Abstract
The present study aimed to explore the mechanism of action of Gegen Qinlian decoction (GGQLD) in experimental non-alcoholic fatty liver disease (NAFLD). A total of 30 rats were randomly divided into five groups: The chow, model, high- and low-dose GGQLD (GGQLD-H and GGQLD-L, respectively) and resveratrol (Resl) groups, and were treated with saline, GGQLD and Resl when a model of high-fat diet (HFD)-induced NAFLD was established. Blood lipid and liver enzymes were detected following treatment for 8 weeks and liver tissue pathology was observed using Oil Red O and haematoxylin and eosin staining. Furthermore, the liver protein and mRNA expression of sirtuin (Sirt)1, peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) were measured using western blotting and reverse transcription-quantitative polymerase chain reaction. Compared with the chow group, the model group demonstrated significantly increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (P<0.01). GGQLD doses and Resl attenuated the elevated serum ALT and AST levels. GGQLD-H and Resl significantly increased the serum high-density lipoprotein cholesterol level compared with that in the model group (P<0.01), while GGQLD-L and Resl significantly decreased serum low-density lipoprotein cholesterol levels (P<0.01). The GGQLDs and Resl groups revealed an evident improvement in Sirt1 protein and mRNA expression. Although GGQLD and Resl significantly decreased NF-κB gene expression compared with the model group (P<0.01), the effect on NF-κB protein expression was not significant. Furthermore, the PGC-1α gene and protein expression in the HFD rat group slightly decreased compared to the levels in the chow group, but the decrease was insignificant. However, an evident increase in PGC-1α mRNA expression was observed in the GGQLD-H group compared with the model group (P<0.01). Histological staining revealed that GGQLD and Resl decreased the lipid droplets in hepatocytes and normalized steatosis in rats fed with a HFD. The results indicated that GGQLD treatment may be a potent strategy for managing NAFLD by managing lipid metabolism and inflammatory and histological abnormalities by triggering the Sirt1 pathway.
Keywords: non-alcoholic fatty liver disease, Chinese herbs, Gegen Qinlian decoction, sirtuin 1, nuclear factor-κB, peroxisome proliferator-activated receptor-γ coactivator-1α
Introduction
Non-alcoholic fatty liver disease (NAFLD) consists of a spectrum of illnesses ranging from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) and irreversible cirrhosis (1). This syndrome has become the most common cause of liver disease (2). Indeed, NAFLD is currently recognized as an aetiological factor in numerous conditions previously labelled as cryptogenic cirrhosis (3). To date, the pathogenesis and progression of NAFLD are not fully understood, while current therapeutic treatment methods require improvement (1,4). Initial theories of the pathogenesis of NAFLD/NASH were based on the ‘2-hit’ hypothesis. Briefly, the ‘first hit’ involves hepatic triglyceride (TG) accumulation or steatosis, while the ‘second hit’ is usually linked to mediators, including inflammatory cytokines and oxidative stress (1). However, there is an increasing recognition of the function that free fatty acids (FFA) have in directly promoting liver injury, leading to the modification of this theory (1). Additionally, a ‘third-hit’ has been included to reflect the inadequate hepatocyte proliferation (1).
In the progression of chronic liver injury, the development of fibrosis/cirrhosis depends on the efficacy of hepatocyte regeneration (5). Hence, cell death with impaired proliferation of hepatocyte progenitors represents the proposed ‘third hit’ in NAFLD pathogenesis (5). Furthermore, hepatic TG accumulation may occur as a result of increased fat synthesis and delivery, as well as decreased export and/or oxidation of fat (6). Additionally, inflammatory cytokines, including tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8, mediate steatohepatitis in patients with NAFLD (7) and may be regulated by the peroxisome proliferator-activated receptor (PPAR)-γ. PPAR-γ may be activated by the PPARγ coactivator (PGC)-1α following sirtuin (Sirt)-1 activation (8).
Chinese herbal medicine (CHM), which has been traditionally used in China and other parts of Asian countries for thousands of years, is now spreading worldwide (9). A specific and basic feature of Chinese medicine is the use of formulas containing several herbs (herbal cocktail) to ameliorate a series of abnormal symptoms associated with a particular disease. Gegen Qinlian decoction (GGQLD), a classical formula from the Treatise on Febrile Diseases, is one of the well-known traditional Chinese medicines and consists of Kudzu root, Rhizoma coptidis, Scutellaria baicalensis Georgi and honey-fried liquorice root (10). Furthermore, it is widely used to treat diarrhoea (11). In previous studies, it was revealed that GGQLD improved diabetes (12,13) and had an anti-inflammatory effect based on its constituents (14–16). Furthermore, glucose and lipid metabolism disorder and inflammation are important in NAFLD. A previous study demonstrated that GGQLD therapeutically managed NAFLD by improving the PPARγ-mediated regulation of lipids and suppression of inflammation (17). However, the mechanisms by which GGQLD triggers PPARγ are unknown, including whether it has a potential Sirt1 agonist activity. Therefore, following the guidelines of the traditional Chinese medicine theory of ‘same treatment for different diseases’ and based on the ameliorating effects of PPARγ in NAFLD management (17), it was hypothesized that GGQLD could improve NAFLD as a Sirt1 agonist. In the present study, this hypothesis was investigated.
Materials and methods
Preparation of GGQLD and resveratrol (Resl)
GGQLD granules were provided by the Department of Pharmacy of Dongfang Hospital, Beijing University of Chinese Medicine (Beijing, China). The granules consisted of the following ingredients of the Gegen Qinlian formula: Kudzu root, Rhizoma coptidis, Scutellaria baicalensis Georgi and main liquorice at 24, 9, 9 and 6 g, respectively. Resl was purchased from Zelangyiy Co., Ltd., (Nanjing, China).
Animals and treatment
A total of 30 male Sprague-Dawley rats (7 weeks old; weight, 200±20 g) were supplied by Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animal experimental procedures were approved by the Animal Ethics Committee of Beijing University of Chinese Medicine under the guidelines issued by Regulations of Beijing Laboratory Animal Management (18). The rats were maintained on a 12-h light/dark cycle under a constant temperature (22±2°C) and humidity of 50±10% with ad libitum access to standard chow diet (control group) or a high-fat diet (HFD; 34% fat, 19% protein and 47% carbohydrate by energy composition; n=6 per group) for 8 weeks to induce NAFLD. Additionally, the GGQLD granules and Resl were dissolved in 100 ml of distilled water, and stored at 2–8°C until further use. The five groups of animals (n=6) were treated orally for 8 weeks as follows: GGQLD low- and high-dose (GGQLD-L and GGQLD-H) groups treated with 5.04 and 10.08 g/kg/day GGQLD, respectively; Resl group, treated with 400 mg/kg/day Resl; and the HFD model and control groups were treated with 10 ml/kg/day saline for 8 weeks from the beginning. The GGQLD and Resl groups were administered the respective treatments at the beginning of the HFD feeding, and the control received the standard chow diet.
Determination of metabolic parameters and liver enzymes
At the end of the treatment, the animals were anaesthetized using 4% chloral hydrate (Sinopharm Chemical Reagent Co. Ltd, Shanghai, China) after a 12-h overnight fast and blood samples were collected from the abdominal aorta of rats. The fasting serum TGs (Jing 20142401132), total cholesterol (TC, Jing 20162400910), high-density lipoprotein cholesterol (HDL-C, Jing 20152400950) and low-density lipoprotein cholesterol (LDL-C, Jing 20142400518) as well as fasting serum alanine aminotransferase (ALT, Jing 20142401158) and aspartate aminotransferase (AST, Jing 20142401158) were analysed using ELISA kits (BioSino Biotechnology and Science, Inc., Beijing, China).
Histological analysis
Paraffin sections of the liver tissues were fixed with 10% formaldehyde solution for 48 h at room temperature, and the tissue slices (thickness, 4 µm) were prepared for hematoxylin staining for 10 min and eosin staining for 2 min at room temperature. Hematoxylin and eosin (H&E) were purchased from Beijing Zeping Bioscience & Technologies Co., Ltd. (Beijing, China). The Oil Red O (ORO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) staining was performed with freshly frozen liver tissues (−196°C, liquid nitrogen) for 3 min at room temperature. The H&E- and ORO-stained slides were visualized using a light microscope in order to investigate architecture of the liver and hepatic lipid droplets. The liver tissue samples from 3–5 rats from each group were prepared, stained and the images were subsequently captured using an Olympus digital camera (BX40; Olympus Corporation, Japan) and the NIS Element SF 4.00.06 software (Nikon Corporation, Tokyo, Japan).
Western blotting of Sirt1, PGC-1α and nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) p65
Proteins in 100 mg liver tissue homogenate were extracted using ice-cold tissue lysis buffer (WB0006; TDY Biotech Co., Ltd., Beijing, China) at 4°C in an electric tissue homogenizer at 250 × g for 5 sec, 5 times at 5 sec intervals, then incubated on ice for for 20 min. The supernatant was extracted by centrifugation at 12,000 × g for 20 min at 4°C. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Promega Corporation, Madison, WI, USA). Samples (30 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were then incubated in blocking buffer (5% skimmed milk powder) for 2 h prior to the addition of primary antibodies, which were then incubated at 4°C overnight. The membranes were immunoblotted with primary antibodies against Sirt1 (1:2,000; ab104833; Abcam, Cambridge, UK), PGC-1α (1:1,000; sc-13067; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), NF-κB (p65; 1:2,000; TDY076) and β-actin (1:10,000; TDY041; both TDY Biotech Co., Ltd.) at 4°C overnight. Peroxidase-conjugated goat anti-rabbit secondary antibodies (1:20,000; cat. no. S001; TDY Biotech, Co., Ltd.) were incubated at room temperature for 2 h and an ECL detection system (EMD Millipore, Billerica, MA, USA) was used according to routine methods (19). The intensities of the protein bands were analysed using Gel-Pro 3.2 software (Media Cybernetics, Inc., Rockville, MD, USA). β-actin protein was used as the internal control to normalize the protein loading. The experiment was performed in triplicate.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) for Sirt1, PGC-1α and NF-κB mRNA expression
Total RNA was isolated from the liver samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and subsequently reverse-transcribed at 42°C for 60 min using the oligodT primers and SuperScript Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). The RT reaction was performed with 1 µg of total RNA in a 12-µl reaction using a standard cDNA synthesis kit (CWBIO, Beijing, China), according to the manufacturer's instructions. The qPCR primer sequences were as follows: Sirt1, forward 5′-CCAGATTTCAAGGCTGTTGGTTCC-3′ and reverse 5′-CCACAGGAACTAGAGGATAAGGCGT-3′; PGC-1α, forward 5′-TTCAGTGTCACCACCGAAATCCTTAT-3′ and reverse 5′-AGAGGATCTACTGCCTGGGGACC-3′; NF-κB, forward 5′-ACGATCTGTTTCCCCTCATC-3′ and reverse 5′-TGCTTCTCTCCCCAGGAATA-3′; and GAPDH, forward 5′-TGGAGTCTACTGGCGTCTT-3′ and reverse 5′-TGTCATATTTCTCGTGGTTCA-3′. For each qPCR, a TaqMan® Real-Time PCR assay (Thermo Fisher Scientific, Inc.) was used and the typical thermal cycling conditions were an initial activation step at 95°C for 5 min, followed by 45 cycles of amplification: 94°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec and a final extension of 72°C for 5 min. To compare the Sirt1, PGC-1α and NF-κB mRNA levels, the cDNA concentrations were normalized to GAPDH PCR products and the data were analysed using the 2−ΔΔCq method (20).
Statistical analysis
Data were expressed as the mean ± standard deviation unless otherwise indicated. The data were analysed using a one-way analysis of variance and a post hoc test. Statistical analysis was performed using SPSS v17.0 (IBM Corp, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of GGQLD on lipid metabolism and liver enzymes in HFD rats
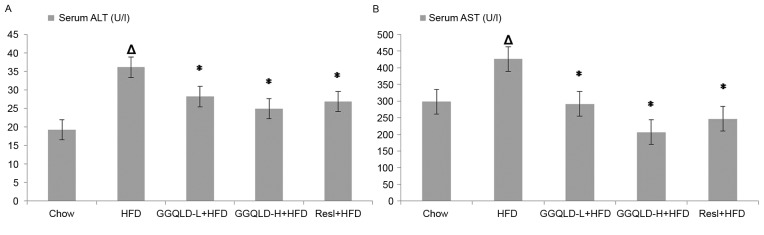
All rats tolerated the experimental procedures well and no mortalities occurred during the study. The serum ALT and AST (36.10±3.92 and 425.85±13.84 U/l, respectively) concentrations in the HFD-fed rats were significantly higher than those in the chow-fed rats (19.27±3.36 and 298.10±5.13 U/l, respectively; P<0.01). Treatment with GGQLD-L and GGQLD-H significantly attenuated ALT levels (28.20±3.01 and 24.98±1.55 U/l, respectively; P<0.01) and AST (291.24±22.36 and 206.68±20.48 U/l, respectively; P<0.01) compared with the HFD-fed rats. Similarly, Resl also significantly reduced the ALT and AST levels (26.88±1.45 and 246.66±20.47 U/l, respectively) compared with rats fed with HFD (P<0.01; Fig. 1).
Figure 1.
Effect of GGQLD on the concentration of ALT and AST. (A) ALT and (B) AST levels in serum. ∆P<0.01 vs. chow; *P<0.01 vs. HFD. GGQLD, Gegen Qinlian decoction; GGQLD-L, Gegen Qinlian decoction low dose; GGQLD-H, Gegen Qinlian decoction high dose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HFD, high-fat diet; Resl, resveratrol.
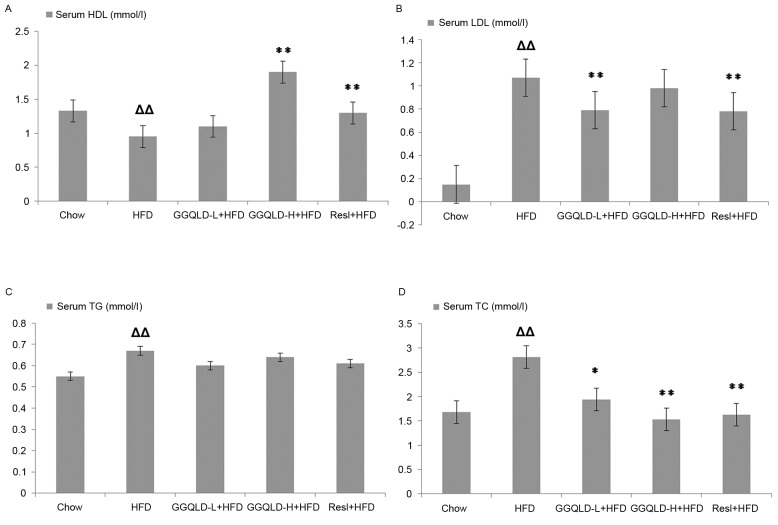
The HDL-C levels rose significantly in the GGQLD-H and Resl groups (1.90±0.10 and 1.30±0.11 mmol/l, respectively) compared with those in the HFD model group (0.95±0.04 mmol/l; P<0.01). Furthermore, GGQLD-H also decreased the LDL-C concentration (0.98±0.15 mmol/l) compared with the HFD group, but not significantly (P>0.05), whereas the decrease in the GGQLD-L and Resl groups (0.79±0.09 and 0.78±0.09 mmol/l, respectively) was significant compared to that of the HFD model group (1.07±0.20 mmol/l; P<0.01). Treatment with GGQLD-L, GGQLD-H and Resl significantly attenuated the elevated TC (1.94±0.39, 1.53±0.11 and 1.63±0.13 mmol/l, respectively) compared with that of the HFD group (2.81±0.79 mmol/l; P<0.05; Fig. 2). Treatment with GGQLD-L, GGQLD-H and Resl had no significant effect on serum TG levels compared with those in the HFD group.
Figure 2.
Effect of GGQLD on the concentration of HDL, LDL, TG and TC. (A) HDL, (B) LDL, (C) TG and (D) TC levels in serum. ∆∆P<0.01 vs. chow; *P<0.05 and **P<0.01 vs. HFD. GGQLD, Gegen Qinlian decoction; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HFD, high-fat diet; GGQLD-L, Gegen Qinlian decoction low dose; GGQLD-H, Gegen Qinlian decoction high dose; Resl, resveratrol.
Histopathological changes in rats
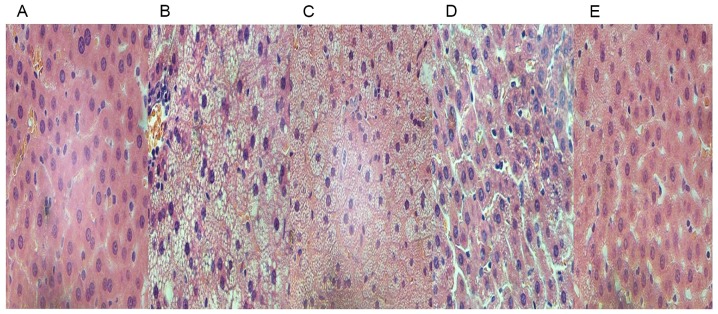
An increase in the number of H&E-positive hepatocytes was evident in the HFD rats compared to those in the chow group (Fig. 3A and B). The H&E-stained images also exhibited steatosis and infiltration with inflammatory cells in the intercellular substance (Fig. 3B). The treatment of HFD rats with GGQLD and Resl reduced the fat deposits in the liver (Fig. 3C-E) and the GGQLD-treated rats revealed histological features similar to the chow group with little steatosis (Fig. 3C and D).
Figure 3.
Results of paraffin section and haematoxylin & eosin staining in vivo (magnification, ×100). Images of liver tissues from (A) chow-fed, (B) high-fat diet model, (C) GGQLD-low dose, (D) GGQLD-high dose and (E) resveratrol-treated rats. GGQLD, Gegen Qinlian decoction.
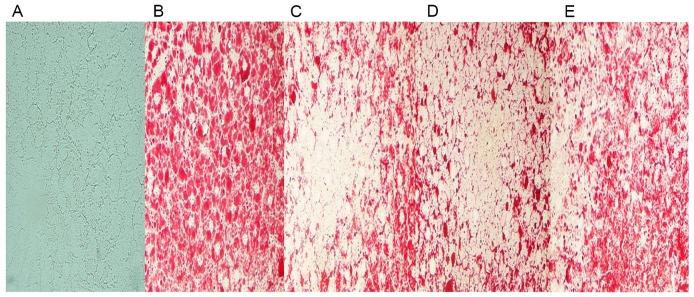
Furthermore, few lipid droplets were detected in the liver sections from the chow group in ORO staining analysis (Fig. 4A). Compared to that of HFD-fed model rats (Fig. 4B), GGQLD and Res1 markedly restrained the deposition of lipid droplets in hepatocytes (Fig. 4C-E).
Figure 4.
Results of frozen liver sections stained with Oil Red O in vivo (magnification, ×400). The red blots demonstrate lipid droplets in hepatocytes. Images from (A) chow-fed, (B) high-fat diet model, (C) GGQLD-low dose, (D) GGQLD-high dose and (E) resveratrol-treated rats. GGQLD, Gegen Qinlian decoction.
GGQLD regulates Sirt1, PGC-1α and NF-κB (p65) expression in rats with HFD-induced NAFLD
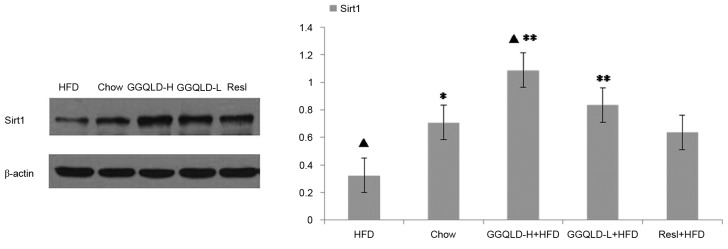
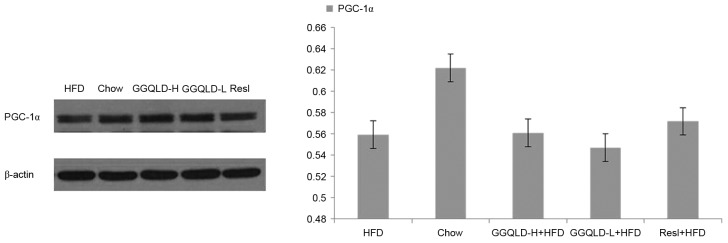
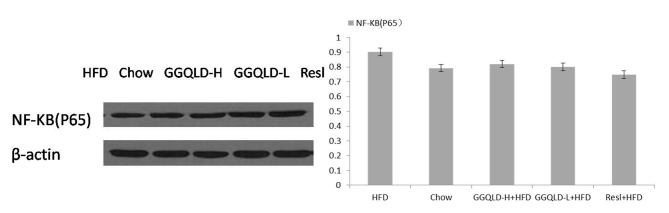
The possibility of GGQLD having a regulatory effect on Sirt1, PGC-1α and NF-κB (p65) expression was investigated. As demonstrated in Fig. 5, the Sirt1 protein level was significantly decreased in the HFD group (P<0.05) compared with the chow group. Notably, both GGQLD groups significantly improved the Sirt1 level compared with the HFD group (P<0.01; Fig. 5). Resl also improved the Sirt1 level compared with the HFD group, however this difference was not significant. As demonstrated in Figs. 6 and 7, although the PGC-1α and NF-κB (p65) levels were improved by GGQLD-L, GGQLD-H and Resl, the effect was not significant (P>0.05 vs. HFD group).
Figure 5.
Western blotting results for Sirt1 expression in liver tissue. ▲P<0.05 vs. chow; *P<0.05 and **P<0.01 vs. HFD. Sirt1, sirtuin 1; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Figure 6.
Western blotting for PGC-1α in liver tissue. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Figure 7.
Western blotting for NF-κB (p65) in liver tissue. NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Validation of GGQLD effects on Sirt1, PGC-1α and NF-κB gene expression using RT-qPCR
To confirm the action of GGQLD on liver Sirt1, PGC-1α and NF-κB protein expression levels, their gene expression levels were also measured. Sirt1 gene expression in the HFD group significantly decreased compared with that of the chow group (P<0.05; Fig. 8). Notably, GGQLD-L, GGQLD-H and Resl significantly increased Sirt1 gene expression compared with the HFD group (P<0.05; Fig. 8). As demonstrated in Fig. 9, PGC-1α gene expression in the HFD group decreased slightly compared with that of the chow group, but not significantly (P>0.05). However, a significant increase in PGC-1α gene expression was evident in the GGQLD-H group (P<0.01) compared with the HFD group. Furthermore, NF-κB gene expression in the HFD rat group increased significantly compared with that of the chow group (P<0.01; Fig. 10). Notably, GGQLD and Resl significantly decreased the NF-κB gene expression compared with the HFD group (P<0.01; Fig. 10).
Figure 8.
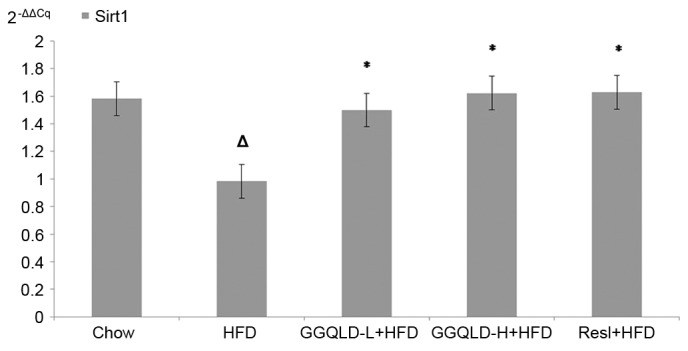
Reverse transcription-quantitative polymerase chain reaction results for Sirt1 expression in liver tissue. ∆P<0.05 vs. chow; *P<0.05 vs. HFD. Sirt1, sirtuin 1; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Figure 9.
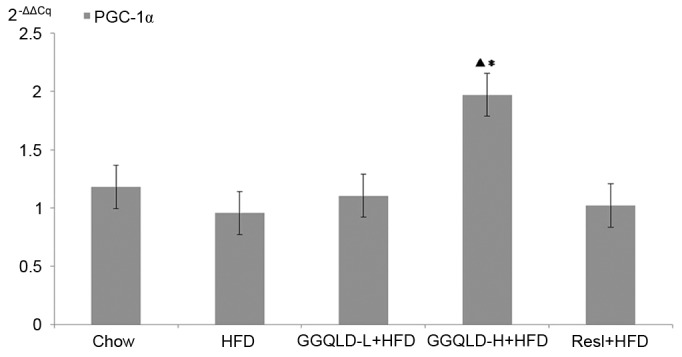
Reverse transcription-quantitative polymerase chain reaction results for PGC-1α expression in liver tissue. ▲P<0.01 vs. chow; *P<0.01 vs. HFD. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Figure 10.
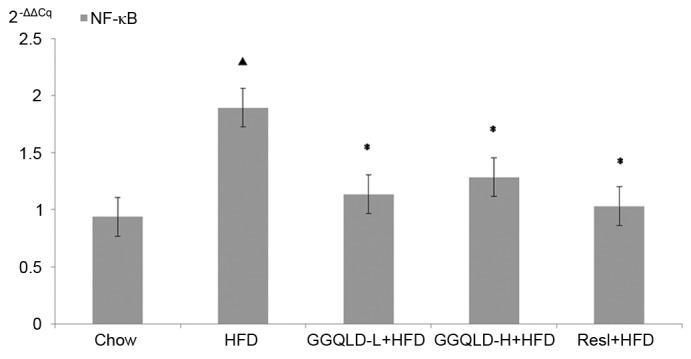
Reverse transcription-quantitative polymerase chain reaction for NF-κB expression in liver tissue. ▲P<0.01 vs. chow; *P<0.01 vs. HFD. NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; HFD, high-fat diet; GGQLD-H, Gegen Qinlian decoction high dose; GGQLD-L, Gegen Qinlian decoction low dose; Resl, resveratrol.
Discussion
NAFLD, which is a multi-factorial disorder associated with a variety of genetic and environmental contributory factors, is considered to be the most common cause of liver disease (21). Whether the initial ‘2-hit hypothesis’ or ‘3-hit hypothesis’ is applied in determining the etiology, insulin resistance, oxidative stress and inflammatory cascades are believed to serve integral roles in the pathogenesis and progression (22). The presence of steatosis induced by the overaccumulation of FFA/TGs and cholesterol is closely associated with chronic hepatic inflammation (23), which is partly mediated by the activation of the inhibitor of the NF-κB kinase subunit β/NF-κB signalling pathway. In murine models of HFD-induced steatosis, increased NF-κB activity is associated with elevated hepatic expression of inflammatory cytokines, including TNF-α and activation of Kupffer cells (23). PPARγ is one of three subtypes of the PPARs. Its agonists stimulate adipose tissue to absorb and store FFAs, and thereby, inhibits liver fatty acid synthesis (24) by activating adenosine monophosphate (AMP)-activated protein kinase (AMPK). PPARγ not only has anti-inflammatory effects but also effectively improves insulin resistance (25). The activation of PPARγ in the immune system modulates the inflammatory response (26). Furthermore, PGC-1α is a key transcriptional regulator of energy homeostasis, and is important in modulating liver fatty acid oxidation (27). The induction of this coactivator has been documented in experimental models of obesity and diabetes (28). The activation of PGC-1α increased the expression of genes that are critical to gluconeogenesis, fatty acid oxidation, lipid transport and mitochondrial biogenesis (29–31).
PGC-1α is not only an important coactivator in liver biology, but may be activated by Sirt1. Sirt1, which is the receptor for Resl, targets numerous proteins, including PPARγ, PGC-1α, uncoupling protein 2 and NF-κB, which serve key roles in various metabolic disorders (32). Furthermore, they lead to the activation of mitochondrial fatty acid oxidation genes. At present, accumulating evidence has indicated an important role for Sirts (33). In total, seven Sirts (Sirt1-7) have been identified in mammals, of which the nuclear-located Sirt1 is the closest homologue of the Sir2 protein, based on a highly-conserved family of nicotinamide adenine dinucleotide+-dependent enzymes that are easily activated by Resl (34). Sirt1 has been demonstrated to increase genomic stability and cellular stress resistance, and it regulates energy metabolism and cellular senescence via deacetylation of target proteins, including p53, forkhead box transcription factors and PGC-1α (35–38). It maintains the oxidation of fatty acids at low glucose concentrations and is a regulator of PGC-1α, which activates PPARγ and induces the transcription of metabolically relevant genes for the oxidation of mitochondrial fatty acid (8). In recent years, numerous studies (39,40) have concluded that Sirt1 activation has a negative regulatory effect on the inflammatory processes. One of the key proteins in these processes is NF-κB, which critically modulates DNA transcription in inflammatory, infectious and apoptotic processes (41). The dysregulation of NF-κB may lead to inflammatory and autoimmune diseases. Increasing evidence has revealed that NF-κB activation contributes to the pathogenesis of NAFLD/NASH and the development of hepatocellular carcinoma (23). The activation of Sirt1 deacetylates the RelA/p65 subunit and thereby inhibits NF-κB signalling (42,43).
An animal model of HFD-induced NAFLD has been widely used to identify the pathogenesis and evaluate novel treatments for NAFLD (44,45). In the present study, 8 weeks of an HFD-induced fatty liver disease in Sprague-Dawley rats, in which key biochemical features and histological abnormalities were revealed by H&E- and ORO-stained liver samples, were consistent with existing reports (44,46). Although studies (47) have focused on therapies for NAFLD, no pharmacological agents have been approved for its treatment. Therefore, the majority of the clinical efforts have currently shifted their focus to strategies associated with metabolic syndromes, namely obesity, diabetes, dyslipidaemia and hypertension (48). Other interventions are directed at specific pathways that are potentially involved in the pathogenesis of NAFLD, including oxidative stress, insulin resistance and pro-inflammatory cytokines (49).
Numerous studies have used adipocytes to demonstrate that Resl has an anti-obesity potential by decreasing adipocyte proliferation, inducing adipocyte apoptosis, inhibiting preadipocyte differentiation, decreasing lipogenesis and promoting lipolysis and fatty acid β-oxidation (50–54). These effects may be mediated by the central regulators of adipogenesis, lipogenesis and fatty acid β-oxidation, including the aforementioned AMPK, Sirt1 and PGC-1α (55). Resl, a deacetylase, is an indirect activator of Sirt1, which increases the intracellular cyclic AMP (cAMP) concentration by inhibiting cAMP phosphodiesterases that downregulate cAMP (56). Additionally, increased cAMP concentrations activate AMPK, which finally binds to the promoter of PGC-1α, demonstrating its important enzymatic role in regulating cellular energy homeostasis (57).
CHM has been demonstrated to exhibit an extremely beneficial effect on numerous diseases (58–60), and the formulations used have a relatively wide safety. Our previous study (14) demonstrated that GGQLD therapeutically managed NAFLD by improving PPARγ dysregulation, thereby regulating lipids and suppressing inflammation. In the present study, treatment with GGQLD for 8 weeks significantly lowered the liver hepatic aminotransferases (ALT and AST) to a level that was comparable to that of the normal chow-fed control group. Additionally, it was effective in impeding fat infiltration, which was evidenced by the decreased hepatic TC, LDL and lipid droplets. Although the pathogenic mechanisms of NAFLD are still under investigation, fat accumulation, particularly TG filtration into hepatocytes, is considered the first step in the development of NAFLD (1). Hence, lipid accumulation serves a confirmed vital role in NAFLD. The results of the biochemical and histological assays demonstrated that GGQLD-L and Resl reduced the serum LDL-C and TC, respectively. Additionally, the HDL-C levels were raised in the GGQLD-H group while the histological staining revealed that GGQLD-L, GGQLD-H and Resl decreased the lipid droplets in the hepatocytes and normalized the steatosis in HFD rats.
Sirt1, a regulator of PGC-1α that activates PPARγ, induces the transcription of metabolically relevant genes for the oxidation of mitochondrial fatty acids (8). This cascade has a negative regulatory effect on inflammatory processes. The present study demonstrated that GGQLD and Resl evidently improved the Sirt1 protein and gene expression level. Although GGQLD and Resl considerably and significantly decreased NF-κB gene expression, the protein expression demonstrated a declining trend. PGC-1α gene and protein expression in the HFD group was slightly decreased compared to that in the chow group but not significantly. However, an evident increase in the PGC-1α gene expression was observed in the GGQLD-H group compared with the chow and HFD groups. Notably, GGQLD increased the Sirt1 protein and gene expression, with effects that were comparable to those of Resl.
Thus, based on the data of the present study, GGQLD had a positive effect on NAFLD by improving the regulation of Sirt1, which has a critical role in lipid and inflammation regulation, thereby additional experimental evidence was provided to support its clinical use. Since the herbal content of GGQLD has been used for thousands of years in traditional medicine, it is considered relatively safe, reliable and tolerable. In summary, the present study explored GGQLD as a potential optional approach to treat NAFLD by managing lipid metabolism, inflammation and histological abnormalities via the Sirt1 pathway. Further experiments would focus on the intestinal immune response in NAFLD based on the involvement of the gut liver axis using systems biology and omics methods.
Acknowledgements
The present study was supported by the Youth Fund of National Natural Science Foundation of China (grant no. 81503407) and the Self-selected subject of the Beijing University of Chinese Medicine Grants (grant nos. 2015-JYB-JSMS125 and 2013-JYBZZ-XS-153).
References
- 1.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: The mist gradually clears. J Hepatol. 2008;48:S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 4.Enjoji M, Yasutake K, Kohjima M, Nakamuta M. Nutrition and nonalcoholic Fatty liver disease: The significance of cholesterol. Int J Hepatol. 2012;2012:925807. doi: 10.1155/2012/925807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 6.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Ling F, Griffin TM, He T, Towner R, Ruan H, Sun XH. Up-regulation of the Sirtuin 1 (Sirt1) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) genes in white adipose tissue of Id1 protein-deficient mice: Implications in the protection against diet and age-induced glucose intolerance. J Biol Chem. 2014;289:29112–29122. doi: 10.1074/jbc.M114.571679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue CC, Zhang AL, Greenwood KM, Lin V, Story DF. Traditional chinese medicine: An update on clinical evidence. J Altern Complement Med. 2010;16:301–312. doi: 10.1089/acm.2009.0293. [DOI] [PubMed] [Google Scholar]
- 10.Shi Z, Li Z, Zhang S, Fu H, Zhang H. Subzero-Temperature Liquid-Liquid Extraction Coupled with UPLC-MS-MS for the simultaneous determination of 12 bioactive components in traditional chinese medicine gegen-qinlian decoction. J Chromatogr Sci. 2015;53:1407–1413. doi: 10.1093/chromsci/bmu226. [DOI] [PubMed] [Google Scholar]
- 11.Ling X, Xiang Y, Tang Q, Chen F, Tan X. Comparative pharmacokinetics of eight major bioactive components in normal and bacterial diarrhea mini-pigs after oral administration of Gegen Qinlian Decoction. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1044–1045:132–141. doi: 10.1016/j.jchromb.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CH, Xu GL, Liu YH, Rao Y, Yu RY, Zhang ZW, Wang YS, Tao L. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine. 2013;20:221–229. doi: 10.1016/j.phymed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Tong XL, Zhao LH, Lian FM, Zhou Q, Xia L, Zhang JC, Chen XY, Ji HY. Clinical observations on the dose-effect relationship of gegen qin lian decoction on 54 out-patients with type 2 diabetes. J Tradit Chin Med. 2011;31:56–59. doi: 10.1016/S0254-6272(11)60013-7. [DOI] [PubMed] [Google Scholar]
- 14.Song S, Qiu M, Chu Y, Chen D, Wang X, Su A, Wu Z. Berberine down-regulates cellular JNK and NF-kappaB activation and this may result in an inhibition of HSV replication. Antimicrob Agents Chemother. 2014;58:5068–5078. doi: 10.1128/AAC.02427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Ye H, Zhu YZ, Guo M, He XX, Zheng XB. Protective effect of baicalin against LPS-induced intestinal injury. Zhongguo Zhong Yao Za Zhi. 2013;38:2854–2858. (In Chinese) [PubMed] [Google Scholar]
- 16.Yang X, Yang J, Zou H. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin Dev Immunol. 2013;2013:268065. doi: 10.1155/2013/268065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YL, Liu LJ, Zhao WH, Li JX. Intervening TNF-α via PPARγ with Gegenqinlian decoction in experimental nonalcoholic fatty liver disease. Evid Based Complement Alternat Med. 2015;2015:715638. doi: 10.1155/2015/715638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regulations of Beijing Laboratory Animal Management. Laboratory Animal Science. 2005;5:1–3. (In Chinese) [Google Scholar]
- 19.Vink EI, Yondola MA, Wu K, Hearing P. Adenovirus E4-ORF3-dependent relocalization of TIF1α and TIF1γ relies on access to the Coiled-Coil motif. Virology. 2012;422:317–325. doi: 10.1016/j.virol.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, Svegliati-Baroni G, Valenti L, Bonino F. Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17122082. pii: E2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: A review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 23.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gastaldelli A, Miyazaki Y, Mahankali A, Berria R, Pettiti M, Buzzigoli E, Ferrannini E, DeFronzo RA. The effect of pioglitazone on the liver: Role of adiponectin. Diabetes Care. 2006;29:2275–2281. doi: 10.2337/dc05-2445. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Shi J, Lu J, Meng G, Zhu H, Hou Y, Yin Y, Zhao S, Ding B. Activation of peroxisome proliferator-activated receptor-gamma potentiates pro-inflammatory cytokine production, and adrenal and somatotropic changes of weaned pigs after Escherichia coli lipopolysaccharide challenge. Innate Immun. 2009;15:169–178. doi: 10.1177/1753425908102014. [DOI] [PubMed] [Google Scholar]
- 27.Nassir F, Ibdah JA. Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:8713–8742. doi: 10.3390/ijms15058713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 alpha expression. Diabetes. 2009;58:1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- 33.Sacconnay L, Carrupt PA, Nurisso A. Human sirtuins: Structures and flexibility. J Struct Biol. 2016;196:534–542. doi: 10.1016/j.jsb.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari A, Herzig KH, Lecklin A, Mervaala E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation. J Nutr Metab. 2011;2011:525094. doi: 10.1155/2011/525094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denu JM, Gottesfeld JM. Minireview series on sirtuins: From biochemistry to health and disease. J Biol Chem. 2012;287:42417–42418. doi: 10.1074/jbc.R112.428862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, III, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui X, Chen Q, Dong Z, Xu L, Lu T, Li D, Zhang J, Zhang M, Xia Q. Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-κB. Cell Death Dis. 2016;7:e2403. doi: 10.1038/cddis.2016.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed Pharmacother. 2017;90:500–508. doi: 10.1016/j.biopha.2017.03.102. [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela R, Illesca P, Echeverría F, Espinosa A, Rincón-Cervera MÁ, Ortiz M, Hernandez-Rodas MC, Valenzuela A, Videla LA. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017;8:1526–1537. doi: 10.1039/C7FO00090A. [DOI] [PubMed] [Google Scholar]
- 42.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J Endocrinol. 2007;194:539–550. doi: 10.1677/JOE-07-0234. [DOI] [PubMed] [Google Scholar]
- 45.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 46.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardi R, Onali S, Thorburn D, Davidson BR, Gurusamy KS, Tsochatzis E. Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): An attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011640. doi: 10.1002/14651858.CD011640.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich CG, Rau M, Jahn D, Geier A. Changes in drug transport and metabolism and their clinical implications in non-alcoholic fatty liver disease. Expert Opin Drug Metab Toxicol. 2017;13:625–640. doi: 10.1080/17425255.2017.1314461. [DOI] [PubMed] [Google Scholar]
- 49.Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2010;3:121–137. doi: 10.1177/1756283X09359964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;364:972–977. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- 52.Chen S, Li Z, Li W, Shan Z, Zhu W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can J Physiol Pharmacol. 2011;89:793–799. doi: 10.1139/y11-077. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z, Zhu W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem. 2012;23:1100–1112. doi: 10.1016/j.jnutbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Costa Cdos S, Rohden F, Hammes TO, Margis R, Bortolotto JW, Padoin AV, Mottin CC, Guaragna RM. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1-3 mRNA expression in human visceral adipocytes. Obes Surg. 2011;21:356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- 55.Maruyama H, Kiyono S, Kondo T, Sekimoto T, Yokosuka O. Palmitate-induced Regulation of PPARγ via PGC1α: A mechanism for lipid accumulation in the liver in nonalcoholic fatty liver disease. Int J Med Sci. 2016;13:169–178. doi: 10.7150/ijms.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons GE, Jr, Pruitt WM, Pruitt K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int J Mol Sci. 2015;16:950–965. doi: 10.3390/ijms16010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MY, Seo CS, Shin IS, Kim YB, Kim JH, Shin HK. Evaluation of oral subchronic toxicity of soshiho-tang water extract: The traditional herbal formula in rats. Evid Based Complement Alternat Med. 2013;2013:590181. doi: 10.1155/2013/590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bose S, Kim H. Evaluation of in vitro anti-inflammatory activities and protective effect of fermented preparations of Rhizoma Atractylodis Macrocephalae on intestinal barrier function against lipopolysaccharide insult. Evid Based Complement Alternat Med. 2013;2013:363076. doi: 10.1155/2013/363076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Qiu XM, Hao Q, Li DJ. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor β mediating nitric oxide production and NF-κB suppression in endothelial cells. Cell Death Dis. 2013;4:e551. doi: 10.1038/cddis.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]