Abstract
Chronic congestive heart failure (CHF) is the end outcome of organic heart diseases and one of the major diseases harmful to human health. Renal sympathetic denervation (RSD) is the anatomical basis of transcatheter renal sympathetic nerve ablation within the renal artery. To date, the roles of norepinephrine and angiotensin II (Ang II) in myocardial apoptosis and their underlying mechanisms have not been well explored. The aim of the present study was to verify the hypothesis that RSD is likely to inhibit myocardial apoptosis by inhibiting the release of norepinephrine and Ang II. An isoproterenol-induced CHF rat model was established, and the effects of RSD on myocardial apoptosis were examined using flow cytometry and TUNEL staining. The expression of factors associated with myocardial apoptosis, including p53, tumor necrosis factor-α (TNF-α), nuclear factor-κB (NF-κB), caspase-2 and −3, were measured using quantitative polymerase chain reaction and western blot analysis. The results indicated that the mRNA levels of p53, TNF-α, NF-κB, caspase-2 and −3 were significantly reduced in the myocardial tissues of rats in the CHF+RSD group when compared with the levels in the CHF+sham group (P<0.01 for all). In addition, the protein levels of p53, TNF-α, NF-κB and caspases-2 and −3 were decreased by 42.6, 41.3, 46.7, 30.0 and 35.8%, respectively, in myocardial tissues of rats in the CHF+RSD group in comparison with the CHF+sham group (P<0.01 for all). Furthermore, myocardial apoptosis was improved in rats in the CHF+RSD group compared with that in the CHF+sham group (P<0.01). In conclusion, the present study provides a theoretical basis for application of RSD in the treatment of CHF.
Keywords: congestive heart failure, myocardial apoptosis, nuclear factor-κB, renal sympathetic denervation, tumor necrosis factor-α
Introduction
Chronic congestive heart failure (CHF) is the end outcome of organic heart diseases and one of the major diseases threatening human health. Heart failure occurs in 0.9% of Chinese adults (1), and has become a significant social and public health issue. Cardiac remodeling is an important pathophysiological process in the event of CHF, and involves co-action of myocardial apoptosis, cardiac hypertrophy and myocardial fibrosis (2). Apoptosis is a gene-controlled autonomous program cell death process involving activation, expression and regulation of a series of genes (3). Myocardial apoptosis occurs in a variety of cardiovascular diseases and is closely associated with arrhythmia and cardiac dysfunctions (4). Although the detailed mechanisms of the apoptotic process are not fully understood, members of the caspase family, including caspases 2, 3, 6, 7, 8, 9 and 10, have been confirmed to serve an essential role in apoptosis (5). Tumor suppressor gene p53 is another known regulatory factor closely associated with myocardial apoptosis (6,7). In addition, overactivation of the sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) may lead to myocardial apoptosis (8). Currently, the suppression of excessive activation of the SNS and RAAS in order to further inhibit myocardial apoptosis is an important focus for the treatment of heart failure.
SNS activation is closely associated with myocardial apoptosis; however, this activation increases the serum nore-pinephrine concentration, leading to overactivation of the norepinephrine-tumor necrosis factor-α (TNF-α)-caspase pathway-mediated myocardial apoptosis (9). In addition, SNS activation increases the release of angiotensin II (Ang II), leading to nuclear factor (NF)-κB-mediated myocardial hypertrophy, fibrosis and apoptosis (10). Zelarayan et al (11) demonstrated that p53 serves an important role in Ang II-induced myocardial apoptosis and may also regulate NF-кB expression. SNS activation is also closely correlated with the prognosis of patients with heart failure. It is often accompanied with increased releases of norepinephrine and neuropeptide Y, peripheral vasoconstriction, reduced renal blood flow, and decreased sodium and water excretion, all of which will facilitate the process of heart failure (9).
SNS activation mainly includes cardiac sympathetic nerve activation (CSNA), renal sympathetic nerve activation (RSNA), central nervous system activation, as well as skeletal muscle sympathetic activation (12). The amount of norepinephrine released in the heart and kidney after SNS activation accounted for 62% of the total norepinephrine in the body, suggesting that CSNA and RSNA play essential roles in the onset and progression of heart failure (13). Following renal sympathetic activation, the renal afferent nerve may activate multi-organ sympathetic nerves, including the cardiac sympathetic nerve, central sympathetic nerve and skeletal muscle sympathetic nerve (14), which further leads to increased norepinephrine release and RAS activation, thus contributing to myocardial remodeling. Renal nerves enter the renal parenchyma and renal artery, forming a neural network in renal tubules and glomerulus. Renal nerves have a relatively simple composition. Anatomical studies have confirmed that 95% of renal sympathetic nerves are located on the surface of renal artery (15). To date, parasympathetic nerves have not been found in renal nerves. Therefore, renal denervation (RD) is equal to renal sympathetic denervation (RSD). A previous study has demonstrated that RSD is the anatomical basis for catheter renal sympathetic nerve ablation within the renal artery and easy to implement (16). Transcatheter renal sympathetic ablation has been used for the treatment of hypertension since 2009, and has been proven to be safe and effective in reducing the blood pressure in patients with refractory hypertension (17). Based on the physiological and pathological effects of RSNA and the fact that renal sympathetic activation is a common pathophysiological channel of heart failure and hypertension, it can be speculated that RSD may also be able to improve myocardial remodeling of patients with heart failure. The recent REACH-Pilot trial included patients with CHF and normal blood pressure, and demonstrated that RSD significantly increased the patients' six-minute walking distance without significantly affecting their blood pressure, thus suggesting that renal sympathetic ablation significantly improved the heart function of patients with heart failure (18). However, renal sympathetic ablation had little adverse impacts on the hemodynamics and renal function, suggesting that its impact on the prognosis of patients with heart failure is not mediated by lowering the blood pressure.
The current RSD therapy for heart failure is relatively novel, with only a small number of inpatients participating and a lack of long-term follow-up observations. Certain researchers have explored the role of RSD in myocardial remodeling. Wang et al (19) demonstrated that RSD significantly reduced the serum Ang II and aldosterone concentrations in a canine model of heart failure induced by rapid pacing, and inhibited atria reconstruction. Furthermore, Clayton et al (20) confirmed that RSD inhibited the expression of myocardial Ang II receptor in a rabbit model of heart failure, increased urinary sodium excretion, lowered the plasma brain natriuretic peptide (BNP) level and significantly improved heart function. Hu et al (21) confirmed that RSD significantly improved myocardial remodeling in a rat model of myocardial infarction, increased the urine output and improved cardiac function of the treated model animals.
Considering the importance of norepinephrine and Ang II in myocardial apoptosis, it can be speculated that RSD is likely to inhibit myocardial apoptosis by reducing norepinephrine and Ang II release. However, to the best of our best knowledge, the effects of RSD on myocardial fibrosis and its underlying mechanism have not been previously studied. Therefore, the present study aimed to use an isoproterenol-induced rat model with pressure overload heart failure in order to: i) Investigate the impacts of RSD on myocardial apoptosis to provide a theoretical basis for clinical treatment of heart failure with RSD; ii) explore the impacts of RSD on the expression of factors associated with myocardial apoptosis, including p53, TNF-α, NF-κB, caspase-2 and −3; and iii) analyze the molecular mechanisms underlying the inhibitory effects of RSD on myocardial apoptosis, attempting to provide a theoretical basis for the treatment of heart failure using renal sympathetic ablation. The present study confirmed that renal sympathetic activation was accompanied with an increased release of norepinephrine and Ang II. In addition, norepinephrine was able to induce myocardial apoptosis through free peroxy radicals-TNF-α-caspase signaling pathway, and Ang II induced myocardial apoptosis by regulating the expression levels of p53 and NF-кB.
Materials and methods
Experimental animals
A total of 70 4-week-old male cleaning grade Sprague-Dawley rats weighing 250–350 g were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). The rats were housed at an environment with temperature of 23±2°C, humidity of 61±5% and light/dark cycle of 12 h, and were fed with a standard diet. All rats were adapted to the environment for 1 week prior to conducting any experiments in compliance with the procedures of the Animal Ethics Committee of Wuhan University (Wuhan, China). The study was approved by appropriate local institutional review boards at the Renmin Hospital of Wuhan University (Wuhan, China) and conformed to the guidelines set forth by the Declaration of Helsinki.
Animal grouping
The rats were randomly divided into four groups, namely the CHF+sham, CHF+RSD, control (in which the rats were untreated) and sham groups, with 15 rats each in the control and sham groups, and 20 rats each in the CHF+sham and CHF+RSD groups. Rats in the sham group were subjected to pseudo-RSD (in which a sham procedure was performed on the rats), while rats in the CHF+sham were subjected to pseudo-RSD followed by induction of heart failure 1 week later. Rats in the CHF+RSD were subjected to RSD followed by induction of heart failure 1 week later.
Conduction of RSD and induction of heart failure
To perform RSD, rats were anesthetized by intraperitoneal injection of 40 mg/kg sodium pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Abdominal skin was prepared, disinfected and covered with towels. An incision was made on the skin along the abdominal linea alba, and subcutaneous tissue and muscles were separated layer by layer to expose the kidney, ureter as well as intrathecal arteries, veins and nerves. The artery and vein sheaths were stripped under a dissecting microscope (magnification, ×25) to disarticulate all visible nerve fibers, which along with their surroundings were further wiped with a cotton ball dipped in 10% phenol solution to achieve sufficient denervation effect. To ensure thorough denervation, the degree of renal denervation was evaluated using an electrical stimulation method (5). Prior to RSD, a significant sympathetic response (indicated by systolic blood pressure increase by 5–10 mmHg, heart rate increase by 8–15 beats/min and pale kidney due to renal artery contraction) was induced by stimulating the proximal renal nerve for 10–30 sec with square wave provided by ML 1001 (15 V, 0.2 msec, 10 Hz) using a 5Fr radiofrequency catheter (Ablaze; Japan Lifeline Co., Ltd., Tokyo, Japan). Subsequent to RSD, the aforementioned responses such as SBP and heart rate completely disappeared. The procedure strictly followed the requirements for aseptic surgery. At 3 days after surgery, 80,000 U penicillin was intraperitoneally injected per day for 10 days to prevent infection. To induce heart failure, 4 mg/kg isoproterenol (cat. no. EY0883; Amquar Biological Technology Co., Ltd., Shanghai, China) was subcutaneously injected once daily for 10 days at 3 days after surgery with caution to avoid withdraw and damaging blood vessel.
Determination of plasma norepinephrine, Ang II and aldosterone levels
At the end of the experimental observation, peripheral blood was withdrawn from each rat into a tube containing 10% EDTA and aprotinin. Subsequent to centrifugation at 1,350 × g for 20 min at 4°C, plasma was collected and stored at −70°C until further use. The plasma contents of norepinephrine, Ang II and aldosterone were measured using ELISA kits according to the instructions provided by the manufacturer (cat. no. PH003RAT; Phygene Life Sciences, Fuzhou, China for Ang II), (ab136933; Abcam, Cambridge, UK for aldosterone), (PH031UNI; Phygene Life Sciences for norepinephrine). The results were converted to the actual content by comparing to the GAPDH as standard.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of differential mRNA expression in myocardial tissues
RT-qPCR analysis was used to detect the differential mRNA expression levels of p53, TNF-α, NF-κB, caspase-2 and −3 between the RSD and normal myocardial tissues. Rats were sacrificed and myocardial tissues were extracted 6 weeks after establishing heart failure from rats in the control, sham, CHF+sham and CHF+RSD groups. The tissues were ground and dissolved in an appropriate amount of RNAiso Plus solution (Takara, Dalian, China). Total RNA was then extracted using TRIzol reagent (Takara) and reverse transcribed into cDNA using the first-strand cDNA kit (cat. no. FSQ-101; Toyobo Co., Ltd., Tokyo, Japan) and oligo(dT)15. RT-qPCR analysis was performed using FastStart Universal SYBR Green kit (Roche Applied Science, Mannhein, Germany) in a 10 µl reaction system containing 2 µl cDNA template, 5 µl SYBR Green Mix with ROX, 200 nM forward reaction primer and 200 nM reverse reaction primer. Table I lists the primer sequences for p53, TNF-α, NF-κB, caspase 2 and caspase 3. Differential expression levels of RCR products were analyzed using melting curves and the 2−ΔΔCq method (22). Rat GAPDH was used as an internal control, and all experiments were performed for at least three times.
Table I.
Primers used in RT-qPCR.
| Gene (rat) | Forward primer | Reverse primer |
|---|---|---|
| p53 | 5′-TCCCCAGCAAAAGAAAAAAC-3′ | 5′-GCACGGGCATCCTTTAATT-3′ |
| TNF-α | 5′-GGCCACCACGCTCTTCTGT-3′ | 5′-CGGGCTTGTCACTCGAGTTT-3′ |
| NF-κB | 5′-ATTTCGATTCCGCTACGTGTG-3′ | 5′-GGGCGTGCAGGTGAATATTT-3′ |
| Caspase-2 | 5′-TGACCAGACTGCACAGGAAAT-3′ | 5′-ACCCCGTAGATGCCACCTT-3′ |
| Caspase-3 | 5′-CCCTGAAATGGGCTTGTGTAT-3′ | 5′-GGGCCATGAATGTCTCTCTGA-3′ |
| GAPDH | 5′-GAGCGAGATCCCGCTAACATC-3′ | 5′-GCGGAGATGATGACCCTTTTG-3′ |
RT-qPCR, reverse transcription-quantitative polymerase chain reaction; TNF, tumor necrosis factor; NF, nuclear factor.
Western blot analysis for the detection of differences in protein expression in the rat myocardium
Western blot analysis was used to detect the protein expression levels of p53, TNF-α, NF-κB, caspase-2 and −3 in the rat myocardium. First, myocardial tissues were extracted from rats in the CHF+sham, CHF+RSD, control and sham groups, respectively. The tissues were dissolved in ice-cold TNEN lysis buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2.0 mM EDTA and 1.0% Nonidet P-40) supplemented with trace of EDTA, protease inhibitors (Roche Applied Science) and 1 mmol/l phenylmethylsulfonyl fluoride. Following incubation at 4°C for 30 min, samples were centrifuged at 12,000 × g for 15 min at 4°C to remove pellets. Next, 100 µl supernatant was mixed with 20 µl of 6X Laemmli buffer (0.3 mol/l Tris-HCl, 6% SDS, 60% glycerol, 120 mmol/l dithiothreitol and tracer dye bromophenol blue) and incubated at 37°C for 10 min. Subsequently, 20-µl aliquots of the samples were subjected to 10% SDS-PAGE and transferred onto a 0.45 µm polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was then incubated with monoclonal rabbit antibodies against p53 (cat. no. ab26, 1:1,000, mouse monoclonal antibody), TNF-α (cat. no. ab6671, 1:1,000, rabbit polyclonal antibody), NF-κB (cat. no. ab32360, 1:5,000, rabbit monoclonal antibody), caspase-2 (cat. no. ab179520, 1:1,000, rabbit monoclonal antibody) and caspase-3 (cat. no. ab13847, 1:500, rabbit polyclonal antibody), as well as with the internal control GAPDH (1:3,000, mouse monoclonal antibody) (all from Abcam), followed by incubation with secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (1:10,000, BL003A) and goat anti-mouse antibody (1:10,000, BL001A) (both from Biosharp, Beijing, China). The labeled proteins were then visualized with SuperSignal West Pico chemiluminescence imaging system (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The protein levels were quantified by calculating the grayscale values of the three independent repeats scanned by Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments were performed for at least three independent repeats.
Quantitative measurement of myocardial apoptosis using flow cytometry
Rat myocardial apoptosis was examined using FITC-labeled Annexin V/flow cytometry. Annexin V (cat. no. KGA106; KeyGen Biotech Co., Ltd., Nanjing, China) is a calcium-dependent phospholipid binding protein with high affinity for binding to phosphatidylserine on the outer membrane of apoptotic cells. FITC-labeled Annexin V is commonly used as a sensitive reagent to quantitatively detect early apoptotic cells by counting the percentage of positive apoptotic cells. In this experiment, myocardial tissues were collected from rats in the CHF+sham, CHF+RSD, control and sham groups and suspended in chilled 1X phosphate-buffered saline (PBS) solution. The tissues were then digested with fibrinogen and collagenase to obtain single cells. A total of 106 cells were mixed with 100 µl binding buffer and incubated with 10 µl FITC-labeled Annexin V (20 µg/ml) in the dark at room temperature for 30 min, followed by incubation with 5 µl PI (50 µg/ml) in the dark for 5 min. Cells were then diluted with 400 µl binding buffer and immediately subjected to FACScan flow cytometry to quantitatively measure the percentage of apoptotic cells using cells without FITC-labeled Annexin V and PI as a negative control.
TUNEL assay and laser scanning confocal microscopy to detect myocardial apoptosis
TUNEL method is commonly used for detecting apoptosis. In the present study, TUNEL assay (Beckman Coulter, Inc., Brea, CA, USA) was applied together with laser scanning confocal microscopy (LSCM) to detect myocardial apoptosis in rat tissues in the CHF+sham, CHF+RSD, control and sham groups. Briefly, the rat heart were extracted, fixed in 4% polyformaldehyde solution, paraffin-embedded and prepared into 5-µm sections. These sections were dewaxed, treated with 3% H2O2 for 10 min to block endogenous peroxidase activity, rinsed three times with 1X PBS for 5 min each and digested with fresh proteinase K working solution (10 mg/l in 10 mmol/l Tris-HCl, pH 7.4–8.0) at room temperature for 20 min. After rinsing again with 1X PBS three times for 5 min each, the sections were incubated with TUNEL solution in the dark at 37°C in a humidified chamber for 60 min, and rinsed with 1X PBS three times for 5 min each time. Subsequently, the samples were incubated with horseradish peroxidase-labeled anti-FITC antibody (cat. no. C1063, Annexin V-FITC kit; Beyotime Institute of Biotechnology, Haimen, China) at 37°C in a humidified chamber in dark for 30 min, rinsed as before and stained with DAB for 5–10 min. After further rinsing as before, the sections were stained with hematoxylin for a few sec, washed with running water, dehydrated, mounted on slides and sealed with neutral gum. Negative controls were prepared in a similar manner, but without TdT in the TUNEL reaction solution. The slides were subjected to LSCM at a magnification of ×400 to observe the level of myocardial apoptosis of rats in each group.
Statistical analysis
Data are expressed as the mean ± standard deviation. SPSS software version l7.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. One-way analysis of variance was conducted to compare experimental groups, followed by Scheffe's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
RSD significantly inhibits the levels of Ang II and aldosterone in the plasma of rats with heart failure
The present study demonstrated that, following renal sympathetic activation, increased norepinephrine and Ang II were released, while myocardial apoptosis was also increased (Table II). In order to determine whether RSD was able to inhibit myocardial apoptosis of rats with heart failure, ELISA was employed to detect the contents of norepinephrine, Ang II and aldosterone in the plasma of rats in the CHF+sham, CHF+RSD, control and sham groups. The results revealed that the expression levels of plasma Ang II and aldosterone decreased from 491.1±14.8 and 517.3±16.0 in CHF+sham rats to 388.3±13.7 and 408.5±12.3 in the CHF+RSD rats, respectively (P<0.01; Table II). The expression level of norepinephrine was also reduced from 264.6±13.6 in rats of the CHF+sham group to 200.7±8.0 in rats of the CHF+RSD group, although this difference was not statistically significant. By contrast, the expression levels of norepinephrine, Ang II and aldosterone demonstrated no significant differences between rats in the control and sham groups (P>0.05). These experimental results are consistent with a previous study by DiBona (17) using a canine model established with rapid pacing. Thus, RSD was confirmed to lower the CHF-induced release of Ang II and aldosterone induced by CHF.
Table II.
Expression levels of norepinephrine, Ang II and aldosterone in the rat plasma.
| Group | n | Norepinephrine | Ang II | Aldosterone |
|---|---|---|---|---|
| Control | 15 | 159.3±11.1 | 208.5±14.3 | 205.3±14.8 |
| Sham | 13 | 168.2±13.7 | 201.6±10.5 | 197.0±9.5 |
| CHF+sham | 12 | 264.6±13.6 | 491.1±14.8a | 517.3±16.0a |
| CHF+RSD | 10 | 200.7±8.0 | 388.3±13.7b | 408.5±12.3b |
P<0.01 vs. control and sham groups
P<0.01 vs. CHF+sham group. Data are expressed as the mean ± standard deviation. Ang II, angiotensin II; CHF, congestive heart failure; RSD, renal sympathetic denervation.
RSD significantly inhibits the expression of p53, TNF-α, NF-κB, as well as caspases-2 and −3, at the mRNA and protein levels
To determine the effects of RSD on the myocardial apoptosis and remodeling of rats with heart failure, RT-qPCR and western blot analyses were applied to detect the expression levels of the apoptosis-associated factors p53, TNF-α, NF-κB, caspase-2 and −3 at the transcriptional and translational levels. The results revealed that RSD significantly inhibited the expression levels of p53, TNF-α, NF-κB, caspase-2 and −3. In addition, myocardial tissues were extracted from rats in the CHF+sham, CHF+RSD, control and sham groups, and used to measure the mRNA expression by qPCR. The results showed that the mRNA expression levels of the apoptotic factors p53, TNF-α, NF-κB, caspase-2 and −3 in the myocardial tissues of rats in the CHF+RSD group were significantly reduced by 71.6, 39.3, 42.3, 59.1 and 60.7%, respectively, compared with those in the CHF+sham group (Fig. 1; P=7.45×10−7, 4.56×10−8, 4.33×10−9, 1.41×10−7 and 3.00×10−6, respectively). Furthermore, western blot analysis revealed that, compared with the CHF+sham group, the protein levels of p53, TNF-α, NF-κB, caspase-2 and −3 were reduced by 42.6, 41.3, 46.7, 30.0 and 35.8%, respectively, in the myocardial tissues of CHF+RSD rats (Figs. 2 and 3; P=0.002, 1.07×10−4, 1.13×10−4, 1.39×10−4 and 0.002, respectively). By contrast, the mRNA and protein levels of p53, TNFα, NF-κB, caspase-2 and −3 were not significantly different between rats in the control and sham groups (P>0.05).
Figure 1.
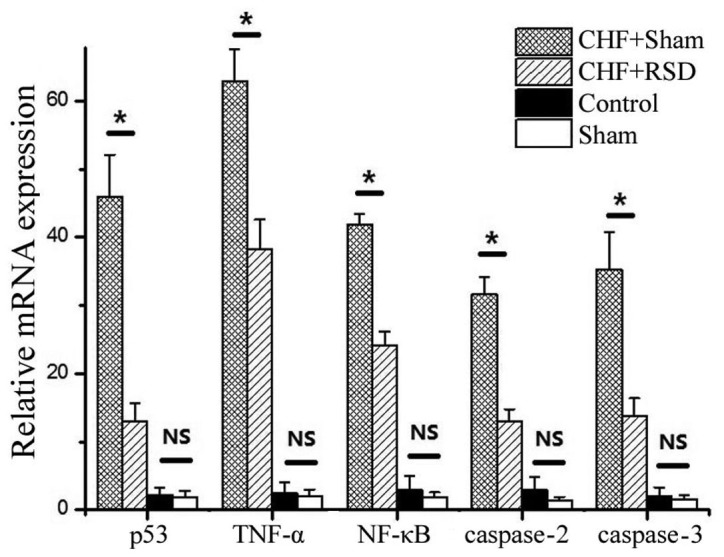
RT-qPCR analysis of apoptosis factors in rat myocardial tissues. The mean mRNA levels of P53, TNF-α, NF-κB, caspase-2 and −3 in myocardial tissues of rats in the CHF+sham, CHF+RSD, control and sham groups are shown from three independent experiments. *P<0.05. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NS, non-significant difference (P>0.05). CHF, congestive heart failure; RSD, renal sympathetic denervation; TNF, tumor necrosis factor; NF, nuclear factor.
Figure 2.
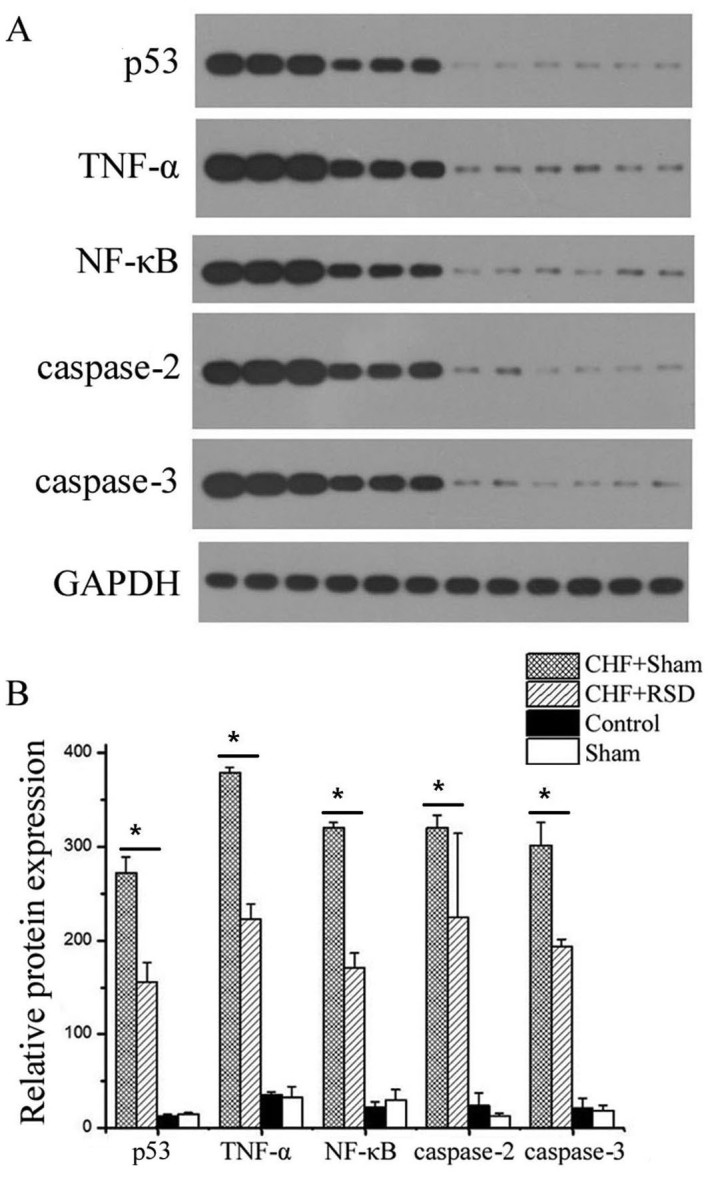
Western blot analysis of apoptosis factors in rat myocardial tissues. (A) Representative western blots and (B) quantified protein expression levels of P53, TNF-α, NF-κB, caspase-2 and −3 in myocardial tissues of rats in the CHF+sham, CHF+RSD, control and sham groups from three independent experiments with similar results. *P<0.05. CHF, congestive heart failure; RSD, renal sympathetic denervation; TNF, tumor necrosis factor; NF, nuclear factor.
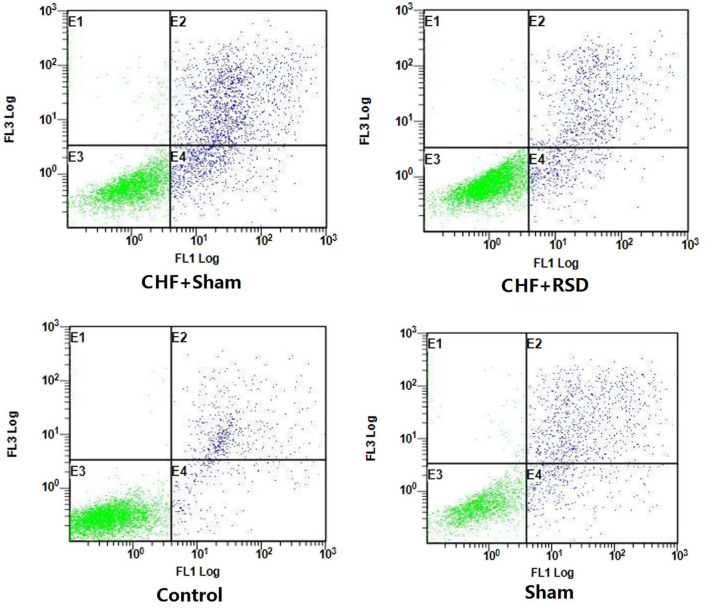
Figure 3.
Apoptosis of myocardial tissues of rats in the CHF+sham, CHF+RSD, control and sham groups analyzed using flow cytometry subsequent to staining with FITC-labeled Annexin V and PI. CHF, congestive heart failure; RSD, renal sympathetic denervation.
Overall, the aforementioned results indicated that RSD was able to significantly inhibit the expression of the apoptotic factors p53, TNFα, NF-κB, caspase-2 and −3 in rat myocardial tissues at both the mRNA and protein levels, and these factors were closely associated with myocardial apoptosis.
RSD inhibits apoptosis in myocardial tissues
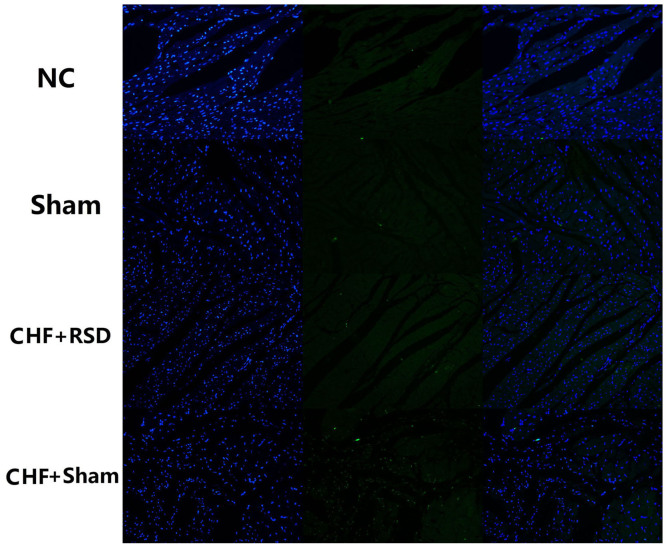
To further determine the impacts of RSD on the apoptotic process of rat myocardial tissues, the apoptotic levels of myocardial tissues of rats in the RSD+sham and CHF+sham groups were detected and compared using Annexin V-FITC/PI-labeled flow cytometry and TUNEL staining/LSCM. The results demonstrated that the apoptosis level of myocardial tissues was decreased from 11.35% in the CHF+sham group to 3.22% in the CHF+RSD group (Fig. 3; P<0.05). However, there was no significantly difference in the apoptosis level between rats in the control and sham groups (Fig. 3; P>0.05). Consistently, TUNEL staining/LSCM also observed that the apoptosis level was significantly decreased in myocardial tissues of rats in the CHF+RSD group compared with that in the CHF+sham group, but showed no significant difference between rats in the control and sham groups (Fig. 4).
Figure 4.
Apoptosis of myocardial tissues of rats in the CHF+sham, CHF+RSD, control and sham groups analyzed using laser scanning confocal microscopy (magnification, ×20) subsequent to staining with TUNEL. Left, DAPI for nuclear staining; middle, FITC for TUNEL; right, merge. CHF, congestive heart failure; RSD, renal sympathetic denervation.
In conclusion, RSD evidently inhibited myocardial apoptosis and intervened in the process of heart failure in the rat tissues.
Discussion
Renal sympathetic activation is the joint pathophysiologic channel of heart failure and hypertension, whereas RSNA serves a crucial role in the onset and progression of heart failure. Subsequent to renal sympathetic activation, renal afferent nerves may induce activation of cardiac, renal, central, skeletal muscle and other multi-organ sympathetic nerves (14), resulting in increased norepinephrine release and RAS system activation, and further promoting the myocardial remodeling process. A previous study has confirmed that catheter-based renal sympathetic nerve ablation can safely and effectively lower the blood pressure of patients with resistant hypertension (16). The REACH pilot study (18) proved that RSD significantly increased the six-minute walking distance of patients with chronic heart failure and normal blood pressure, but could not evidently change their blood pressure, indicating that RSD ablation of renal arteries may significantly improve the cardiac function of heart failure patients with no adverse effects on the hemodynamics and renal function. Previous results have also indicated that the effect of RSD on the prognosis of patients with heart failure is not mediated by the decrease in blood pressure, which suggests the potential application of non-pharmacological treatment for patients with heart failure (18). Recently, Li et al applied RSD to patients with heart dysfunction, myocardial fibrosis, and neurological immune response. The authors observed that RSD significantly improved the myocardial fibrosis and left ventricular and atrial hypertrophy, while inhibiting the SNS, RAAS and arginine vasopressin of rats with transverse aortic constriction (23).
The present study examined the levels of norepinephrine, Ang II and aldosterone in the plasma of rats in different groups. Consistent with the aforementioned results, the present study observed that the expression levels of Ang II and aldosterone in the plasma of rats in the CHF+RSD group were significantly decreased compared with rats in the CHF+sham group. Furthermore, to study the effect of RSD on myocardial apoptosis, a model of rats with heart failure due to pressure load induced by isoproterenol was constructed, and changes in the expression of apoptosis-associated factors were examined. The results revealed that RSD evidently suppressed the expression of apoptosis-associated factors at the mRNA and protein levels. RT-qPCR analysis showed that the mRNA levels of apoptotic factors p53, TNF-α, NF-κB, caspase-2 and −3 in the myocardial tissues of CHF+RSD rats were clearly reduced by 1.6, 39.3, 42.3, 59.1 and 60.7%, respectively, when compared with those in the CHF+sham group. Western blot analysis also observed that the protein levels of these factors in the myocardial tissues of CHF+RSD rats were significantly decreased by 42.6, 41.3, 46.7, 30.0 and 35.8%, respectively, compared with the CHF+sham group. These findings indicated that RSD can effectively reduce myocardial apoptosis after heart failure and improve myocardial remodeling of rats with heart failure. A study by Hu et al investigating rats with heart failure and myocardial infarction also suggested that RSD had an important effect on myocardial remodeling (21,24), which is in a good agreement with the present study results.
TUNEL staining/LSCM was also performed in the present study, and demonstrated that RSD significantly improved myocardial apoptosis in rats with heart failure. In addition, FITC-Annexin V/PI double-labeled flow cytometry found that the apoptosis levels of myocardial tissues of CHF+RSD rats were lowered by 3.22% compared with those of CHF+sham rats (P<0.05), while no significant differences were detected between the control and sham groups (P>0.05). Thus, the results of the two methods investigating myocardial apoptosis were consistent.
Currently, the use of RSD for heart failure therapy is still a novel method. A limited number of inpatients have been treated with RSD and followed up for long term. Grec et al pointed out that RSD treatment not only provides an insight on the treatment of resistant hypertension, but also brings new hope for patients with cardiovascular metabolic diseases (25). Since norepinephrine and Ang II are two important factors for myocardial apoptosis, RSD is likely to achieve its role of inhibiting myocardial apoptosis by suppressing the release of norepinephrine and Ang II. The present study has proven the following: i) Renal sympathetic activation results in increased release of norepinephrine, which further induces myocardial apoptosis through the release of Ang II, which exerts the same role by regulating the expression of p53 and NF-кB; ii) RSD inhibited renal sympathetic activation, thus significantly attenuating the expression levels of p53, TNF-α, NF-κB, caspase-2 and −3, and improving myocardial apoptosis.
In conclusion, the present study confirmed that, by suppressing the release of norepinephrine and Ang II, RSD can significantly inhibit myocardial apoptosis in rats with norepinephrine-induced heart failure, lower the expression levels of important myocardial apoptosis factors p53, TNF-α, NF-κB, caspase-2 and −3, and improve the heart failure conditions of rats with heart failure at the tissue level. The present study not only provides data supporting the use of RSD to treat heart failure, but also provides an insight into the use of non-pharmacological treatment for heart failure.
Acknowledgements
The present study was supported by a grant from the Foundation of Health and Family planning Commission of Hubei Province (no. WJ2015MB293).
References
- 1.Gu DF, Huang GY, He J. Investigation on epidemiology of heart failure in China and the incidence rate. Chin Cardiovascular Dis Magazine. 2003;31:3–6. (In Chinese) [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoynezhad A, Jalali Z, Tortolani AJ. A synopsis of research in cardiac apoptosis and its application to congestive heart failure. Tex Heart Inst J. 2007;34:352–359. [PMC free article] [PubMed] [Google Scholar]
- 5.Bae S, Yalamarti B, Kang PM. Role of caspase-independent apoptosis in cardiovascular diseases, Frontiers in bioscience. A J Virtual Library. 2008;13:2495–2503. doi: 10.2741/2861. [DOI] [PubMed] [Google Scholar]
- 6.Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O'Neill L, Cirielli C, Lakatta EG, Crow MT. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest. 1997;99:2635–2643. doi: 10.1172/JCI119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang EY, Gang H, Aviv Y, Dhingra R, Margulets V, Kirshenbaum LA. p53 mediates autophagy and cell death by a mechanism contingent on Bnip3. Hypertension. 2013;62:70–77. doi: 10.1161/HYPERTENSIONAHA.113.01028. [DOI] [PubMed] [Google Scholar]
- 8.Braam B, Cupples WA, Joles JA, Gaillard C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev. 2012;17:161–175. doi: 10.1007/s10741-011-9246-2. [DOI] [PubMed] [Google Scholar]
- 9.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat cardiomyocytes through a reactive oxygen species-TNF alpha-caspase signaling pathway. Cardiovasc Res. 2004;62:558–567. doi: 10.1016/j.cardiores.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 2004;61:143–151. doi: 10.1016/j.cardiores.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Zelarayan L, Renger A, Noack C, Zafiriou MP, Gehrke C, van der Nagel R, Dietz R, de Windt L, Bergmann MW. NF-kappaB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res. 2009;84:416–424. doi: 10.1093/cvr/cvp237. [DOI] [PubMed] [Google Scholar]
- 12.Booth LC, Ramchandra R, Calzavacca P, May CN. Role of prostaglandins in determining the increased cardiac sympathetic nerve activity in ovine sepsis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R75–R81. doi: 10.1152/ajpregu.00450.2013. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee A, Mir SA, Dutta D, Mitra A, Pathak K, Sarkar S. Analysis of p53 and NF-κB signaling in modulating the cardiomyocyte fate during hypertrophy. J Cell Physiol. 2011;226:2543–2554. doi: 10.1002/jcp.22599. [DOI] [PubMed] [Google Scholar]
- 14.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: Evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.CIR.73.4.615. [DOI] [PubMed] [Google Scholar]
- 15.Mompeo B, Maranillo E, Garcia-Touchard A, Larkin T, Sanudo J. The gross anatomy of the renal sympathetic nerves revisited. Clin Anat. 2016;7:660–664. doi: 10.1002/ca.22720. [DOI] [PubMed] [Google Scholar]
- 16.Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: Renal denervation-an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–841. doi: 10.1097/HJH.0b013e3283599beb. [DOI] [PubMed] [Google Scholar]
- 17.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opinion Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: Primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zhao Q, Huang H, Tang Y, Xiao J, Dai Z, Yu S, Huang C. Effect of renal sympathetic denervation on atrial substrate remodeling in ambulatory canines with prolonged atrial pacing. PLoS One. 2013;8:e64611. doi: 10.1371/journal.pone.0064611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton SC, Haack KK, Zucker IH. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am J Physiol Renal Physiol. 2011;300:F31–F39. doi: 10.1152/ajprenal.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Ji M, Niu C, Aini A, Zhou Q, Zhang L, Jiang T, Yan Y, Hou Y. Effects of renal sympathetic denervation on post-myocardial infarction cardiac remodeling in rats. PLoS One. 2012;7:e45986. doi: 10.1371/journal.pone.0045986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Li ZZ, Jiang H, Chen D, Liu Q, Geng J, Guo JQ, Sun RH, Zhu GQ, Shan QJ. Renal sympathetic denervation improves cardiac dysfunction in rats with chronic pressure overload. Physiol Res. 2015;64:653–662. doi: 10.33549/physiolres.932912. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Yan Y, Zhou Q, Ji M, Niu C, Hou Y, Ge J. Effects of renal denervation on the development of post-myocardial infarction heart failure and cardiac autonomic nervous system in rats. Int J Cardiol. 2014;172:e414–e416. doi: 10.1016/j.ijcard.2013.12.254. [DOI] [PubMed] [Google Scholar]
- 25.Grec V, Buksa M. Are we on the path to solve the enigma of resistant hypertension: Renal sympathetic denervation. Med Arch. 2013;67:454–459. doi: 10.5455/medarh.2013.67.454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]