Abstract
Wristbands are increasingly used for assessing personal chemical exposures. Unlike some exposure assessment tools, guidelines for wristbands, such as preparation, applicable chemicals, and transport and storage logistics, are lacking. We tested the wristband’s capacity to capture and retain 148 chemicals including polychlorinated biphenyls (PCBs), pesticides, flame retardants, polycyclic aromatic hydrocarbons (PAHs), and volatile organic chemicals (VOCs). The chemicals span a wide range of physical–chemical properties, with log octanol–air partitioning coefficients from 2.1 to 13.7. All chemicals were quantitatively and precisely recovered from initial exposures, averaging 102% recovery with relative SD ≤21%. In simulated transport conditions at +30 °C, SVOCs were stable up to 1 month (average: 104%) and VOC levels were unchanged (average: 99%) for 7 days. During long-term storage at −20 °C up to 3 (VOCs) or 6 months (SVOCs), all chemical levels were stable from chemical degradation or diffusional losses, averaging 110%. Applying a paired wristband/active sampler study with human participants, the first estimates of wristband–air partitioning coefficients for PAHs are presented to aid in environmental air concentration estimates. Extrapolation of these stability results to other chemicals within the same physical–chemical parameters is expected to yield similar results. As we better define wristband characteristics, wristbands can be better integrated in exposure science and epidemiological studies.
Keywords: flame retardants, PCBs, pesticides, personal exposure, polycyclic aromatic hydrocarbons, volatile organic compounds
Introduction
Chemical Exposure on Health
Accurately assessing personal exposure to environmental toxicants is central to the challenge of linking chemical exposure and health. For example, exposure to polycyclic aromatic hydrocarbons (PAHs) has been associated with obesity, cancer, decreased intelligence quotient, and respiratory distress,1, 2, 3 whereas exposures to flame retardants has been associated with cardiotoxicity, reduced hormone levels, and neurotoxicity.4, 5, 6 Yet, for most environmental chemicals, there is little information about the frequency and magnitude of personal exposure.7, 8 The lack of low-cost, easy-to-use personal sampling technology currently hinders several fields of research, including epidemiology, exposure science, toxicology, and disaster assessment.
Existing Technologies
Many types of active air sampling devices have been developed to assess chemical exposure, but tend to be costly and complicated to operate.9 Fixed stationary active air samplers are common, but may increase uncertainty when estimating individual exposure to pollutants as compared with personal samplers.10, 11, 12, 13 To address the need to measure personal chemical exposure, studies have used active air monitoring backpacks14, 15, 16, 17 or active samplers on lapels.18 Active backpacks include a battery pack and pump that continuously sample air, but the requirement to carry a backpack can be a burden for study participants.14 Other concerns include pump noise and weight that can affect compliance,14, 19, 20 equipment malfunction during deployment, and cost to the researcher. Active backpack equipment costs ∼$3000 and calibration equipment can cost over $2500. There may be additional costs for pump refurbishment, replacement batteries, and calibration certifications. Although there can be tradeoffs between cost and quality for some approaches, there may be other sampling techniques, such as passive sampling, to measure chemical exposure that provide a quality compromise.
Biological samples such as blood or urine are favored by researchers trying to measure the totality of personal environmental chemical exposures.7, 8, 19 However, it is challenging to identify biomarkers with robust specificity and sensitivity.20, 21 In addition, it is difficult to accurately control for inter- and intra-individual variability because of factors influencing metabolic capacity, chemical toxicokinetics, and exposure event timing.22, 23, 24 Furthermore, biomarker analysis for non-persistent chemicals with short biologic half-lives can lead to exposure misclassification.24, 25 Biomarkers also do not indicate the source of chemical exposure, but rather integrate all exposure routes such as inhalation, ingestion, and dermal contact.26 There are relatively few measurable biomarkers that can be directly linked to exposures. Policy decisions may be informed by biomarkers, but they cannot be used to regulate. Biological sample collection, such as phlebotomy,25 can also be invasive and may yield low participation. External measures are needed to characterize and quantify chemicals in an individual’s environment.
Passive Sampling
For over two decades, passive sampling devices have been used to sample the bioavailable fraction of organic contaminants in numerous environmental media.27, 28, 29, 30 Passive samplers have been used to sample non-polar and semipolar chemicals in air,31, 32, 33 water,34, 35, 36 and sediments.37, 38 Passive sampling badges and tubes have been previously used to assess personal exposure to some volatile organic chemicals (VOCs).39, 40 Both SVOCs and VOCs occur in air and diffuse into the lipophilic membrane of passive samplers. Concentrations increase until equilibrium is reached with the surrounding sample matrix.27 Chemicals absorb into the polymer and permeate throughout the polymer during deployment.27 Chemicals in the lipophilic polymer represent the unbound fraction of contaminants, making passive samplers a biologically relevant surrogate of chemical exposure.41, 42, 43, 44
History and Characteristics of Wristbands
A recent advancement in passive sampling devices is the invention of the personal wristband chemical monitor, derived from commercially available silicone wristbands.33 Silicone wristbands have been used increasingly to provide personal exposure assessments, and are often partnered with demographic data assessed by questionnaires to infer lifestyles and behaviors that are associated with chemical concentrations.45, 46, 47, 48 To date, wristbands have been used to measure exposure to PAHs with occupationally exposed roofers,33 flame retardants with preschool children,45 and pesticides with farmers in developing countries.47, 48
Because accurate measurement of chemical exposure is a critical component for estimating health effects, wristbands can complement current methods for personal chemical exposure. For example, wristbands have been paired with conventional methods in two separate studies. Hammel et al.46 compared wristbands with hand wipes and urine samples for organophosphate flame retardants (OPFRs), and found more significant correlations between OPFRs in wristbands and corresponding urinary metabolites than by using hand wipes. Similarly in a manuscript in preparation, Dixon et al.compared wristbands with air monitoring backpacks and urine samples for PAHs, and found more significant correlations between PAHs in wristbands and corresponding urinary metabolites than between backpack and urine samples. In both these studies, Wristbands correlate well with metabolites in urine samples, thus demonstrating the utility of wristbands as a biologically relevant exposure tool.
Establishing Wristband Practices and User Guidelines
As the use of wristbands increases, it is critical to establish a framework for methods and applications, especially as there are alternative ways to prepare, transport, store, and extract chemicals from these samplers. Some sampling technologies are delicate or susceptible to compromise during transport or storage, or their stability and robustness is not well characterized, leading to increased uncertainty for users.49 There is a need to provide practical guidelines for preparation, application, transport, and storage of wristbands. It is beneficial to know the conditions needed to retain quality data while not using unnecessary, burdensome protocols. In addition, once samples reach the laboratory, it is not always possible to analyze the samples immediately. Essential information on the criteria for transport and storage of wristbands will increase the utility of this technology for exposure science, epidemiology, and toxicology studies.
The objectives of this study are: (1) to demonstrate that when the wristband is properly prepared it provides a simple, chemically clean foundation for quantitative organic chemical monitoring; (2) to assess the transport and storage stability of captured chemicals in the wristband, and (3) to estimate wristband–air partitioning coefficients, Ksa, for a commonly studied chemical class, PAHs. Together, these aims provide practical guidelines for future wristband users in study design, field deployment, and laboratory analysis.
Materials and methods
Standards, Solvents, and Reagents
Solvents were purchased from Fisher Scientific (Pittsburgh, PA, USA). Ethyl acetate was Optima and n-hexane was GC-Resolv grade. Analytical grade standards, purity ≥98%, were obtained from Accustandard (New Haven, CT, USA) and selected to represent a range of physicochemical properties. A complete list of chemicals, CAS numbers, and physicochemical parameters are provided in Supplementary Table S1. Internal standards and extraction surrogates are detailed in Supplementary Table S2. Polytetrafluoroethylene (PTFE) transport/storage bags and closures were purchased from Welch Fluorocarbon (Dover, NH, USA).
Wristband Sampler Conditioning and Preparation
Before use, silicone wristbands (width: 1.3 cm; inner diameter: 5.8 cm, https://24hourwristbands.com, Houston, TX, USA) were baked at 300 °C for 180 min, under vacuum at 0.1 Torr (Vacuum Oven, Blue-M, model no. POM18VC-2, with Welch Duo-seal pump, model no. 1405). The vacuum oven was flushed with 99.99% nitrogen at 15, 30, 45, 60, 90, 120, and 180 min intervals during baking. An in-line liquid nitrogen cold trap was used between the vacuum oven and pump to prevent volatilized impurities from damaging the pump. Wristbands were then stored in sealed metal containers at 4 °C. Air-tight PTFE bags are used for storage before and after deployment.
Wristband Sampler Analysis
For deployed wristbands used in the partitioning study, wristbands were cleaned with two sequential rinses of 18 MΩ·cm water and one isopropanol rinse to remove superficial fouling or particles. To illustrate the effectiveness of this cleaning process, microscopic images were obtained (Supplementary Figure S1). After deployment, cleaned wristbands were stored in amber glass jars at −20 °C until extraction.
SVOCs were recovered from wristbands with two 100 ml rounds of ethyl acetate extraction at ambient temperature as previously described.33 The ethyl acetate was combined and reduced to nominally 1 ml with nitrogen evaporators (Turbo-Vap L, Biotage, Charlotte, NC, USA and N-EVAP 111, Organomation Associates, Berlin, MA, USA). For VOC analyses, wristbands were thermally extracted directly as described below in ‘Volatile organic chemicals (VOCs)’ section.
Wristband Capture and Transport and Storage Stability
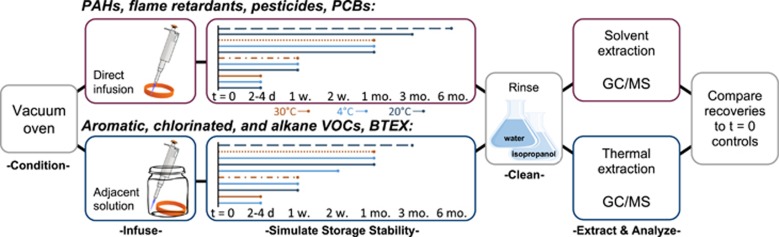
To simulate capture and stability of chemicals within the wristband samplers, 112 wristbands were analyzed. Stability in this context includes degradation of chemical(s) within the wristband or loss(es) through diffusion processes. Wristbands were infused with 148 chemicals using one of two methods: a direct application method of pipetting standards onto the wristbands was used for SVOCs, and a vapor infusion method where gas vapors were allowed to equilibrate with wristbands in a closed glass vessel was used for VOCs. Both methods are further detailed in the Supplementary Information. After infusion, four wristbands were extracted, representing the initial concentrations (t=0). Two wristband extracts from separate treatment groups were compromised and thus excluded from subsequent analyses as indicated by n=3 in Supplementary Table S6. All other wristbands were stored in clean, air-tight PTFE bags under various temperatures and time point scenarios depicted in Figure 1. Replication consisted of four wristbands extracted for each scenario. Field transport simulations were 2–4 days and 1 week following storage at −20 °C, 4 °C, and +30 °C, and an additional 14 days for VOCS at 4 °C. Long-term storage stability of chemicals in wristbands was evaluated at −20 °C, and included 3 months (82–88 days) for VOCs and 6 months (182 days) for SVOCs.
Figure 1.
Recovery, transport, and storage stability experimental design. Dashed blue line represents long-term storage, dotted orange line represents accelerated storage stability, and dash–dot line represents transport stability. The cleaning step was only used for wristbands deployed outside the laboratory.
Accelerated storage stability studies are generally performed at higher temperatures to estimate degradation rates at longer times.50 The degradation rate based on accelerated stability studies for the pharmaceutical industry estimates that many reaction rates increase by a factor of two for every 10 °C increase.50 Our recommended storage, −20 °C, was used as the basis for calculation, and the accelerated storage stability studies were performed at +30 °C for 1 month. Based on the accelerated study that is 50 °C higher (25) for 28 days (28 × 25=896), the 28-day +30 °C data may roughly estimate chemical stability at ~900 days at −20 °C.
Instrumental Analysis
Instrumental analysis for the chemicals was performed with five different established analytical methods. For each analytical method additional details are provided in the Supplementary Information, including Supplementary Table S2.
Polycyclic aromatic hydrocarbons (PAHs)
A total of 50 alkylated and unsubstituted PAHs were measured with a modified GC-MS/MS with an Agilent 7890B gas chromatograph (Santa Clara, CA, USA) interfaced with an Agilent 7000C mass spectrometer equipped with a triple-axis detector gas chromatograph coupled to tandem mass spectrometer (GC-MS/MS) as detailed elsewhere.51 PAHs included 2-, 3-, 4-, 5-, and 6-ring PAHs.
Flame retardants
Triphenylphosphate and seven polybrominated diethyl ether (PBDE) flame retardants were analyzed with an Agilent 7890A gas chromatograph coupled to a 5975C mass spectrometer under electron impact ionization (70 eV). The analytical conditions are further detailed in Kile et al.45 and Supplementary Information.
Pesticides
A total of 47 pesticides or pesticide degradation products were measured: 27 are classified as insecticides, 7 herbicides, 7 fungicides, and 6 pesticide degradation products. The method reflects our interest in insecticides for their potential human health impacts and includes 21 organochlorine, 3 organophosphate, and 3 pyrethroid insecticides. Pesticides were analyzed on an Agilent 6890N GC with dual 7683 injectors, dual columns, DB-XLB and DB-17MS columns (Agilent), and dual microelectron capture detectors (μ-ECD).47
Polychlorinated biphenyls (PCBs)
Thirteen PCBs including tetra-, penta-, hexa-, and hepta-chlorinated PCBs were measured with an Agilent 7890A gas chromatograph coupled to a 5975C mass spectrometer under electron impact ionization (70 eV).
Volatile organic chemicals (VOCs)
A total of 31 alkanes, aromatic and halogen substituted aromatic hydrocarbons were extracted directly with a Markes International M-CTE250I microchamber thermal extractor onto Tenex TA Carbograph 5TD thermal desorption tubes (Markes International, Cincinnati, OH, USA), conditions as detailed in Supplementary Table S3. Sorbed tubes were transferred to the Markes International Series 2 Ultra TC auto-sampler interfaced through a Series 2 Unity with an Agilent 7890A gas chromatograph and mass spectrometer. Instrument conditions are detailed in Supplementary Table S2.
All target chemicals were quantified by the relative response of the internal standard to target chemicals in a 4–9-point calibration curve (R2 >0.97, Supplementary Information and Supplementary Table S2).
Paired Wristband and PUF Active Air Monitoring Samplers
Concentrations in the paired study are used to calculate Ksa, a partition coefficient between wristbands and air. Wristbands (prepared as described in O’Connell et al.33) and air monitoring backpacks (as described previously in Perera et al.52) were deployed simultaneously for 48 h on 22 women in an epidemiological birth cohort at the Columbia Center for Children’s Environmental Health in New York City. Informed consent was obtained in agreement with the Columbia University Institutional Review Board (IRB), the IRB of record. Further details of the air monitoring samplers and limits of detection and quality control are provided in Supplementary Information and Supplementary Table S4. Briefly, a sampler holder (URG-200-25A, URG, Chapel Hill, NC, USA) with a precleaned polyurethane foam (PUF) plug, which collects gas-phase organic molecules, was attached over the shoulder on a backpack strap. Twelve PAH concentrations were above the limit of detections in both the wristband and PUF and were used in subsequent analysis.
Informed consent was obtained in agreement with the Columbia University Institutional Review Board (IRB), the IRB of record.
Statistical Analysis
Treatment recoveries were scaled as a percentage of the mean control (t=0) treatments. For the 148 chemicals, there are 1450 time and temperature scenarios. Mean percent recoveries were compared with t=0 control treatments with Dunnett’s tests. Each family of Dunnett’s tests, defined as all comparisons for one chemical, controls for false discovery rates from multiple comparisons, is robust to non-normality, and is appropriate for our sample sizes.53 In the paired study, linear regression was used to predict Ksa from the Koa of PAHs at equilibrium. Variance among PAHs in the Ksa model was not different (Levene’s test P-value=0.90). Statistical analyses were performed with JMP Pro 12.0.1. Significance was set at α=0.05. Standard deviation from pilot data indicated that treatment groups of n=3 were adequate, and an additional replicate was included (n=4) in case of sample loss.
QC Samples
To ensure data quality, over 30% of the total samples analyzed were for quality control (QC) purposes. Blank wristband samples were collected during wristband conditioning and cleaning steps. Solvent extraction blanks were collected by performing the extraction process without wristbands. A mix of surrogate standards were added to all wristbands before extraction to quantify chemical recoveries during the extraction process and averaged 101% across methods (median=98%). To account for any instrument background responses, injections of n-hexane solvent were included in all analytical batches. To monitor instrument performance, continuing calibration verification standards (CCVs) were analyzed before and after analytical batches. CCVs consist of a solution with known concentrations for all target chemicals and all were within 20% of the true value for at least 80% of the chemicals, indicating that instrument performance remained consistent throughout the analyses. Additional QC pertaining to the paired wristband and active PUF samples is detailed in the Supplementary Information.
Results
Chemically Clean Foundation for Quantitative Organic Chemical Monitoring
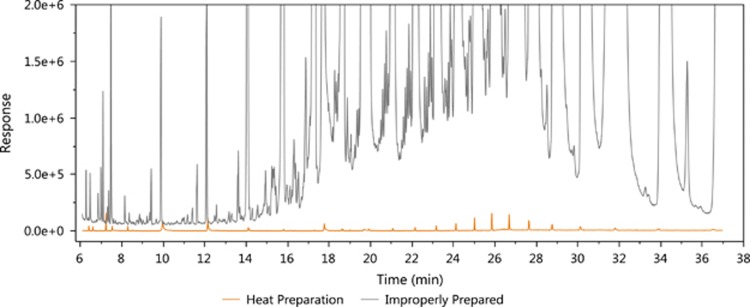
After iterative rounds of optimization, we were able to remove oligomers and other chemical contaminants by baking the wristbands under vacuum. We monitored the total mass reduction, the total ion chromatogram (TIC) shown in Figure 2, as well as strength and elasticity properties (Supplementary Figures S2 and S3). For weight reduction, an average of 4% (SD 1.3%, n=60) was measured after the method was finalized (Supplementary Figure S3c). By examining the TIC (Figure 2), there is graphical evidence that significant amounts of oligomers were removed. Prepared wristbands were analyzed in five quantitative methods, and none of the 148 target chemicals were above detection limit for any wristband (n=6). Cleaned and conditioned wristbands were also screened in a 1400 chemical automated mass spectral deconvolution identification system with deconvolution reporting software, further described in Supplementary Information (n=6). Using the 1400 analyte method, only 4 chemicals were detected: di-n-butylphthalate, dicyclohexyl phthalate, bis(2-ethyhexyl)phthalate, and methylphenol. In all cases, the 4 chemicals detected after cleaning were present at low levels (<500 p.p.b.), and would need to be subtracted from concentrations measured in deployed wristband samples if quantitated and reported in additional studies. Elasticity, strength, and color were adequate for use, and further test details of these parameters are provided in Supplementary Information and Supplementary Table S5. Microscopic images of the wristband surface after deployment indicate that there are few particulates on the wristband surface before cleaning (Supplementary Figure S1a and b), and even less after cleaning (Supplementary Figure S1c and d).
Figure 2.
Conditioned wristband in orange shown in the total ion chromatogram (TIC) below, compared with an improperly prepared wristband (gray trace). Note that a wristband without any cleaning would be even further off scale.
Recovery, Transport, and Storage Stability
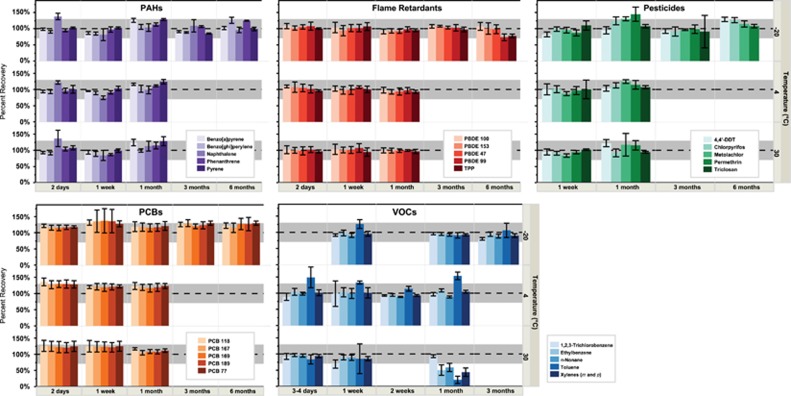
A total of 148 chemicals were recovered from the initial infusions (Table 1), with <21% relative standard deviation (RSD). For all time points and chemical classes, the majority of stability estimates are within 30% of starting concentrations (Figure 3). For the 148 chemicals, there were 1450 time and temperature scenarios in total. Of these, 88 scenarios (6%) were both significantly different and less than t=0 controls. However, not all significant differences are meaningful in the context of storage stability. For example, of 88 scenarios, only 29 were both significant and below 70% of initial recoveries. These 29 instances include, in particular, 17 VOCs from the 1-month time point at +30 °C that exhibited low concentrations (average 66% for VOCs). Of the remaining significantly decreased time points (n=58), they represent sporadic decreases that are inconsistent with subsequent time points. For example, at −20 °C, 2,3-dimethylanthracene is significantly different from starting concentrations, but is still recovered on average ∼78%. This suggests that some variability is inherent in the infusion process or analytical methods, and does not specifically reflect chemical stability in the wristbands. Furthermore, ∼18% of the total 1450 scenarios were statistically different and greater than t=0 control, and are reasonably estimated to be an artifact of variability in the infusion process and/or analytical method for those particular compounds. Supplementary Table S6 gives percent recoveries for all time and temperature scenarios. Overall, given potential variability from the infusion, extraction, retention in the polymer, and the analytical method, relative SDs averaged only 9% (median 7%, range <0.1 to 59%) illustrating good precision for most chemicals across a wide breadth of compound diversity.
Table 1. Summary data from percent recovery, transport, and stability across 148 chemicals.
| Chemical group | Log Koa range |
Initial recovery from exposure |
Transport stability 1 week, +30 °C |
Accelerated storage stability: 1 month, +30 °C |
Long-term storage or archive: 3–6 months, −20 °C |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery range (%) | Recovery (%) AVG; median | RSD (%) Range | Stability range (%) | Stability (%) AVG; median | RSD (%) range | Stability range (%) | Stability (%) AVG; median | RSD (%) range | Stability range (%) | Stability (%) AVG; median | RSD (%) range | ||
| PAHs (50) | 5.0–13.7 | 40–138 | 101; 103 | 0–20 | 72–103 | 89; 90 | 2–19 | 89–150 | 117; 116 | 2–23 | 85–142 | 107; 104 | 1–11 |
| Flame retardants (7) | 8.5–13.3 | 103–168 | 121; 113 | 3–7 | 95–108 | 103; 104 | 9–15 | 97–103 | 100; 100 | 2–8 | 74–108 | 95; 101 | 7–18 |
| Pesticides (47) | 6.8–13.5 | 66–160 | 120; 119 | 3–21 | 81–155 | 98; 95 | 2–21 | 69–135 | 116; 120 | 2–30 | 105–140 | 120; 120 | 4–10 |
| PCBs (13) | 8.6–11.7 | 92–112 | 96; 95 | 7–9 | 121–128 | 124; 124 | 10–16 | 104–118 | 110; 110 | 3–8 | 116–168 | 130; 130 | 5–34 |
| VOCs (31) | 2.7–5.2 | 81–185 | 102; 90 | 0–20 | 66–99 | 85; 88 | 8–54 | 20–94 | 64; 67 | 4–57 | 73–107 | 93; 91 | 3–21 |
| Total (148) | 2.7–13.7 | 40–168 | 102; 99 | 0–21 | 66–155 | 95; 93 | 2–54 | 20–150 | 104; 113 | 2–57 | 73–168 | 110; 103 | 3–34 |
Figure 3.
Select chemicals from five broad classes of chemicals. Sets include stability at 4, 7, 28, 82, and 182 (or 183) days (x axis) and −20 °C, 4 °C, and +30 °C are organized as three groups on the y axis. The percentage recovery for each chemical is given by the y axis (scale on left). Stability out to 183 days is reported for −20 °C for all SVOC chemical classes. Recoveries at 2–4, 7, and 28 days at both 4 °C and +30 °C are given for all chemical classes. Each bar represents n=4 for each chemical at each time and temperature, with error bars representing standard error. The gray bar across each graph bounds ±30% of the true value (100%). Within pesticides are representative chemicals from insecticides, herbicides, and fungicides. Within the flame retardants are both an organophosphate and PBDEs. PCBs include tetra-to hepta-chlorinated congeners, and PAHs include 2–6-ring chemicals. VOCs include aromatic, chlorinated, and alkane chemicals.
Wristband–Air Partition Coefficient Estimates
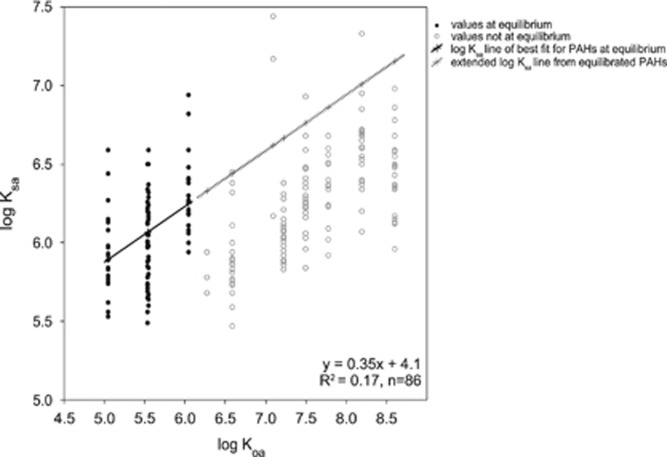
The paired wristband and active air monitoring backpack study provide a relationship between calculated log Ksa (a silicone wristband–air partition coefficient) and log Koa for the measured PAHs (wristband and PUF data presented in Supplementary Information). The concentrations of 4 PAHs, naphthalene, 2-methylnaphthalene, 1-methylnaphthalene, and acenaphthene, are estimated to be at equilibrium with air. When wristbands were worn for both 24 h and 7 days by the same individual, it was demonstrated that these 4 lower molecular weight PAHs reached equilibrium after 24 h because concentrations of these 4 PAHs in the wristbands were equal whether worn for 24 h or 7 days (data not shown). Over the 48 h of exposures in the paired wristband and PUF study, the 4 equilibrated PAHs are related to log Koa (Eq. (1), Figure 4) with:
Figure 4.
Linear regression plot of log Koa and estimated log Ksa for 12 PAHs in participants who wore paired wristbands and active air-monitoring backpacks. Values in gray circles were generally expected to fall below the line of best fit since they were not at equilibrium at the end of deployment. PAH, polycyclic aromatic hydrocarbon.
The other 8 PAHs not at equilibrium primarily fall, as expected, below the predicted fit (Figure 4). Using the equation from the 4 PAHs at equilibrium, the estimated Ksa for all 12 PAHs are calculated (Table 2).
Table 2. The predicted K sa values (± 95% prediction interval) for 12 PAHs using Eq. (1).
| PAH | log Koa | Predicted log Ksa (l/kg) |
|---|---|---|
| Naphthalene | 5.0 | 5.9±0.3 |
| 2-Methylnaphthalene | 5.5 | 6.1±0.3 |
| 1-Methylnaphthalene | 5.5 | 6.1±0.3 |
| Acenaphthene | 6.0 | 6.2±0.3 |
| Acenaphthylene | 6.3 | 6.3±0.4 |
| Fluorene | 6.6 | 6.4±0.4 |
| Anthracene | 7.1 | 6.6±0.4 |
| Phenanthrene | 7.2 | 6.7±0.5 |
| 2-Methylphenanthrene | 7.5 | 6.8±0.5 |
| 1-Methylphenanthrene | 7.8 | 6.9±0.5 |
| Pyrene | 8.2 | 7.0±0.6 |
| Fluoranthene | 8.6 | 7.2±0.6 |
Discussion
Properly Conditioned and Prepared Wristbands, a Chemically Clean Tool for Chemical Monitoring
Removal of oligomers and other chemical contaminants that have adsorbed into the wristband is necessary before use because these chemicals adversely affect analytical sensitivity and overall analytical instrument operation. Without proper wristband conditioning, sensitivity is decreased, and the instrument requires excessive maintenance. Higher temperatures or lack of vacuum generally resulted in loss of elasticity or color. However, loss of color has no effect on analytical performance. Although solvent cleaning has been successfully used,33 it is more expensive using 42 liters of solvents per 100 wristbands, entails more staff interaction time with the solvent exchanges, and requires disposal of the solvents. Lack of sufficient cleaning led Hammel et al.46 to resort to a three-solvent, solid–liquid extraction, and a florisil solid-phase cleanup step of postdeployed wristbands to achieve adequate data objectives. Vacuum-heat conditioning produced clean wristbands, reduced laboratory expenses, and decreased staff time while providing wristbands that can be extracted with a single solvent at room temperature or directly through thermal extraction.
Recovery, Transport, and Storage Stability
Demonstrate chemicals are captured and quantitatively and precisely recovered
Through previous efforts in our laboratory, we know wristbands sequester a wide variety of chemicals including flame retardants,45 PAHs,33 pesticides,47 and personal care products.48 Currently, there are few sensor technologies for measuring individual exposure to a wide range of chemicals. Some examples of existing technologies include: brominated flame retardants, OPFRs, and PAHs by active and passive air samplers,14, 17, 52, 54, 55, 56 dust and hand wipe samplers,55, 57 and biological samples.58, 59, 60, 61 The wristband sampler provides one of the only individual sampling technologies available that has been shown to capture both VOCs and SVOCs ranging 10 orders of magnitude of Koa.33, 45, 47, 48, 62 To our knowledge, this is the first demonstration of individual personal samplers that can be used to analyze PCBs, organochlorine pesticides, organophosphate pesticides, pyrethroid pesticides, PBDEs and triphenylphosphate, PAHs, alky substituted PAHs, consumer and industrial chemicals, as well as BTEX and aromatic, chlorinated, and alkane VOCs. While explicitly testing 148 chemicals, extrapolation of these results to other chemicals within the same physical–chemical boundaries is expected to yield similar results, especially for chemicals within chemical classes like PAHs, PCBs, PBDEs, pyrethroids, and alkanes. For example, although we only explicitly tested a single penta-PCB, we would reasonably expect to recover all penta-PCB congeners.
Transport and storage stability of VOCs and SVOCs captured in the wristband
We examined wristband transport and storage stability conditions to determine whether temperature or duration influences the recovery of organic chemicals from the wristband samplers. Chemical stability and potential degradation of VOCs and SVOCs during transport and storage are commonly cited problems in complex environmental matrices.63 For instance, epidemiological studies collecting biological samples in the field often require special transport considerations, including freezing (e.g., dry ice or colder) samples immediately upon collection that can further complicate a study design and reduce compliance.64 Furthermore, longer-term storage stability data are not always obtained or reported for sensors, biological matrices, or sampling techniques that can contribute to increased uncertainty or comparability between studies. The lack of degradation or loss by diffusion of VOCs and SVOCs even with elevated temperature (e.g., 4 °C or +30 °C) over simulated transport conditions provides many practical advantages with regard to study design and compliance. Storage at +30 °C resulted in some loss of VOCs after 1 month that had average recoveries of 66%. Consequently, we would not recommend keeping wristbands for VOC analysis at +30 °C for longer than 7 days.
An accelerated storage stability study at +30 °C for 1 month found 124 of the 148 chemicals were within the 70% to 130% recovery range, and of the 24 chemicals outside the range, 17 were VOCs. Noteworthy, all 31 VOCs were stable at −20 °C at the longest period tested (3 months) and all 117 SVOCs were above 70% recovery at −20 °C for 6 months. We might hypothesize that at longer time periods (e.g., 900 days) some chemical loss might occur especially for VOCs.
Here we demonstrated with simulated long-term storage at −20 °C for 6 months that 105 out of 117 SVOCs were within 70–130% recovery range. All 12 chemicals that were outside this range were above 130% recovery, generally by only a few percent, suggesting SVOCs are stable in wristbands under these conditions. We also demonstrated that all 31 VOCs are stable at −20 °C for at least 3 months. The stability of VOCs and SVOCs while stored provides many practical advantages over other sensors or matrices. For instance, even water USEPA SVOC and VOCs methods 8270 and 8260 maintains that extractions be completed in 7 and 14 days, respectively. The clean wristband polymer may provide a stabilizing matrix platform for many chemicals as compared with more complex matrices.
Estimate of Silicone–Air Partitioning Coefficients
Although wristbands concentrations can be compared among participants in a study without further calculations, an additional application would be to estimate air concentrations (ng/m3) around the individual. For passive samplers, partition coefficients are developed in order to calculate air concentrations from chemical concentrations in the passive sampler. In the present study, paired wristband passive samplers and low-volume personal active samplers were used to estimate Ksa, a silicone wristband–air partition coefficient, for specific PAHs.65 Deriving Ksa values will help researchers calculate concentrations of chemicals in air (i.e., PAHs) from wristbands.
The linear regression of log Koa and estimated log Ksa for 12 PAHs in the paired sampler study indicates that there is a range of Ksa values for each PAH. This is expected because of differences in wristband personal environments across the 22 study participants. Despite this variability, a relationship emerges between the PAH log Koa and the estimated log Ksa values (Eq. (1)).65 Four PAHs were used because they were in equilibrium by the end of the deployment period. For PAHs that did not reach equilibrium during the study, one would expect the Ksa to be low because the PAH concentration in the wristband silicone has not yet attained equilibrium. As expected, 8 PAHs not at equilibrium in the wristbands all fall below the line of best fit in Figure 3 because their true Ksa values cannot be calculated until they reach equilibrium. It is consistent to anticipate that the measured log Ksa of those 8 PAHs, once at equilibrium, would fall along the line of best fit. Future research is warranted to confirm this relationship, and to determine whether Ksa/Koa trends are similar for other chemical classes.
Numerous researchers have determined partition coefficients between passive samplers and air using active sampling equipment with models that include several chemical classes, such as PAHs, PCBs, pesticides, and chlorobenzenes.27, 65, 66 In these previous works, linear relationships are drawn between log Ksa and log Koa, with low sample size, but R2>0.8. In contrast, our Eq. (1) is derived from 86 data points across 4 PAHs and 22 individuals, resulting in a low model fit R2=0.17. Interestingly, when we perform a separate linear regression for each of the 22 individuals, model fits are substantially improved with average R2=0.59 (range: 0.06–0.92, Supplementary Figure S4). However, by using all 22 individuals to predict Ksa, we incorporate variability among individuals when combined into a single regression. Variation is expected to be greater among the wristbands in this study between individuals as both inhalation and dermal exposure sources can be sampled. Moreover, the localized wrist environment likely differs between individuals from external factors such as clothing worn around the sampler or temperature differences during absorption.
Study Limitations
One challenge is accurately calculating an environmental (or a personal exposure) concentration of a chemical from what is measured in any passive sampling device. This requires either achieving equilibrium for all chemicals, which is not practical, or by adding labeled chemicals to the wristband before deployment to act as performance reference chemicals (PRCs). The amount of PRC that diffuses out of the wristband during deployment can be used to estimate the status of equilibrium for that chemical. With the use of Ksa, as developed here, and PRCs, the environmental concentration can be estimated. PRCs are used to calculate sample-specific, chemical sampling rates by the wristband. The PRC approach is accepted by the scientific community.27, 65, 67, 68, 69 The rate at which PRCs diffuse from wristbands is controlled by conditions of the external environment, the properties of the chemical itself, and characteristics of the sampler, such as wristband mass and thickness. PRCs can be added to wristbands in concentrations that are generally regarded as safe. For example, pyrene is a chemical with a relative potency factor for cancer risk of zero70 and could be used as a PRC if used in low ng/g wristband concentrations and approved by IRB before deployment.70, 71
Passive sampling produces time-integrated concentrations of chemicals.28, 29 This can be either a strength or a weakness of the approach, depending on the goals of the study. It is a strength if the objective is to assess a person’s total individual chemical exposure over a period of time, or to measure an average concentration of a chemical over a given time period.32, 72 It is a weakness, however, if the objective is to capture the elevated concentration at a specific moment during a pulse of contamination.
Wristbands used in this paired study to develop Ksa values were worn on the wrist, and potentially incorporate some dermal along with the gas-phase PAH exposure routes. Additional work is needed to parse out the influence of dermal and gas-phase PAH exposure to silicone–air partition coefficients that likely varies by chemical. In future studies, wristbands may also be worn on clothing such as a lapel, as demonstrated in O’Connell et al.,33 when environmental exposure studies are intended to include only measures of inhalation exposures.
Conclusions
We present a quantitative methodology that can measure volatile and semivolatile chemicals in wristband samplers that would complement current monitoring methods. Many chemicals classes were illustrated spanning a log Koa range of 2.1 to 13.7, and it is expected that other chemicals, although not explicitly tested in this study, would have similar stability characteristics. General guidelines for transport in air-tight bags for 1 or 2 weeks, even at +30 °C, are acceptable. Storage at 4 °C or −20 °C for SVOC and −20 °C for VOCs out to 6 and 3 months, respectively, is also acceptable. Based on the accelerated storage stability results, longer storage times at −20 °C will likely also be successful and studies to confirm this are currently underway. We also provide the first estimates of wristband–air partition coefficients for future use in determining environmental concentrations. Wristbands are a non-invasive, easy-to-use candidate technology that measures personal exposure to multiple non-polar and semipolar, volatile and semivolatile organic chemical mixtures.
Acknowledgments
This research was supported by FSES Program at Oregon State University, and by the National Institute of Environmental Health Sciences (NIEHS) under award numbers 1R21ES024718 and 4R33ES024718. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH. HMD was supported in part by NIEHS Fellowship T32ES007060 and the ARCS Foundation. We thank Blair Paulik, Alan Bergmann, David Minick, Josh Willmarth, and Lindsay Denluck at Oregon State, Darrell Holmes and Lehyla Calero at CCCEH, and David Camann at Southwest Research Institute.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
KAA and SGO disclose a financial interest in MyExposome that is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by OSU in accordance with its policy on research conflict of interest. The other authors declare no conflict of interest.
Supplementary Material
References
- Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001-2006). Environ Health Perspect 2014; 122: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environ Health 2013; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström C-E, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 2002; 110 (Suppl 3): 451–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci 2013; 133: 144–156. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocrine Disruptors 2013; 1: e26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA et al. Is the PentaBDE replacement, tris (1, 3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol 2011; 256: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP. The exposome: from concept to utility. Int J Epidemiol 2012; 41 (1): 24–32. [DOI] [PubMed] [Google Scholar]
- Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol 2011; 21: 5–9. [DOI] [PubMed] [Google Scholar]
- Fan Z, Jung KH, Lioy PJ. Development of a passive sampler to measure personal exposure to gaseous PAHs in community settings. Environ Sci Technol 2006; 40: 6051–6057. [DOI] [PubMed] [Google Scholar]
- McBride SJ, Ferro AR, Ott WR, Switzer P, Hildemann LM. Investigations of the proximity effect for pollutants in the indoor environment. J Exposure Anal Environ Epidemiol 1999; 9: 602–621. [DOI] [PubMed] [Google Scholar]
- Wallace L. Indoor particles: a review. J Air Waste Manage Assoc 1996; 46: 98–126. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu X, Jung KH, Ohman-Strickland P, Bonanno LJ, Lioy PJ. Ambient concentrations and personal exposure to polycyclic aromatic hydrocarbons (PAH) in an urban community with mixed sources of air pollution. J Expo Sci Environ Epidemiol 2011; 21: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect 2004; 112: 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin P, Jones KC, Strandberg B. Occupational and indoor air exposure to persistent organic pollutants: a review of passive sampling techniques and needs. J Environ Monit 2007; 9: 501–509. [DOI] [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 2004; 126: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Maugeri U, Mrozek-Budzyn D, Mroz E, Klimaszewska-Rembiasz M et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr Allergy Immunol 2010; 21: 4p2 e723–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC et al. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J Expo Sci Environ Epidemiol 2012; 22: 70–81. [DOI] [PubMed] [Google Scholar]
- Tsai P-J, Vincente JH. A study of workers' exposures to the inhalable and ‘total’aerosol fractions in the primary nickel production industry using mannequins to simulate personal sampling. Ann Occup Hyg 2001; 45: 385–394. [DOI] [PubMed] [Google Scholar]
- Wild CP, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ Mol Mutag 2013; 54: 480–499. [DOI] [PubMed] [Google Scholar]
- Paulik LB, Anderson KAConsiderations for measuring exposure to chemical mixtures. In: Rider C, Simmons JE (eds). Chemical Mixtures and Combined Chemical and Nonchemical Stressors: Exposure, Toxicity, Analysis and Risk. Springer: New York, NY, in press. [Google Scholar]
- Mayeux R. Biomarkers: potential uses and limitations. NeuroRx 2004; 1: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PB, Burke TA, Hubal EAC, Cura JJ, McKone TE. Using biomarkers to inform cumulative risk assessment. Environ Health Perspect 2007; 115: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect 2015; 123: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward LL, Hays SM, Smolders R, Koch HM, Cocker J, Jones K et al. Sources of variability in biomarker concentrations. J Toxicol Environ Health 2014; 17: 45–61. [DOI] [PubMed] [Google Scholar]
- Paustenbach D, Galbraith D. Biomonitoring and biomarkers: exposure assessment will never be the same. Environ Health Perspect 2006; 114: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Barr DB, Calafat AM. Characterizing children's exposures: beyond NHANES. Neurotoxicology 2005; 26: 547–553. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Booij K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices. Springer Science & Business Media. 2006. [Google Scholar]
- Petty JD, Huckins JN, Zajicek JL. Application of semipermeable membrane devices (SPMDs) as passive air samplers. Chemosphere 1993; 27: 1609–1624. [Google Scholar]
- Greenberg MS, Chapman PM, Allan IJ, Anderson KA, Apitz SE, Beegan C et al. Passive sampling methods for contaminated sediments: Risk assessment and management. Integr Environ Assess Manage 2014; 10: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GA, Gravell A, Vrana B, Harman C, Budzinski H, Mazzella N et al. Measurement of environmental pollutants using passive sampling devices–an updated commentary on the current state of the art. Environ Sci Processes Impacts 2014; 16: 369–373. [DOI] [PubMed] [Google Scholar]
- Paulik LB, Donald CE, Smith BW, Tidwell LG, Hobbie KA, Kincl L et al. Emissions of polycyclic aromatic hydrocarbons from natural gas extraction into air. Environ Sci Technol 2016; 50: 7921–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell LG, Allan SE, O’Connell SG, Hobbie KA, Smith BW, Anderson KA. PAH and OPAH flux during the deepwater horizon incident. Environ Sci Technol 2016; 50: 7489–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA. Silicone wristbands as personal passive samplers. Environ Sci Technol 2014; 48: 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Anderson KA. Impact of the deepwater horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol 2012; 46: 2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CA, Khairy MA, Muir DC, Lohmann R. Significance of population centers as sources of gaseous and dissolved PAHs in the lower Great Lakes. Environ Sci Technol 2014; 48: 7789–7797. [DOI] [PubMed] [Google Scholar]
- O'Connell SG, McCartney MA, Paulik LB, Allan SE, Tidwell LG, Wilson G et al. Improvements in pollutant monitoring: Optimizing silicone for co-deployment with polyethylene passive sampling devices. Environ Pollut 2014; 193: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Lao W, Maruya KA, Burgess RM. Calculating the diffusive flux of persistent organic pollutants between sediments and the water column on the Palos Verdes Shelf Superfund Site using polymeric passive samplers. Environ Sci Technol 2014; 48: 3925–3934. [DOI] [PubMed] [Google Scholar]
- Liu H-H, Bao L-J, Feng W-H, Xu S-P, Wu F-C, Zeng EY. A multisection passive sampler for measuring sediment porewater profile of dichlorodiphenyltrichloroethane and its metabolites. Anal Chem 2013; 85: 7117–7124. [DOI] [PubMed] [Google Scholar]
- Namieśnik J, Zabiegała B, Kot-Wasik A, Partyka M, Wasik A. Passive sampling and/or extraction techniques in environmental analysis: a review. Anal Bioanal Chem 2005; 381: 279–301. [DOI] [PubMed] [Google Scholar]
- Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health 2007; 80: 711–719. [DOI] [PubMed] [Google Scholar]
- Anderson K, Hillwalker W. Bioavailability. Encyclopedia of Ecology 2008. pp 348–357.
- Paulik LB, Smith BW, Bergmann AJ, Sower GJ, Forsberg ND, Teeguarden JG et al. Passive samplers accurately predict PAH levels in resident crayfish. Sci Total Environ 2016; 544: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij K, Smedes F, Van Weerlee EM, Honkoop PJ. Environmental monitoring of hydrophobic organic contaminants: the case of mussels versus semipermeable membrane devices. Environ Sci Technol 2006; 40: 3893–3900. [DOI] [PubMed] [Google Scholar]
- Forsberg ND, Smith BW, Sower GJ, Anderson KA. Predicting polycyclic aromatic hydrocarbon concentrations in resident aquatic organisms using passive samplers and partial least-squares calibration. Environ Sci Technol 2014; 48: 6291–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M et al. Using silicone wristbands to evaluate preschool children's exposure to flame retardants. Environ Res 2016; 147: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ Sci Technol 2016; 50: 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC et al. Silicone wristbands detect individuals' pesticide exposures in West Africa. R Soc Open Sci 2016; 3: 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann AJ, North PE, Vasquez L, Bello H, Ruiz M, Anderson KA. Multi-class chemical exposure in rural Peru using silicone wristbandsJ Exp Sci Environ Epidemiol 2017. [DOI] [PMC free article] [PubMed]
- Calafat AM, Needham LL. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ Health Perspect 2009; 117: 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH harmonised tripartite guideline: Stability testing of new drug substances and productsInternational Conference on Harmonization: Geneva. 2003. [Google Scholar]
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J Chromatogr A 2015; 1419: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai W-Y, Kinney P, Camann D, Barr D et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect 2003; 111: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch D, Tiku ML. Robustness of Statistical Methods and Nonparametric Statistics. Springer Science & Business Media. 2012. [Google Scholar]
- Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environ Sci Technol 2004; 38: 34–41. [DOI] [PubMed] [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Östman C. Organophosphate and phthalate esters in air and settled dust–a multi-location indoor study. Indoor Air 2011; 21: 67–76. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012; 88: 1119–1153. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014; 116: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ, Rawn DF. The brominated flame retardants, PBDEs and HBCD, in Canadian human milk samples collected from 1992 to 2005; concentrations and trends. Environ Int 2014; 70: 1–8. [DOI] [PubMed] [Google Scholar]
- Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 2010; 12: 943–951. [DOI] [PubMed] [Google Scholar]
- Cooper E, Covaci A, Van Nuijs A, Webster T, Stapleton H. Analysis of the flame retardant metabolites bis (1, 3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 2011; 401: 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol 2008; 42: 1377–1384. [DOI] [PubMed] [Google Scholar]
- Turner MC, Nieuwenhuijsen M, Anderson K, Balshaw DM, Cui Y, Dunton G et al. Assessing the exposome with external measures: commentary on the state of the science and research recommendations. Annu Rev Public Health 2017; 38: 215–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 2006; 40: 6247–6254. [DOI] [PubMed] [Google Scholar]
- Cariou R, Antignac J-P, Marchand P, Berrebi A, Zalko D, Andre F et al. New multiresidue analytical method dedicated to trace level measurement of brominated flame retardants in human biological matrices. J Chromatogr A 2005; 1100: 144–152. [DOI] [PubMed] [Google Scholar]
- Khairy MA, Lohmann R. Field validation of polyethylene passive air samplers for parent and alkylated PAHs in Alexandria, Egypt. Environ Sci Technol 2012; 46: 3990–3998. [DOI] [PubMed] [Google Scholar]
- Lohmann R. Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ Sci Technol 2011; 46: 606–618. [DOI] [PubMed] [Google Scholar]
- Khairy MA, Lohmann R. Field calibration of low density polyethylene passive samplers for gaseous POPs. Env Sci Process Impact 2014; 16: 414–421. [DOI] [PubMed] [Google Scholar]
- Vrana B, Schüürmann G. Calibrating the uptake kinetics of semipermeable membrane devices in water: Impact of hydrodynamics. Environ Sci Technol 2002; 36: 290–296. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Booij K, Paschke A, Vrana B, Mills GA, Greenwood R. Field performance of seven passive sampling devices for monitoring of hydrophobic substances. Environ Sci Technol 2009; 43: 5383–5390. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Development of a Relative Potency Factor (Rpf) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures (External Review Draft). U.S. Environmental Protection Agency: Washington, DC, EPA/635/R-08/012A, 2010.
- Forsberg ND, Stone D, Harding A, Harper B, Harris S, Matzke MM et al. Effect of native American fish smoking methods on dietary exposure to polycyclic aromatic hydrocarbons and possible risks to human health. J Agric Food Chem 2012; 60: 6899–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairy MA, Lohmann R. Field validation of polyethylene passive air samplers for parent and alkylated PAHs in Alexandria, Egypt. Environ Sci Technol 2012; 46: 3990–3998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.