Abstract
The objective of the present study was to investigate acute kidney injury (AKI) induced by myocardial ischemia/reperfusion (MIR) in diabetic rats and elucidate its underlying mechanism. A rat model of MIR was established by left anterior descending coronary artery occlusion for 30 min, followed by reperfusion for 2 h. Rats were randomly divided into four groups: i) Sham group, ii) sham + MIR group, iii) diabetic group and iv) diabetes + MIR group. Myocardial injury was detected by plasma creatine kinase isoenzyme MB and lactate dehydrogenase assays. AKI induced by MIR in diabetic rats was characterized by increases in cystatin C and β2-microglobulin levels. Oxidative stress injury was accompanied by an increase of malondialdehyde levels and a decrease of total antioxidative capacity in the renal tissues. Immunohistochemistry and western blot analysis demonstrated that the expression of DJ-1 and nuclear factor erythroid 2-related factor 2 (Nrf2) were significantly increased in the diabetes + MIR group compared with that in the sham + MIR and diabetic groups. Taken together, these results suggested that AKI induced by MIR in diabetic rats may be associated with activation of the DJ-1/Nrf2 pathway.
Keywords: DJ-1, nuclear factor erythroid 2-related factor-2, acute kidney injury, myocardial ischemia-reperfusion, diabetes
Introduction
Acute kidney injury (AKI), a syndrome associated with high post-operative mortality in humans, is one of the most serious complications of cardiac surgery (1,2). The incidence of AKI due to coronary revascularization or shock has been increasing worldwide. Clinical and experimental studies have demonstrated that myocardial ischemia-reperfusion (MIR) causes AKI (2,3). The mechanism underlying the decline in renal function during MIR was thought to be multifactorial in origin, and associated with hypoperfusion, loss of pulsatile perfusion, hemolysis and a systemic inflammatory response. These factors may increase reactive oxygen species (ROS) (4). In endogenous or exogenous renal injury such as MIR, excessively produced ROS may cause AKI (5). However, the mechanism underlying this damage in the kidney during MIR has remained to be fully elucidated.
Diabetes is one of the most frequent co-morbidities in patients undergoing cardiac surgery and is an independent predictor of AKI in patients with normal pre-operative kidney function (6). Furthermore, insulin-requiring diabetes is also an independent predictor of AKI after cardiac transplantation (7). Clinical studies have demonstrated that hyperglycemia is associated with a significantly higher incidence of AKI. Insulin should be administered if glucose levels are increased (8,9). Intraoperative hyperglycemia should be avoided, as it may lead to excessive production of ROS, which prominently occurs in the kidney and causes acute or progressive renal damage (10).
Attenuation of oxidative stress is known to have an important role in the renoprotective effect mediated by a variety of treatment interventions (11). Previous studies by our group have indicated that DJ-1, which is a multifunctional protein that has potent anti-oxidant properties, protected against oxidative injury in diabetic kidneys and in MIR (12,13). Another study demonstrated that DJ-1 protein was required for the stabilization of nuclear factor erythroid 2-related factor-2 (Nrf2), which has a central role in the protective effect against oxidative injury, and subsequent production of Nrf2-regulated enzymes (14). Hence, it was of interest to investigate the role of the DJ-1/Nrf2 pathway in AKI induced by MIR in diabetic rats.
Materials and methods
Materials
A total of 40 male Sprague Dawley rats (weight, 250–300 g; age, 9–12 weeks) were purchased from Wuhan University, Animal Biosafety Level 3 Laboratory (Wuhan, China). All rats were housed under standard laboratory conditions at 22–24°C, a relative humidity of 50±15%, and a 12 h light/dark cycle. All animals had free access to standard rat chow and water. The experimental protocol of the present study was reviewed and approved by the Animal Care and Use Committee of Wuhan University (Wuhan, China), and was in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. Streptozotocin (STZ) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies against DJ-1 were purchased from Cell Signaling Technology, Inc. (cat no. 5933; Beverly, MA, USA). Antibodies to Nrf2 were purchased from Santa Cruz Biotechnology, Inc. (cat no. sc722; Dallas, TX, USA). GAPDH and Lamin B were purchased from Cell Signaling Technology, Inc. (cat nos. 5174 and 13435), and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (cat no. sc2357). All other chemicals were obtained from commercial sources and were of the highest grade available.
Induction of diabetes
Experimental diabetes was induced in the animals by a single intraperitoneal administration of STZ dissolved in 0.1 mol/l citrate buffer (pH 4.5) at a dose of 65 mg/kg. Normal rats received an equal volume of citrate buffer. Three days post-STZ injection, tail vein blood samples were collected and glucose levels were measured with a Onetouch glucometer (Johnson & Johnson, New Brunswick, NJ, USA). The rats were considered diabetic and used for the study only if their glucose levels were >15 mmol/l (15). Rats were maintained for 8 weeks after vehicle or STZ injection.
Surgical preparation
Animals were intraperitoneally anesthetized with pentobarbital sodium (50 mg/kg) (16) followed by a tracheotomy and artificial ventilation. Blood pressure was recorded from the left femoral artery using a pressure transducer with the heart rate monitored by an electrocardiogram throughout the procedure. The left femoral vein was cannulated for administration of drugs. A fourth-intercostal space thoracotomy was performed and the pericardium was excised to expose the heart. The left anterior descending coronary artery (LAD) was ligated 2 mm above the left auricle by a 6–0 silk suture. A small polypropylene tube was placed between the ligature and the LAD. The artery was occluded for 30 min by tightening the ligature. After ischemia for 30 min, the ligature was loosened to allow reperfusion for 2 h. The sham group underwent the same surgical procedures, apart from tying the 6–0 silk suture. After 2 h of reperfusion, the rats were sacrificed, and renal tissues and blood samples were obtained for further analysis.
Experimental protocol
Rats were randomly assigned into one of four experimental groups (n=8 per group) as follows: i) Sham-operated group (sham group), non-diabetic rats that underwent isolation of the LAD without occlusion as a control; ii) MIR group, non-diabetic rats subjected to 30 min of myocardial ischemia and 2 h of reperfusion after the LAD had been isolated and occluded (sham + MIR group); iii) diabetic rats that underwent isolation of the LAD without occlusion (diabetic group); iv) diabetic rats subjected to 30 min of myocardial ischemia and 2 h of reperfusion after the LAD had been isolated and occluded (diabetic + MIR group).
Measurement of serum glycosylated hemoglobin (HbA1c), creatine kinase isoenzyme MB (CK-MB) and lactate dehydrogenase (LDH) levels
Blood samples were collected at the end of reperfusion and centrifuged at 1,000 × g for 10 min at 4°C. Serum was separated and stored at −20°C. HbA1c was measured using an Olympus automatic analyzer (cat no. AU5400; Olympus Corporation, Tokyo, Japan). CK-MB and LDH levels were measured using commercial kits (cat nos. 01010605082 and 01010113477; Beijing Kemeidongya Biotechnology Ltd., Beijing, China) according to the manufacturer's instructions.
Renal histopathological assessment
The left kidney was cut into sections and fixed in 4% formaldehyde. After embedding in paraffin, 4-µm sections were stained with hematoxylin at 37°C for 3 min and eosin at 37°C for 10 sec for light microscopy (original magnification, ×200; Olympus BX50; Olympus Corporation).
Histological assessment of tubular necrosis was semi-quantitatively determined using a method modified from McWhinnie et al (17) using the following scoring system: 0, normal histology; 1, tubular cell swelling, brush border loss and nuclear condensation, with up to one-third of the tubular profile exhibiting nuclear loss; 2, same as for score 1, but more than one-third and less than two-thirds of the tubular profile displaying nuclear loss; and 3, same as for score 1, but more than two-thirds of the tubular profile exhibiting nuclear loss.
Measurement of serum cystatin C (Cys C) and β2-microglobulin (β2-MG) levels
Blood samples were collected at the end of reperfusion and centrifuged at 3,000 rpm, for 10 min at 4°C. Serum was separated and stored at −20°C. Cys C and β2-MG levels were measured using ELISA assay kits (cat nos. E-EL-R783 and E-EL-R1085c; Elabscience Biotechnology Co., Ltd., Wuhan, China) according to the manufacturer's instructions.
Assessment of total antioxidative capacity (T-AOC) and malondialdehyde (MDA) in renal tissues
The renal tissues were harvested and immediately homogenized on ice in 5 volumes of normal saline. The homogenates were centrifuged at 1,200 × g for 10 min. The T-AOC and MDA levels in the supernatant were measured using T-AOC and MDA assay kits (cat nos. A015-2 and a003-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Immunohistochemical assessment of renal DJ-1 and Nrf2
Paraffin-embedded renal sections were stained using the streptavidin-biotin complex (streptavidin peroxidase, SP) immunohistochemistry technique for DJ-1 and Nrf2 detection using SP immunohistochemistry kits (cat no. I003-2; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. Brown staining in the cytoplasm and/or nucleus was considered an indicator of positive expression. The results were semi-quantitatively evaluated with Image-Pro® Plus version 6.0 software (Media Cybernetics, Rockville, MD, USA) according to optical density values of positive staining.
Western blot analysis
Cytoplasmic and nuclear proteins were extracted from frozen renal tissues with a nuclear extraction kit (cat no. P0027; Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer's instructions. GAPDH was used as internal control of cytoplasmic protein and Lamin B was used as an internal control of nuclear protein. A BCA protein assay kit was used to determine the protein concentration (cat no. P0012S; Beyotime Institute of Biotechnology). Equal amounts of protein (30 µg) were separated by 12% SDS-PAGE at 100 V for 3 h. After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes at 200 mA for 2 h. The transferred membranes were incubated overnight at 4°C with rabbit anti-mouse polyclonal antibodies for DJ-1 and Nrf2 (each at 1:800 dilution) in Tris-buffered saline containing Tween-20 (TBS-T) containing 5% skimmed milk. After washing three times in TBS-T, membranes were incubated with anti-rabbit immunoglobulin G conjugated to horseradish peroxidase at a dilution of 1:2,000 in TBS-T containing 5% skimmed milk for 2 h at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence (PerkinElmer, Inc., Waltham, MA, USA) and captured on X-ray film. The optical density of the bands was measured with a BandScan V4.0 imaging analysis system (http://bandscan.software.informer.com/).
Statistical analysis
Mean ± standard error of the mean values were calculated to summarize all outcome measurements. Statistical analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The statistical significance of the differences between groups was evaluated using unpaired Student's t-tests for pair-wise comparisons, or a two-way analysis of variance followed by the Tukey post hoc test for multiple comparisons. The level of significance was set at P<0.05 for all statistical tests.
Results
Body weight, kidney weight, fasting blood glucose and glycosylated hemoglobin (HbA1c) content
At the end of the study, the body weight of diabetic rats was significantly decreased as compared with that of normal rats. The change of kidney weight was not obvious in diabetic rats during the experimental period. The ratio of kidney weight to body weight in diabetic rats was significantly increased as compared to that in the normal rats. A significant increase in fasting blood glucose in diabetic rats was observed when compared with that in the control group. HbA1c was markedly higher in diabetic rats than that in normal rats (Table I).
Table I.
Effects of different time on body weight, kidney weight, fasting blood glucose and HbA1c levels in rats.
| Parameter | Sham | Sham + MIR | Diabetic | Diabetes + MIR |
|---|---|---|---|---|
| Body weight (g) | 275.77±12.02 | 262.83±14.82 | 179.78±14.52a,b | 176.93±12.77a,b |
| Kidney weight (g) | 1.03±0.05 | 0.95±0.13 | 1.01±0.12 | 1.03±0.87 |
| Kidney/body | ||||
| weight ratio (×10−3) | 3.72±0.13 | 3.78±0.82 | 5.64±0.82a,b | 5.32±0.64a,b |
| Glucose (mg/dl) | 7.69±0.84 | 8.65±1.59 | 25.88±2.52a | 26.15±1.52a |
| HbA1c (%) | 4.67±0.72 | 4.66±0.26 | 13.68±2.98a | 12.74±1.58a |
Values are expressed as the mean ± standard error of the mean (n=8 per group).
P<0.05 vs. sham group.
P<0.05 vs. sham + MIR group. Hb, hemoglobin; MIR, myocardial ischemia/reperfusion.
Serum CK-MB and LDH levels
To examine damage of cardiomyocytes induced by MIR, the activities of the cardiac enzyme CK-MB and LDH as indices of myocardial cellular injury were measured at the end of reperfusion in the different groups. Compared with the sham group, MIR caused a significant increase in LDH and CK-MB in the sham + MIR group, as did the diabetic condition, but to a significantly lesser extent (P<0.05). The damage evoked by MIR was further enhanced in the diabetes + MIR group (P<0.05 vs. all other groups; Fig. 1).
Figure 1.
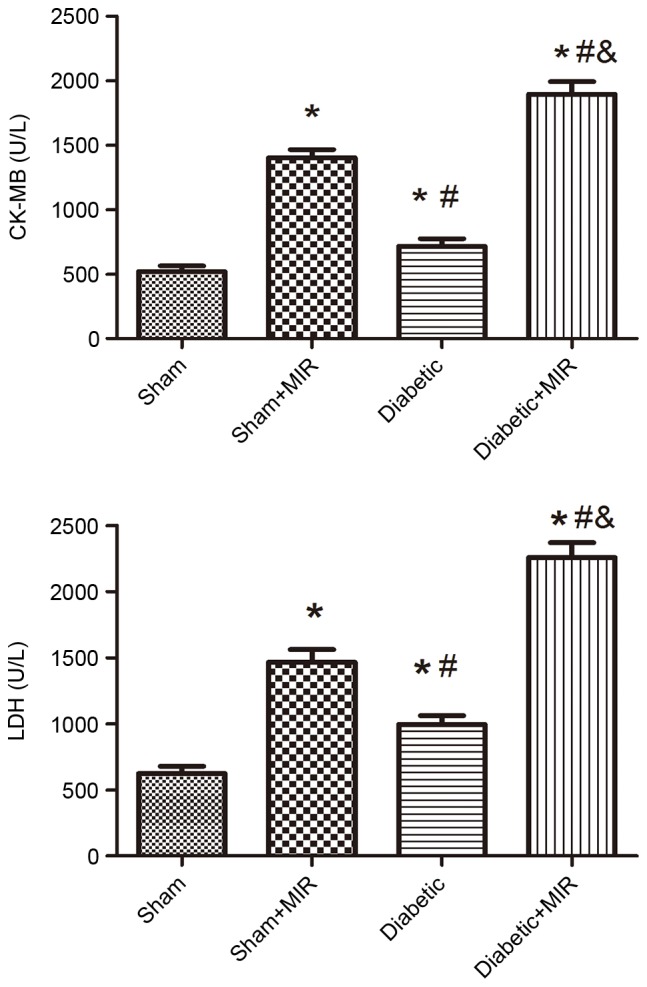
Serum CK-MB and LDH levels in the different treatment groups. Values are expressed as the mean ± standard error of the mean (n=8). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. LDH, lactate dehydrogenase; CK-MB, creatine kinase isoenzyme MB; MIR, myocardial ischemia/reperfusion.
Renal histopathology
As presented in Fig. 2, the renal tubules exhibited pathological changes, including edema, necrosis and vacuolization in the sham + MIR and diabetic groups. A significant aggravation of histological edema, necrosis and vacuolization was seen in the diabetes + MIR group.
Figure 2.
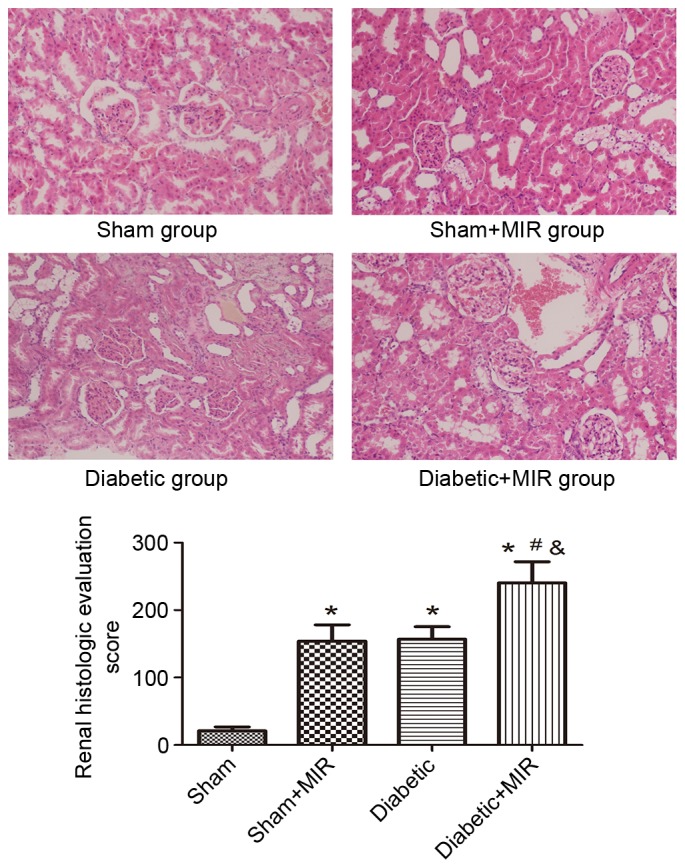
Renal histological evaluation in the various treatment groups (hematoxylin and eosin staining; magnification, ×200). Values are expressed as the mean ± standard deviation (n=8 per group). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. MIR, myocardial ischemia/reperfusion.
When compared with the renal histological evaluation score of kidneys obtained from normal rats, the sham + MIR and the diabetic groups exhibited in a significant increase in renal histologic evaluation score individually (P<0.05). This increase was significantly enhanced in the diabetes + MIR group (P<0.05 vs. all other groups).
Serum Cys C and β2-MG levels
Serum Cys C and β2-MG levels were measured to evaluate the renal injury induced by MIR in diabetic rats. MIR and diabetes individually significantly increased serum Cys C and β2-MG levels in comparison with those in the sham group, which was further aggravated in the diabetes + MIR group (P<0.05; Fig. 3).
Figure 3.
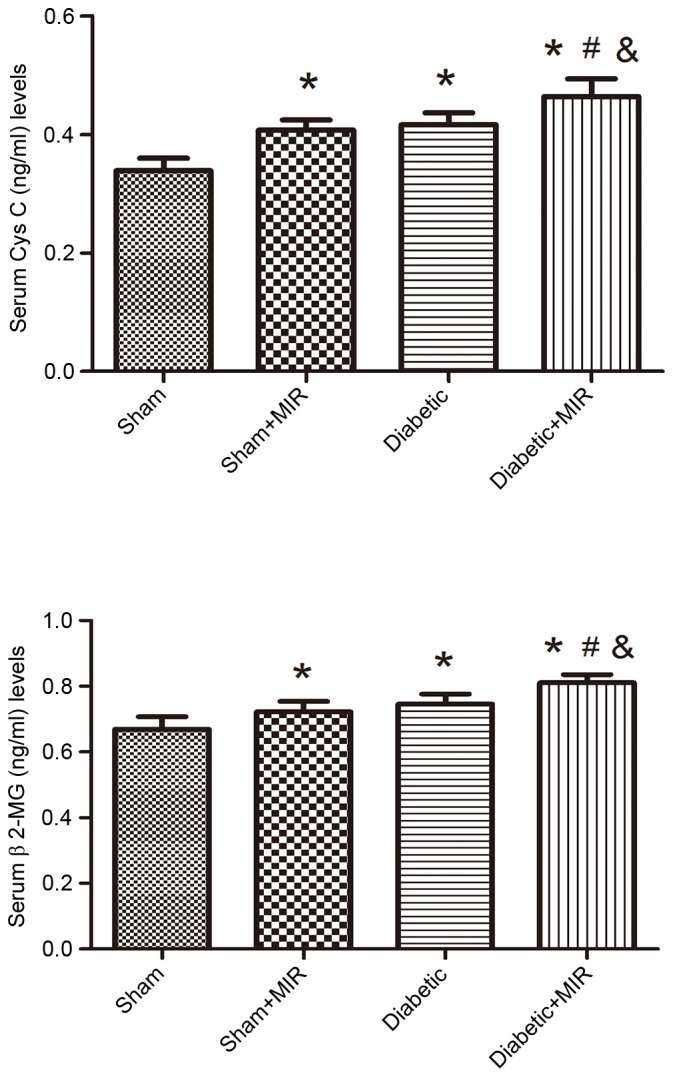
Serum Cys C and β2-MG levels in the different treatment groups. Values are expressed as the mean ± standard error of the mean (n=8). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. Cys C, cystatin C; β2-MG, β2-microglobulin; MIR, myocardial ischemia/reperfusion.
T-AOC and MDA levels in renal tissues
As presented in Fig. 4, MIR and the diabetic condition significantly decreased the T-AOC and significantly increased MDA levels in rats, compared with those in the sham group, and these changes were significantly aggravated in the diabetes + MIR group (P<0.05).
Figure 4.
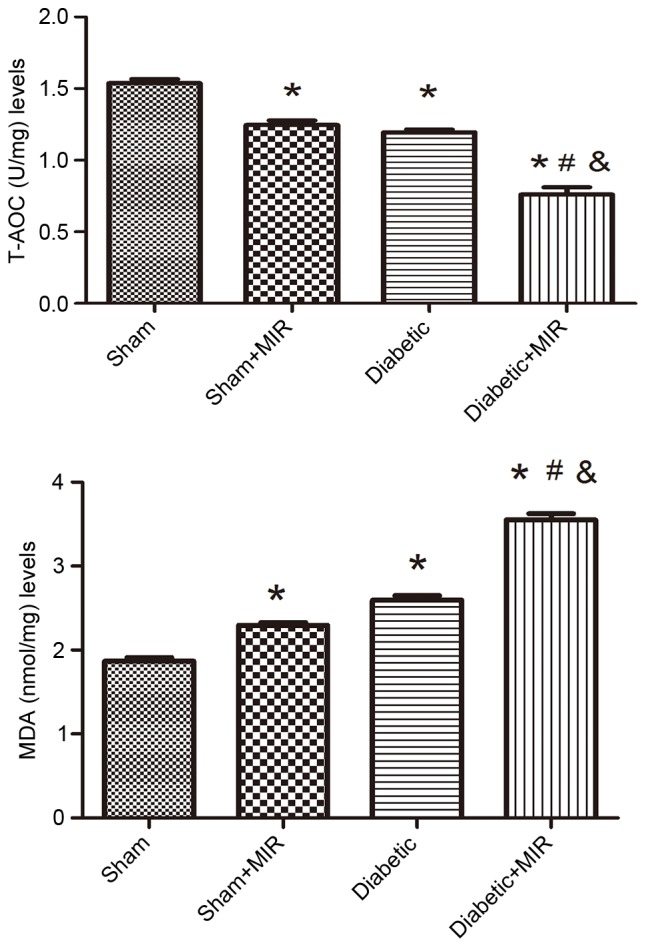
T-AOC and MDA levels in renal tissues of the different treatment groups. Values are expressed as the mean ± standard error of the mean (n=8). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. MIR, myocardial ischemia/reperfusion; MDA, malondialdehyde; T-AOC, total antioxidative capacity.
DJ-1 and Nrf2 expression in renal tissues
Immunohistochemical analysis of the expression of DJ-1 in the sham group revealed small brown granules in the cytoplasm, while significant expression of DJ-1, as indicated by brown staining in the cytoplasm, was observed in the diabetes + MIR group (P<0.05 vs. sham group; Fig. 5). No significant difference was observed between Sham + MIR and Diabetic groups (Fig. 5).
Figure 5.
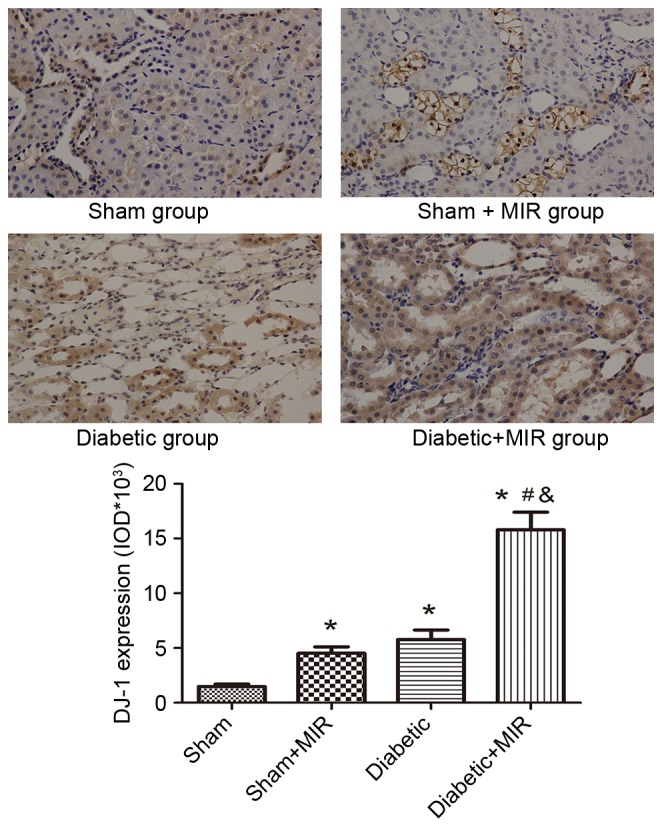
Expression of DJ-1 in renal tissues assessed by immunohistochemistry (streptavidin peroxidase staining; magnification, ×400). Values are expressed as the mean ± standard error of the mean (n=8). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. MIR, myocardial ischemia/reperfusion; IOD, integrated optical density.
The expression of Nrf2 in the sham group displayed as light brown immunostaining in the cytoplasm and no staining in the nuclei. Significant expression of Nrf2, as indicated by strong brown staining in the cytoplasm and nuclei, was observed in the diabetes + MIR group (P<0.05; Fig. 6). No significant difference was observed between Sham + MIR and Diabetic groups (Fig. 6).
Figure 6.
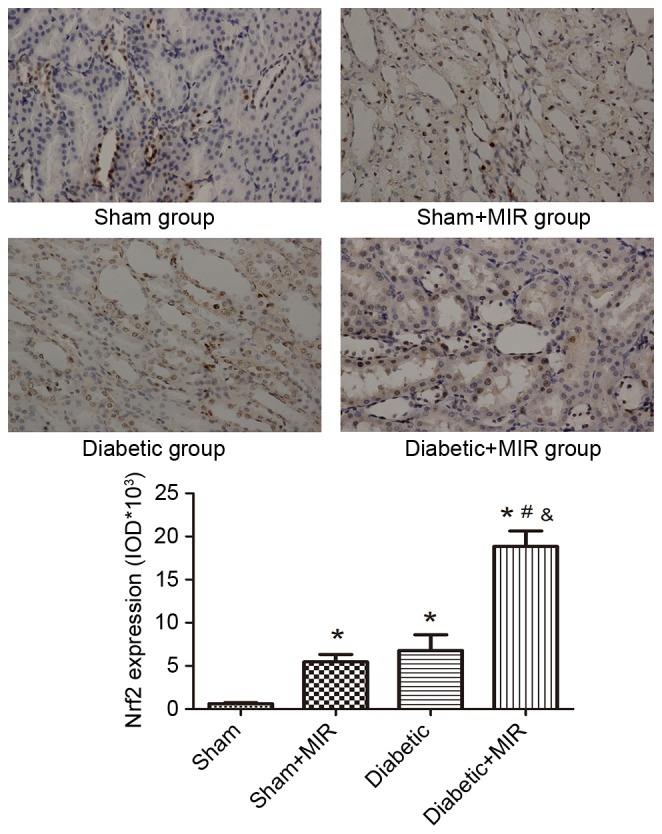
Expression of Nrf2 in renal tissues assessed by immunohistochemistry (streptavidin peroxidase staining; magnification, ×400). Values are expressed as the mean ± standard error of the mean (n=8). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. MIR, myocardial ischemia/reperfusion; IOD, integrated optical density; Nrf2, nuclear factor erythroid 2-related factor 2.
In line with these results, western blot analysis revealed weak expression of DJ-1 and Nrf2 in the kidneys of animals of the sham group. By contrast, significant increases in the protein expression of Nrf2 and DJ-1 were found in the diabetes + MIR group (P<0.05; Fig. 7). No significant difference was observed in Nrf2 expression between Sham + MIR and Diabetic groups (Fig. 7); however, the expression of DJ-1 was significantly higher in the diabetic group compared with the Sham + MIR group (P<0.05; Fig. 7).
Figure 7.
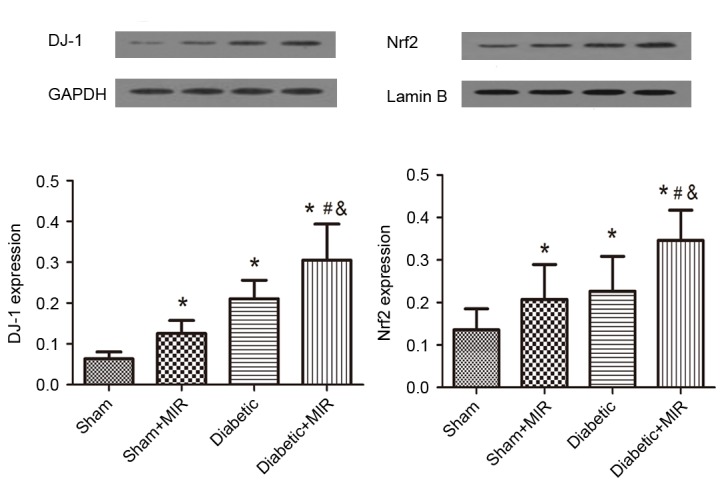
Expression of DJ-1 and Nrf2 in renal tissues assessed by western blot analysis. Values are expressed as the mean ± standard error of the mean (n=6). *P<0.05 vs. sham group, #P<0.05 vs. sham + MIR group, &P<0.05 vs. diabetic group. MIR, myocardial ischemia/reperfusion; Nrf2, nuclear factor erythroid 2-related factor 2.
Discussion
Previous studies have revealed that acute myocardial infarction with AKI was strongly associated with long-term mortality, which has been increasing worldwide. However, at present, no effective strategy is available to halt the acute worsening of renal function in patients with decompensated heart failure or those undergoing cardiac surgery, where even a small change in renal function was associated with increased mortality (18). Furthermore, diabetes mellitus, one of the most common complications of patients undergoing cardiac surgery and one of the major causes of nephropathy, was found to be an independent predictor of AKI (19). Great progress has been made through the identification of Cys C and β2-MG as diagnostic markers for kidney injury, even in the absence of a subsequent manifestation of kidney dysfunction (20). In the present study, the damage of cardiomyocytes induced by MIR was further enhanced in the diabetes + MIR group, as indicated by the increase of the cardiac enzyme CK-MB and LDH as indices of myocardial cell injury. In line with this, the increase in Cys C and β2-MG levels induced by MIR was more pronounced in diabetic rats. In addition, the kidneys of diabetic MIR rats exhibited glomerular hypertrophy and renal tubular edema on histological analysis, and the renal histological evaluation score was also increased. All of these results demonstrated that the MIR-induced renal injury model presenting with renal hypertrophy, renal tubular and glomerular damage was successfully created, and that these pathologies were aggravated in diabetic rats.
The mechanism underlying the renal injury during MIR is thought to be multifactorial in origin, and associated with hypoperfusion, loss of pulsatile perfusion, hemolysis and a systemic inflammatory response. These factors may increase ROS (21). Oxidative stress is becoming increasingly recognized as an important causative factor of renal damage induced by MIR (3,4). Diabetes may decrease the AOC and increase oxidative stress, which induces the generation of ROS and aggravates renal injury after cardiac surgery (22). T-AOC is an important marker of oxidation, which mainly reflects non-enzymatic but includes the activity of a minority of small molecular enzymatic systems (23). MDA is a naturally occurring product of lipid peroxidation and prostaglandin biosynthesis that is mutagenic and carcinogenic (24). The results of the present study demonstrated an increase of MDA levels and a decrease of T-AOC levels in renal tissues following MIR. Oxidative stress was aggravated in diabetic rats. These findings demonstrated that a decreased anti-oxidant activity already existed in renal injury induced by MIR and that the anti-oxidant capacity was further weakened in diabetic rats.
Activation of DJ-1/Nrf2 has been established to have an important protective role in oxidative stress (25,26). In addition, a previous study by our group has found that the renoprotective effects in diabetic rats may be enhanced by activation of Nrf2 (12). Disruption of DJ-1 decreased Nrf2 protein stability, whereas overexpression of DJ-1 restored protein stability by decreasing ubiquitination of Nrf2. DJ-1 protein was found to be required for the stabilization of Nrf2 and cell survival by a variety of substrates (26). The results of the present study demonstrated a significant increase in the expression of DJ-1 and Nrf2 in the kidneys of rats following MIR. In diabetic rats, the expression of DJ-1 and Nrf2 was further increased. All of the above indicated that the DJ-1/Nrf2 signaling pathway has an important role in AKI induced by MIR in diabetic rats.
In conclusion, the present study indicated that MIR-induced renal dysfunction was exacerbated under diabetic conditions and involved upregulation of the DJ-1/Nrf2 signaling pathway. This may provide a novel therapeutic strategy for the treatment of complications of cardiac surgery in diabetic patients.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81501648).
References
- 1.Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, Archbold RA, Uppal R, Yaqoob MM. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int. 2015;87:473–481. doi: 10.1038/ki.2014.259. [DOI] [PubMed] [Google Scholar]
- 2.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, Kwak YL. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–154. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Parlakpinar H, Ozer MK, Cicek E, Cigremis Y, Vardi N, Acet A. Renal damage in rats induced by myocardial ischemia/reperfusion: Role of nitric oxide. Int J Urol. 2006;13:1327–1332. doi: 10.1111/j.1442-2042.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 4.Ozer MK, Parlakpinar H, Vardi N, Cigremis Y, Ucar M, Acet A. Myocardial ischemia/reperfusion-induced oxidative renal damage in rats: Protection by caffeic acid phenethyl ester (CAPE) Shock. 2005;24:97–100. doi: 10.1097/01.shk.0000168525.97716.28. [DOI] [PubMed] [Google Scholar]
- 5.Chen TH, Liao FT, Yang YC, Wang JJ. Inhibition of inducible nitric oxide synthase ameliorates myocardial ischemia/reperfusion injury-induced acute renal injury. Transplant Proc. 2014;46:1123–1126. doi: 10.1016/j.transproceed.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Yi Q, Li K, Jian Z, Xiao YB, Chen L, Zhang Y, Ma RY. Risk factors for acute kidney injury after cardiovascular surgery: Evidence from 2,157 cases and 49,777 controls-a meta-analysis. Cardiorenal Med. 2016;6:237–250. doi: 10.1159/000444094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J. 2015;170:895–902. doi: 10.1016/j.ahj.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Mendez CE, Der Mesropian PJ, Mathew RO, Slawski B. Hyperglycemia and acute kidney injury during the perioperative period. Curr Diab Rep. 2016;16:10. doi: 10.1007/s11892-015-0701-7. [DOI] [PubMed] [Google Scholar]
- 9.Fiaccadori E, Sabatino A, Morabito S, Bozzoli L, Donadio C, Maggiore U, Regolisti G. Hyper/hypoglycemia and acute kidney injury in critically ill patients. Clin Nutr. 2016;35:317–321. doi: 10.1016/j.clnu.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Cao A, Wang L, Chen X, Guo H, Chu S, Zhang X, Peng W. Ursodeoxycholic acid ameliorated diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress. Biol Pharm Bull. 2016;39:1300–1308. doi: 10.1248/bpb.b16-00094. [DOI] [PubMed] [Google Scholar]
- 11.Wang JY, Yang JH, Xu J, Jia JY, Zhang XR, Yue XD, Chen LM, Shan CY, Zheng MY, Han F, et al. Renal tubular damage may contribute more to acute hyperglycemia induced kidney injury in non-diabetic conscious rats. J Diabetes Complications. 2015;29:621–628. doi: 10.1016/j.jdiacomp.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Shen ZY, Meng QT, Liu HZ, Duan WN, Xia ZY. The role of DJ-1/Nrf2 pathway in the pathogenesis of diabetic nephropathy in rats. Ren Fail. 2016;38:294–304. doi: 10.3109/0886022X.2015.1120119. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Zhou B, Xia ZY, Zhao B, Lei SQ, Yang QJ, Xue R, Leng Y, Xu JJ, Xia Z. Hyperglycemia-induced inhibition of DJ-1 expression compromised the effectiveness of ischemic postconditioning cardioprotection in rats. Oxid Med Cell Longev. 2013;2013:564902. doi: 10.1155/2013/564902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: A clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. 2013;2013:836760. doi: 10.1155/2013/836760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo Z, Guo T, Jiang J, McNeill JH. N-acetylcysteine attenuates PKCbeta2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc Res. 2007;73:770–782. doi: 10.1016/j.cardiores.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Yang J, Ding JW, Chen LH, Wang YL, Li S, Wu H. Sequential expression of TLR4 and its effects on the myocardium of rats with myocardial ischemia-reperfusion injury. Inflammation. 2008;31:304–312. doi: 10.1007/s10753-008-9079-x. [DOI] [PubMed] [Google Scholar]
- 17.McWhinnie DL, Thompson JF, Taylor HM, Chapman JR, Bolton EM, Carter NP, Wood RF, Morris PJ. Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation. 1986;42:352–358. doi: 10.1097/00007890-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Duan SY, Xing CY, Zhang B, Chen Y. Detection and evaluation of renal biomarkers in a swine model of acute myocardial infarction and reperfusion. Int J Clin Exp Pathol. 2015;8:8336–8347. [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, Yu Q, Zhang J, Huang W, Liu Y, Pei H, Liu J, Sun L, Yang L, Li C, et al. Increased myocardial ischemia-reperfusion injury in renal failure involves cardiac adiponectin signal deficiency. Am J Physiol Endocrinol Metab. 2014;306:E1055–E1064. doi: 10.1152/ajpendo.00428.2013. [DOI] [PubMed] [Google Scholar]
- 20.O'Neal JB, Shaw AD, Billings FT., IV Acute kidney injury following cardiac surgery: Current understanding and future directions. Crit Care. 2016;20:187. doi: 10.1186/s13054-016-1352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–867. doi: 10.1038/ki.2011.156. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Auriemma S, Fabbri A, D'Onofrio A, Katz N, McCullough PA, Ricci Z, Shaw A, Ronco C. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Wang D, Liu X, Pei W, Li J, Cao Y, Zhang J, An Y, Nie J, Tong J. Oxidative DNA damage is involved in cigarette smoke-induced lung injury in rats. Environ Health Prev Med. 2015;20:318–324. doi: 10.1007/s12199-015-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czerska M, Mikołajewska K, Zieliński M, Gromadzińska J, Wasowicz W. Today's oxidative stress markers. Med Pr. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- 25.Chan JY, Chan SH. Activation of endogenous antioxidants as a common therapeutic strategy against cancer, neurodegeneration and cardiovascular diseases: A lesson learnt from DJ-1. Pharmacol Ther. 2015;156:69–74. doi: 10.1016/j.pharmthera.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Yan YF, Yang WJ, Xu Q, Chen HP, Huang XS, Qiu LY, Liao ZP, Huang QR. DJ-1 upregulates anti-oxidant enzymes and attenuates hypoxia/re-oxygenation-induced oxidative stress by activation of the nuclear factor erythroid 2-like 2 signaling pathway. Mol Med Rep. 2015;12:4734–4742. doi: 10.3892/mmr.2015.3947. [DOI] [PubMed] [Google Scholar]


