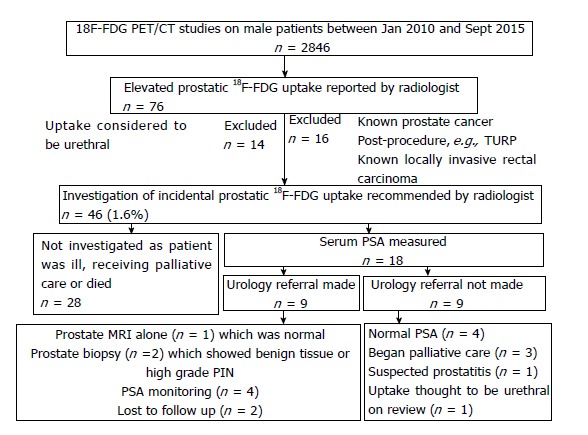
Flowchart showing clinical outcomes in patients with elevated prostatic 18F-labelled fluorodeoxyglucose uptake.
DISCUSSION
Prostate cancer is the commonest male cancer[15]. There is therefore a potentially high incidence of synchronous prostatic tumour in patients undergoing 18F-FDG PET/CT for other indications. However, PET/CT lacks specificity and sensitivity for primary detection of prostate cancer; consequently it is unclear how patients with incidental tracer uptake in the prostate should be managed. Our study has shown that focal 18F-FDG uptake is not indicative of prostate cancer in this cohort, with SUVmean and SUVmax values significantly overlapping between malignant and benign conditions, and that the reporting of incidental prostatic uptake did not affect subsequent clinical management of any patient in our institute over a 5-year period.
Sector-based analysis showed that, in individual patients, malignant prostatic sectors had a trend to higher 18F-FDG uptake than benign sectors. However, this difference was not statistically significant and total prostate 18F-FDG uptake in men with prostate cancer was comparable to that from age-matched controls. Comparison of 18F-FDG uptake across patient subgroups showed no correlation between 18F-FDG uptake and histopathological findings. Although some authors have suggested that 18F-FDG uptake weakly correlates with Gleason score, the small numbers in our study did not demonstrate this finding[9,16]. In fact, we observed a higher SUVmax and SUVmean in patients with no biopsy, benign biopsy or high grade PIN than in patients with prostate cancer. This may be partially explained by increased 18F-FDG uptake in prostatitis, where there is also increased glucose uptake within the inflammatory tissue[17].
Incidental and unexplained prostate uptake was found in 1.6% of all 18F-FDG PET/CT studies in male patients, which is comparable to the rate reported previously[3-5]. These patients had a median SUVmax of 7.8, which is suspicious for tumour; other authors have suggested an SUVmax greater than 6.0 should be considered as a cut-off value for high-grade prostate cancer[9]. Only 40% of patients with incidental and unexplained prostatic uptake were investigated with a serum PSA. Twenty percent of patients were referred to a urologist, and only one-third of these patients underwent further investigation with either biopsy or MRI. This may reflect the fact that the existing cancer diagnosis is the primary factor in determining clinical prognosis, and that the subsequent detection of prostate cancer would not significantly affect patient management due to unsuitability for radical therapy. Another possibility is a reluctance to perform a transrectal prostate biopsy in patients undergoing chemotherapy due to the risk of sepsis. In some patients, incidental prostatic uptake was not investigated for different reasons, e.g., uptake was thought to represent tracer in the urethra upon review (Figure 6), or uptake resolved on repeat PET/CT (Figure 7). Ultimately over a 5-year period in our centre, involving nearly three thousand 18F-FDG PET/CT studies, no change in patient management occurred as a result of an incidental finding of elevated prostatic 18F-FDG uptake. Therefore, our retrospective study questions the need to investigate incidental prostatic uptake of 18F-FDG in men undergoing PET/CT.
Figure 6.
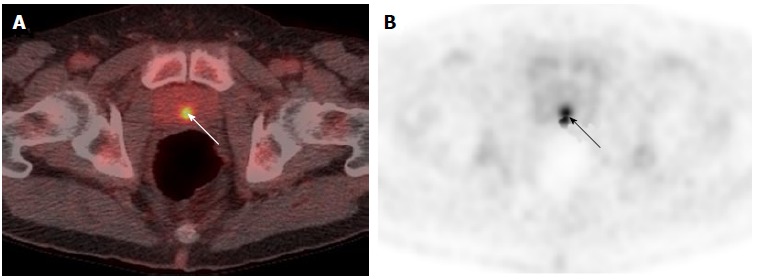
Midline uptake on 18F-labelled fluorodeoxyglucose positron emission tomography/computed tomography in a 71-year-old man with oesophageal carcinoma and serum prostate specific antigen of 4.58 ng/mL. A, B: Fused PET/CT and PET-only imaging shows focal uptake in the midline of the prostate (arrowed). The uptake was considered to be tracer in the urethra given its anatomical location. PET/CT: Positron emission tomography/computed tomography.
Figure 7.
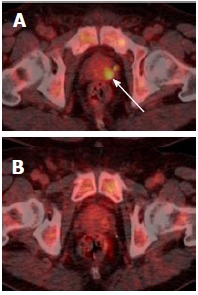
Resolving focal prostatic uptake in a 61-year-old man with Stage IV high-grade non-Hodgkin’s lymphoma and serum prostate-specific antigen of 2.85 ng/mL. A: Fused PET/CT imaging performed after 2 cycles of chemotherapy shows focal uptake (arrowed) in the left side of the prostate at the level of the midgland on fused PET/CT; B: Repeat PET/CT performed 4 mo later following completing of 6 cycles of chemotherapy demonstrates resolution of this focal uptake. PET/CT: Positron emission tomography/computed tomography.
Our study has some limitations. Firstly, as a retrospective study our population consisted of patients who underwent 18F-FDG PET/CT primarily for other malignancies, and therefore the time difference between PET/CT and MRI was long in some cases (up to 46 mo). This timescale is similar to previously reported retrospective studies and given that the natural history of prostate cancer is one of a slow-growing tumour, most prostate cancers will be present for years before clinical presentation[10,18]. Secondly, the number of eligible patients in our study was small. Thirdly, patients in our study had ultrasound-guided biopsy or MRI/ultrasound fusion biopsy, which are less sensitive in detecting prostate cancer than whole-mount histology derived from prostatectomy samples.
In conclusion, 18F-FDG uptake has low clinical utility in distinguishing benign and malignant prostatic disease. Reporting incidental prostatic uptake did not affect subsequent patient management or clinical outcomes in this cohort of patients. This study suggests there may be little benefit in investigating incidental elevated prostatic 18F-FDG uptake on PET/CT which should be addressed with future large prospective studies.
COMMENTS
Background
18F-labelled fluorodeoxyglucose (18F-FDG) uptake on positron emission tomography/computed tomography (PET/CT) is used extensively in the diagnosis, staging and monitoring of many cancers. Incidental elevated prostatic 18F-FDG uptake is found in a significant proportion of men undergoing PET/CT for unrelated reasons. 18F-FDG PET/CT is not routinely used in prostate cancer because of the relatively low metabolic activity of prostate cancer, the proximity to tracer in the urethra and the presence of significant 18F-FDG uptake in benign and inflammatory prostatic disease.
Research frontiers
The significance of incidental prostatic uptake, together with the need for further investigation, is unclear.
Innovations and breakthroughs
The results suggest that incidental prostatic uptake has no significant correlation with prostate magnetic resonance imaging or biopsy findings. In a cohort of nearly 3000 men over 5 years, reporting incidental prostatic 18F-FDG uptake did not alter patient management or clinical outcomes.
Applications
The results suggest there is little benefit in investigating incidental elevated prostatic 18F-FDG uptake.
Peer-review
This is an interesting study which investigates the clinical significance of incidental FDG uptake. Although the number of eligible patients was small, this is an well written retrospective study.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by Addenbrooke’s Hospital, Cambridge.
Informed consent statement: The requirement for informed consent for data analysis was waived.
Conflict-of-interest statement: The authors have no competing interests.
Data sharing statement: No additional data available.
Peer-review started: September 23, 2016
First decision: November 21, 2016
Article in press: March 22, 2017
P- Reviewer: Kucherlapati MH, Lim SM S- Editor: Kong JX L- Editor: A E- Editor: Zhao LM
Contributor Information
Madhurima R Chetan, School of Clinical Medicine, University of Cambridge, Cambridge CB2 0SP, United Kingdom.
Tristan Barrett, Department of Radiology, Addenbrooke’s Hospital and University of Cambridge, Cambridge CB2 0QQ, United Kingdom.
Ferdia A Gallagher, Department of Radiology, Addenbrooke’s Hospital and University of Cambridge, Cambridge CB2 0QQ, United Kingdom.
References
- 1.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–233. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 2.Picchio M, Mapelli P, Panebianco V, Castellucci P, Incerti E, Briganti A, Gandaglia G, Kirienko M, Barchetti F, Nanni C, et al. Imaging biomarkers in prostate cancer: role of PET/CT and MRI. Eur J Nucl Med Mol Imaging. 2015;42:644–655. doi: 10.1007/s00259-014-2982-5. [DOI] [PubMed] [Google Scholar]
- 3.Kang PM, Seo WI, Lee SS, Bae SK, Kwak HS, Min K, Kim W, Kang DI. Incidental abnormal FDG uptake in the prostate on 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography scans. Asian Pac J Cancer Prev. 2014;15:8699–8703. doi: 10.7314/apjcp.2014.15.20.8699. [DOI] [PubMed] [Google Scholar]
- 4.Seino H, Ono S, Miura H, Morohashi S, Wu Y, Tsushima F, Takai Y, Kijima H. Incidental prostate 18F-FDG uptake without calcification indicates the possibility of prostate cancer. Oncol Rep. 2014;31:1517–1522. doi: 10.3892/or.2014.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon T, Jeong IG, You D, Hong JH, Ahn H, Kim CS. Prevalence and clinical significance of incidental (18)F-fluoro-2-deoxyglucose uptake in prostate. Korean J Urol. 2015;56:288–294. doi: 10.4111/kju.2015.56.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NHS England. NHS Commissioning B02. PET-CT. [accessed. 2016. p. Jan 22]. Available from: https: //www.england.nhs.uk/commissioning/spec-services/npc-crg/group-b/b02/ [Google Scholar]
- 7.Czernin J, Allen-Auerbach M, Nathanson D, Herrmann K. PET/CT in Oncology: Current Status and Perspectives. Curr Radiol Rep. 2013;1:177–190. doi: 10.1007/s40134-013-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertagna F, Sadeghi R, Giovanella L, Treglia G. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin. 2014;53:249–258. doi: 10.3413/Nukmed-0668-14-05. [DOI] [PubMed] [Google Scholar]
- 9.Reesink DJ, Fransen van de Putte EE, Vegt E, De Jong J, van Werkhoven E, Mertens LS, Bex A, van der Poel HG, van Rhijn BW, Horenblas S, et al. Clinical Relevance of Incidental Prostatic Lesions on FDG-Positron Emission Tomography/Computerized Tomography-Should Patients Receive Further Evaluation? J Urol. 2016;195:907–912. doi: 10.1016/j.juro.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Brown AM, Lindenberg ML, Sankineni S, Shih JH, Johnson LM, Pruthy S, Kurdziel KA, Merino MJ, Wood BJ, Pinto PA, et al. Does focal incidental 18F-FDG PET/CT uptake in the prostate have significance? Abdom Imaging. 2015;40:3222–3229. doi: 10.1007/s00261-015-0520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang I, Chong A, Jung SI, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K, Ryu SB. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013;27:140–145. doi: 10.1007/s12149-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Hu S, Cheng J, Xu J, Shi W, Zhu B, Zhang Y, Yao Z, Pan H, Zhang Y. Prevalence and risk of cancer of incidental uptake in prostate identified by fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Clin Imaging. 2014;38:470–474. doi: 10.1016/j.clinimag.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Han EJ, H O J, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluoro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010;83:915–920. doi: 10.1259/bjr/19887771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerety EL, Lawrence EM, Wason J, Yan H, Hilborne S, Buscombe J, Cheow HK, Shaw AS, Bird N, Fife K, et al. Prospective study evaluating the relative sensitivity of 18F-NaF PET/CT for detecting skeletal metastases from renal cell carcinoma in comparison to multidetector CT and 99mTc-MDP bone scintigraphy, using an adaptive trial design. Ann Oncol. 2015;26:2113–2118. doi: 10.1093/annonc/mdv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 16.Chang JH, Lim Joon D, Lee ST, Hiew CY, Esler S, Gong SJ, Wada M, Clouston D, O’Sullivan R, Goh YP, et al. Diffusion-weighted MRI, 11C-choline PET and 18F-fluorodeoxyglucose PET for predicting the Gleason score in prostate carcinoma. Eur Radiol. 2014;24:715–722. doi: 10.1007/s00330-013-3045-1. [DOI] [PubMed] [Google Scholar]
- 17.Lin KH, Chen YS, Hu G, Tsay DG, Peng NJ. Chronic bacterial prostatitis detected by FDG PET/CT in a patient presented with fever of unknown origin. Clin Nucl Med. 2010;35:894–895. doi: 10.1097/RLU.0b013e3181f49e57. [DOI] [PubMed] [Google Scholar]
- 18.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, Feuer E, de Koning H. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]


