Abstract
Mucin 1 (MUC1) is a transmembrane glycoprotein that is aberrantly unregulated in numerous types of cancer, including non-small cell lung cancer (NSCLC), and serves a key role as an oncogene in the tumorigenesis of various human adenocarcinomas. Studies have indicated that MUC1 is involved in cell proliferation, invasion and migration. However, the role of MUC1 in NSCLC progression remains poorly understood. The aim of the present study was to investigate the role of MUC1 in stable MUC1-low-expression NSCLC cell lines that were generated by transfection with MUC1-siRNA. Cell Counting Kit-8 assay was preformed to determine the proliferation ability of NSCLC cells, while cell apoptosis was detected using flow cytometry. In addition, the mRNA and protein expression levels of MUC1 were detected by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively. Western blot analysis was also used for detection of other associated proteins. The results demonstrated that, compared with the control group, the cell proliferation ability was significantly declined in the MUC1 inhibition group, and the cell apoptosis rate was markedly increased. Inhibition of MUC1 gene in NCI-H1650 cells suppressed cell proliferation and induced cell apoptosis. In addition, the protein expression levels of vascular endothelial growth factor (VEGF) and VEGF-C were notably decreased by MUC1 inhibition, indicating the anti-angiogenic effect of MUC1 downregulation. Furthermore, inhibition of MUC1 gene with MUC1-siRNA significantly suppressed the phosphorylation of protein kinase B and extracellular signal-regulated kinase. In conclusion, the findings indicated that silencing of MUC1 gene may inhibit the development of NSCLC cells.
Keywords: mucin 1, non-small cell lung cancer, proliferation, apoptosis, anti-angiogenic effect
Introduction
Lung cancer is a malignant tumor that severely threatens human health (1), and is characterized by high morbidity and mortality rates. Furthermore, lung cancer accounts for the highest incidence of male cancer and mortality rate of all malignant tumors in males, and the second highest in females worldwide. The high morbidity and mortality of lung cancer is closely associated with the alterations observed in the biological behavior of lung cancer cells. Non-small cell lung cancer (NSCLC) comprises approximately 80–85% of all the lung cancer cases (2); however, the treatment of NSCLC remains challenging, with a low overall 5-year survival rate (3,4). As a result of poor early diagnosis and the high rates of recurrence and metastasis, the curative effect of surgical treatment for NSCLC is poor (5). In addition, the incidence rate of NSCLC has been reported to be rising in recent decades (6). Therefore, it is urgent to explore novel therapeutic means for NSCLC treatment.
Mucin 1 (MUC1), a large trans-membrane protein, is mainly expressed on the apical border of normal secretory epithelial cells (7). It is synthesized as a single polypeptide, while N-terminal (MUC1-N) and C-terminal (MUC1-C) are its two subunits. MUC1 is frequently overexpressed in epithelial cancer (8), and previous evidence has verified that the abnormal upregulation of MUC1 in tumors is involved in the regulation of cancer transformation, occurrence, metastasis and invasion. Studies have also indicated that MUC1 interacts with various signals that participate in cell adhesion, growth and survival, including protein kinase C, c-Src, Grb2/SOS and various member of the human epidermal growth factor receptor family (9,10). Furthermore, MUC1 serves critical roles in the regulation of the phosphoinositide 3-kinase/protein kinase B (AKT) and B-cell lymphoma-xL pathway activation.
A previous study (11) suggested that MUC1 may be a novel target for lung cancer treatment, and may be involved in the regulation of lung cancer cell growth. However, the exact role of MUC1 in NSCLC progression and the underlying molecular mechanisms involved remain poorly understood. Thus, in the present study, the aim was to investigate the role of MUC1 in the development of NSCLC and further explore the underlying molecular mechanisms.
Materials and methods
Cell culture
The human NSCLC cell line NCI-H1650 (CRL-5883) and normal human lung epithelial cell line BEAS-2B (TCP-2030) were obtained from the American Type Culture Collection (Manassas, VA, USA). BEAS-2B cells were grown in bronchial epithelial cell growth medium (Lonza, Basel, Switzerland). NCI-H1650 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (Corning Incorporated, Corning, NY, USA), 1% penicillin-streptomycin solution (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a 5% CO2 incubator at 37°C. Cell were passaged every 2–3 days.
Cell transfection
At 24 h before the transfection, 1×105 NCI-H1650 cells/well were seeded in a 6-well plate. To investigate the role of MUC1 in the development of NSCLC, MUC1-siRNA or a control-siRNA (synthesized by Shanghai GenePharma Co., Ltd., Shanghai, China) were transfected into NCI-H1650 cells with 30 µl Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. Following incubation for 48 h, the transfected NCI-H1650 cells were used for subsequent analysis.
Cell proliferation assay
NCI-H1650 cell proliferation was determined by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay, in line with the manufacturer's instructions. Briefly, at 48 h post-transfection with MUC1-siRNA or the control-siRNA, NCI-H1650 cells were collected, reseeded into 96-well plates at a density of 3×103 cells/well and then incubated for 24 h at 37°C under 5% CO2. Subsequently, CCK-8 solution (10 µg/ml) was added to the cell culture medium in each well and incubated at 37°C for 2 h. The optical density value at 450 nm was assayed using a spectrophotometer. All experiments were performed in triplicate.
Cell apoptosis assay
For the analysis of cell apoptosis, at 48 h after transfection, the NCI-H1650 cells were collected, washed three times with phosphate-buffered saline (PBS) solution and fixed with 70% ethanol for 30 min. Subsequently, the cells were again washed three times with PBS and then labeled with Annexin V-FITC and propidium iodide (Cell Signaling Technology, Inc., Boston, MA, USA) following the manufacturer's instructions. Cells were subsequently analyzed by flow cytometry (FACSCalibur) and CellQuest Pro software (both from BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocols. Experiments were repeated three times.
Western blot analysis
In order to detect the protein expression levels of MUC1, vascular endothelial growth factor (VEGF), VEGF-C, p-AKT and p-extracellular signal-regulated kinase (p-ERK), western blot analysis was performed according to standard procedures. Briefly, cellular total protein was extracted from each sample using radioimmunoprecipitation assay buffer (BioVision, Inc., Milpitas, CA, USA). Protein concentration was determined by the BCA method, then proteins (40 µg) were electrophoresed by 12% SDS-PAGE, transferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA) and blocked in PBS solution supplemented with 5% fat-free milk for 1 h at 25°C. Subsequently, membranes were blotted at 4°C overnight with the following primary antibodies: MUC1 (14161; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), VEGF (SAB1402390; 1:1,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), VEGF-C (2445; 1:1,000), p-AKT (13038; 1:1,000) and p-ERK (5683; 1:1,000) (all from Cell Signaling Technology, Inc.), with GAPDH (166574; 1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) serving as the internal control. Blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies: Anti-rabbit (7074) and anti-mouse (14709) (1:5,000; both from Cell Signaling Technology, Inc.) at 25°C for 2 h. Protein bands were visualized using a chemiluminescence phototope-HRP kit (Pierce; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The mean density of the bands was quantified using an ImageJ v2.1.4.7 (National Institutes of Health, Bethesda, MD, USA) after the film was scanned.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cultured cells was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. The first-strand cDNA was generated from total RNA using the PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). Subsequently, qPCR was conducted with the SYBR Premix Ex Taq II reagent (Takara Bio, Inc.). GAPDH served as an internal control for detection of the mRNA expression of MUC1. The sequences of the primers used were as follows: GAPDH forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse, 5′-AGAGGCAGGGATGATGTTCTG−3′ with a product size of 208 bp; MUC1 forward, 5′-CGCCGAAAGAACTACGGGCAGCTG-3′ and reverse, 5′-CAAGTTGGCAGAAGTGGCTGCCAC-3′ with a product size of 100 bp. The thermal cycling conditions were: 60 sesc at 95°C, followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 45 sec. Relative quantification was performed using the 2−ΔΔCq method (12). All qPCR reactions were repeated three times.
Statistical analysis
Data are expressed as the mean ± standard deviation. All statistical analyses were performed using SPSS version 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Student's t-test was used to evaluate the differences between groups. A value of P<0.05 was considered as an indicator of a statistically significant difference.
Results
MUC1 expression in human NSCLC cells
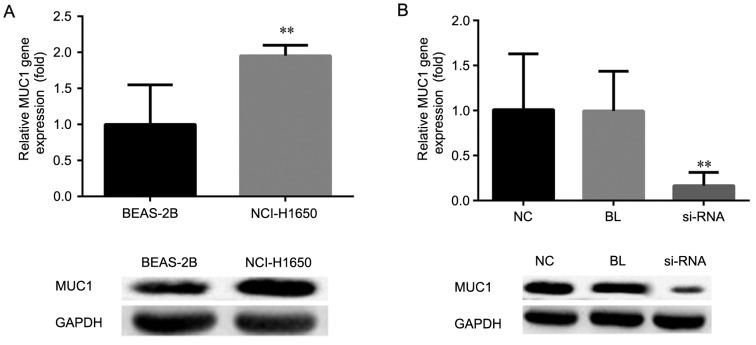
To investigate the expression of MUC1 in human NSCLC cells, RT-qPCR and western blot analysis were performed, respectively. As shown in Fig. 1A, compared with the normal human lung epithelial cell line BEAS-2B, the mRNA and protein expression levels of MUC1 in the human NSCLC cell line NCI-H1650 were significantly increased (P<0.01).
Figure 1.
mRNA and protein expression levels of MUC1 were determined by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively, in (A) NCI-H1650 and BEAS-2B cells, and (B) NCI-H1650 cells transfected with MUC1-siRNA or control-siRNA, at 48 h after transfection. All data are presented as the mean ± standard deviation of three independent experiments. **P<0.01, vs. NC and BL groups. MUC1, mucin 1; siRNA, small interfering RNA; NC, negative control group (transfected with control-siRNA); BL, blank control group (non-transfected); siRNA, MUC1-siRNA group (transfected with MUC1-siRNA).
In order to investigate the role of MUC1 in human NSCLC, MUCI was silenced by using MUC1-siRNA. At 48 h after cell transfection, the effective downregulation of the mRNA and protein expression levels of MUC1 was confirmed by RT-qPCR and western blot analysis, respectively, as compared with the non-transfected and control-transfected cells (P<0.01; Fig. 1B).
MUC1 downregulation inhibits cell proliferation
CCK-8 assay was performed for cell proliferation determination. To investigate the role of MUC1 in NSCLC cell proliferation, MUC1-siRNA or control-siRNA were transfected into NCI-H1650 cells. At 48 h after the cell transfection, the cell proliferation ability was detected in the different groups. The data suggested that, compared with the non-transfected and control-transfected groups, the cell proliferation ability of cells with MUC1 downregulation by siRNA transfection was significantly declined (P<0.01; Fig. 2). Thus, these results indicated that downregulation of MUC1 in NCI-H1650 cells inhibited cell proliferation.
Figure 2.
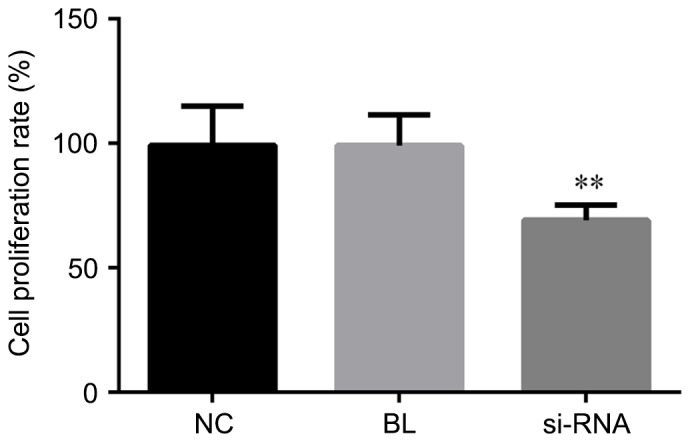
MUC1 downregulation inhibits the proliferation in NCI-H1650 cells. Cell proliferation was analyzed by Cell Counting Kit-8 assay at 48 h after transfection with MUC1-siRNA or control-siRNA. All data are presented as the mean ± standard deviation of three independent experiments. **P<0.01, vs. NC and BL groups. MUC1, mucin 1; siRNA, small interfering RNA; NC, negative control group (transfected with control-siRNA); BL, blank control group (non-transfected); siRNA, MUC1-siRNA group (transfected with MUC1-siRNA).
MUC1 downregulation promotes cell apoptosis
To investigate the effect of MUC1 on cell apoptosis, the apoptosis rate was measured by flow cytometry assay at 48 h after NCI-H1650 cell transfected with MUC1-siRNA or control. As shown in Fig. 3, the apoptosis rate of the MUC1-siRNA-transfected NCI-H1650 cells was significantly higher in comparison with that of cells transfected with control-siRNA or non-transfected cells. These data indicated that MUC1 downregulation was able to promote NCI-H1650 cell apoptosis.
Figure 3.
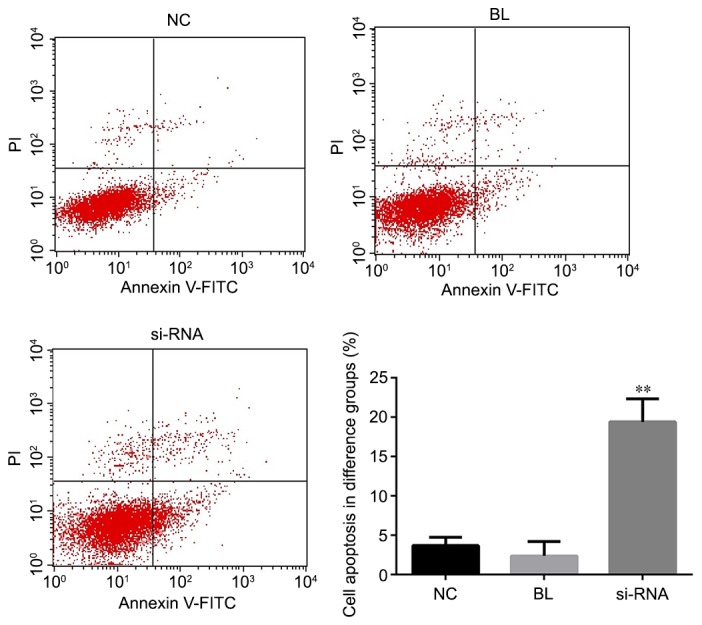
MUC1 downregulation increases the apoptosis of NCI-H1650 cells. At 48 h after transfection with MUC1-siRNA or control-siRNA, flow cytometry was conducted to detect the cell apoptosis, and cell apoptosis rate (early apoptosis rate + late apoptosis rate) was calculated. All data are presented as the mean ± standard deviation of three independent experiments. **P<0.01, vs. NC and BL groups. MUC1, mucin 1; siRNA, small interfering RNA; NC, negative control group (transfected with control-siRNA); BL, blank control group (non-transfected); siRNA, MUC1-siRNA group (transfected with MUC1-siRNA).
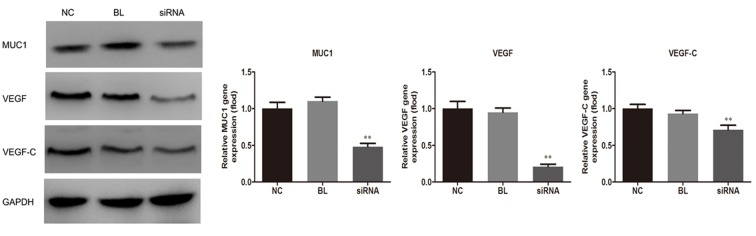
MUC1 downregulation inhibits VEGF and VEGF-C expression levels
The current study next examined the effect of suppressed expression of MUC1 on the expression of VEGF. As shown in Fig. 4, the protein expression levels of VEGF and VEGF-C were significantly reduced in NCI-H1650 cells in the siRNA group when compared with non-transfected and the control-transfected groups (P<0.01), indicating the anti-angiogenic effect of MUC1 downregulation.
Figure 4.
MUC1 downregulation suppresses the expression levels of VEGF and VEGF-C in NCI-H1650 cells transfected with MUC1-siRNA or control-siRNA. At 48 h after transfection, VEGF and VEGF-C expression levels were measured using western blot analysis. All data are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. NC and BL groups. MUC1, mucin 1; VEGF, vascular endothelial growth factor; siRNA, small interfering RNA; NC, negative control group (transfected with control-siRNA); BL, blank control group (non-transfected); siRNA, MUC1-siRNA group (transfected with MUC1-siRNA).
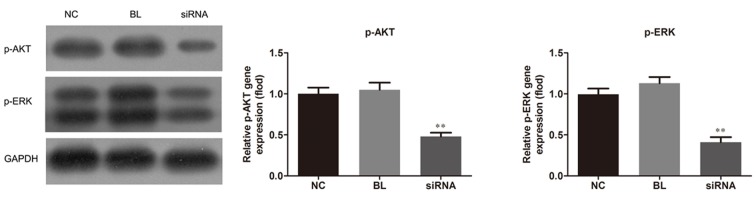
MUC1 downregulation affects AKT and ERK activation
Since AKT and MAPK signaling pathways have been strongly involved in tumor angiogenesis (13–15), the present study also investigated the effect of MUC1 downregulation on the activation of these two pathways. Western blot analysis revealed that MUC1 siRNA transfection caused an evident decrease in the phosphorylation of AKT and ERK, the protein expression levels of p-AKT and p-ERK were reduced in the siRNA group when compared with the non-transfected and control-transfected groups (P<0.01), which is indicative of activation suppression of AKT and ERK by MUC1 downregulation (Fig. 5).
Figure 5.
MUC1 downregulation inhibits the activation of AKT and ERK signaling pathways in NCI-H1650 cells. At 48 h after the transfection with MUC1-siRNA or control-siRNA, p-AKT and p-ERK expression levels were measured by western blot analysis. All data are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. NC and BL groups. MUC1, mucin 1; AKT, protein kinase B; ERK, extracellular signal-regulated kinase; p, phosphorylated; siRNA, small interfering RNA; NC, negative control group (transfected with control-siRNA); BL, blank control group (non-transfected); siRNA, MUC1-siRNA group (transfected with MUC1-siRNA).
Discussion
In the present study, it was demonstrated that MUC1 protein and mRNA levels were overexpressed in an NSCLC cell line. The study further investigated the effect of downregulation of MUC1 by siRNA transfection on lung cancer progression using ab in vitro cell line model. It was demonstrated that MUC1 downregulation in NCI-H1650 cells inhibited cell proliferation, induced cell apoptosis, inhibited VEGF and VEGF-C production, and suppressed the activation of AKT and ERK pathways. These results strongly suggest that MUC1 downregulation inhibited NSCLC progression.
MUC1 has been verified to be upregulated in a variety of cancer types (16–18). The high expression level of MUC1 in lung cancer has been closely correlated with early recurrence, poor prognosis and high metastatic potential (19). Ren et al (20) suggested that downregulation of MUC1 in lung cancer A549 cells increased their sensitivity to genotoxic drugs in vitro and in vivo. In addition, Gao et al (21) reported that MUC1 knockdown suppresses lung cancer growth and metastasis via inhibiting cell proliferation and inducing apoptosis. According to these previous findings, the present study observed that MUC1 downregulation inhibited the NSCLC NCI-H1650 cell proliferation and promoted cell apoptosis.
Angiogenesis is an important process required for tumor genesis, growth, invasion and metastasis. VEGF, a member of the platelet-derived growth factor family, serves critical roles in inducing angiogenesis and vessel permeability (22). Furthermore, VEGF-C is a ligand of the VEGF receptor, VEGF-R3, and is the only factor known to cause lymphangiogenesis (23,24). In a previous study (25), the level of VEGF-C was suggested to be associated with lymphangiogenesis and metastasis, as well as with the patient prognosis in various types of cancer. In the present study, it was observed that MUC1 downregulation inhibited VEGF and VEGF-C production, indicating that MUC1 served important roles in the regulation of VEGF and VEGF-C expression levels and in lung cancer angiogenesis.
Various signaling pathways participate in cancer development though the regulation of MUC1. For instance, MUC1 is able to promote various cancer cell growth and survival via activation of the β-catenin and nuclear factor-kB pathway (26). Additionally, AKT and MAPK pathways have been reported to be strongly involved in tumor angiogenesis (12–14), and thus analysis of these two pathways was conducted in the present study. The data demonstrated that downregulation of MUC1 inhibited the activation of AKT and ERK pathways, indicating the participation of these two signaling pathways in the biological function of MUC1.
In conclusion, the current study provided evidence that MUC1 downregulation inhibits NSCLC cell proliferation, facilitates NSCLC cell apoptosis, and suppresses VEGF and VEGF-C production. The AKT and ERK signaling pathways were observed to participate in the function of MUC1. These data, combined with previous findings, indicate that MUC1 is involved in multiple aspects of tumor progression, thus, representing a novel therapeutic target for NSCLC treatment.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, Perez CA. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/S0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YY, Hu ZG, Zeng FJ, Han J. Clinical profile of cyclooxygenase-2 inhibitors in treating non-small cell lung cancer: A Meta-Analysis of nine randomized clinical trials. PLoS One. 2016;11:e0151939. doi: 10.1371/journal.pone.0151939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenchel K, Sellmann L, Dempke WC. Overall survival in non-small cell lung cancer-what is clinically meaningful? Transl Lung Cancer Res. 2016;5:115–119. doi: 10.3978/j.issn.2218-6751.2016.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quéré G, Descourt R, Robinet G, Autret S, Raguenes O, Fercot B, Alemany P, Uguen A, Férec C, Quintin-Roué I, Le Gac G. Mutational status of synchronous and metachronous tumor samples in patients with metastatic non-small-cell lung cancer. BMC Cancer. 2016;16:210. doi: 10.1186/s12885-016-2249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Gum J, Jr, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 9.Al Masri A, Gendler SJ. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene. 2005;24:5799–5808. doi: 10.1038/sj.onc.1208738. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, Wang X. Critical roles of mucin-1 in sensitivity of lung cancer cells to tumor necrosis factor-alpha and dexamethasone. Cell Biol Toxicol. 2017;33:361–371. doi: 10.1007/s10565-017-9393-x. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Cook KM, Figg WD. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsham AM, Plas DR, Thompson CB, Simon MC. Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res. 2004;64:3500–3507. doi: 10.1158/0008-5472.CAN-03-2239. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu M, Bai O, Liu W, Xie X, Wang Z, et al. RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene. 2010;29:5404–5415. doi: 10.1038/onc.2010.270. [DOI] [PubMed] [Google Scholar]
- 16.Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, Joshi MD, MacDermed D, Weichselbaum R, Kufe D. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene. 2010;29:920–929. doi: 10.1038/onc.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Retterspitz MF, Monig SP, Schreckenberg S, Schneider PM, Hölscher AH, Dienes HP, Baldus SE. Expression of {beta}-catenin, MUC1 and c-met in diffuse-type gastric carcinomas: Correlations with tumour progression and prognosis. Anticancer Res. 2010;30:4635–4641. [PubMed] [Google Scholar]
- 18.Kaira K, Murakami H, Serizawa M, Koh Y, Abe M, Ohde Y, Takahashi T, Kondo H, Nakajima T, Yamamoto N. MUC1 expression in thymic epithelial tumors: MUC1 may be useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch. 2011;458:615–620. doi: 10.1007/s00428-011-1041-x. [DOI] [PubMed] [Google Scholar]
- 19.Ohgami A, Tsuda T, Osaki T, Mitsudomi T, Morimoto Y, Higashi T, Yasumoto K. MUC1 mucin mRNA expression in stage I lung adenocarcinoma and its association with early recurrence. Ann Thorac Surg. 1999;67:810–814. doi: 10.1016/S0003-4975(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 20.Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/S1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, McConnell MJ, Yu B, Li J, Balko JM, Black EP, Johnson JO, Lloyd MC, Altiok S, Haura EB. MUC1 is a downstream target of STAT3 and regulates lung cancer cell survival and invasion. Int J Oncol. 2009;35:337–345. [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 23.Kajita T, Ohta Y, Kinura K, Tamura M, Tanaka Y, Tsunezuka Y, Oda M, Sasaki T, Watanabe G. The expression of vascular endothelial growth factor C and its receptors in non-small cell lung cancer. Br J Cancer. 2001;85:255–260. doi: 10.1054/bjoc.2001.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 25.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 26.Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. MUC1 oncoprotein promotes growth and survival of human multiple myeloma cells. Int J Oncol. 2008;33:153–159. [PMC free article] [PubMed] [Google Scholar]