Abstract
Transforming growth factor (TGF)-β1 has an essential role in bronchitis and the induction of bronchial remodelling, which are critical processes in the pathogenesis of asthma. However, the role of interleukin (IL)-15 in asthma inflammation remains unclear. The aim of the present study was to evaluate the effect of TGF-β1 mRNA expression on IL-15 mRNA expression in asthmatic patients and to assess the role of IL-15 in the clinical course of asthma. The study included 221 participants, comprising 130 patients with asthma and 91 healthy volunteers. The participants were subjected to testing using spirometry, as well as the Asthma Control Test™ and Borg Scale. The expression of TGF-β1 and IL-15 mRNA was analyzed in blood samples using reverse transcription-quantitative polymerase chain reaction. Statistical analysis indicated that IL-15 and TGF-β1 mRNA expression each differed significantly between the patient and control groups (P=0.0016 and P=0.033, respectively). A significant correlation was identified between IL-15 expression and TGF-β1 expression (R=0.41, P=0.0005). No correlation was observed between IL-15 expression and the degree of asthma severity, the results of spirometric examination or the frequency of asthma exacerbations. Further analysis revealed that IL-15 expression was elevated following the administration of inhaled glucocorticosteroids (iGCs; P=0.024), and reduced following methylxanthine treatment (P<0.001). The occurrence of dyspnoea differed between the study and control groups, and this was not found to be associated with IL-15 expression. Since IL-15 expression was correlated with TGF-β1 expression among asthmatic patients, and IL-15 expression was elevated following iGC administration, the results of the study suggest that IL-15 activity might be associated with the pathogenesis of asthma.
Keywords: interleukin-15, transforming growth factor-β, asthma, inflammation, airway remodelling, airway obstruction
Introduction
Bronchial asthma is a chronic, heterogeneous inflammatory disease of the respiratory tract that leads to bronchial hyperreactivity and obturation (1,2). It is characterized by considerable phenotypic and endotypic variability (3).
The immunological processes responsible for inflammation are an important issue in asthma. One of the key immunomodulating cytokines is transforming growth factor (TGF)-β, which has three isoforms (TGF-β1, 2 and 3). TGF-β1 is a protein from the TGF-β subfamily, which comprises >30 cytokines. TGF-β1 regulates the production of the extracellular matrix and stimulates fibroblast production through the mothers against decapentaplegic (SMAD) cascade (4,5). By inducing the expression of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), TGF-β1 regulates the production of fibroblast growth factor 2 or induction of the connective tissue growth factor, and induces fibronectin synthesis through the direct activation of c-Jun N-terminal kinase (6,7).
TGF-β1 also plays an important role in airway remodelling in asthma, as it increases the proliferation of airway smooth muscle cells via the mitogen-activated protein kinase pathway and by upregulation of the α5β1-integrin receptor, which plays a key role in the process of proliferation (7). TGF-β1 exerts its pro-inflammatory effects through the induction of eosinophil, macrophage, B lymphocyte, neutrophil and mastocyte chemotaxis (8). It is a proliferative factor for mastocytes and neutrophils and may activate their degranulation (9). The induced inflammation is manifested by the suppression of T-helper (Th)2 lymphocytes and inhibition of eosinophil differentiation (8,9). As TGF-β1 has numerous functions in the body, a change in its expression has the ability to seriously affect the course of asthma.
Interleukin (IL)-15 is another notable cytokine with regard to asthma. It is a 4α-helix protein that is structurally similar to IL-2, a growth factor and modulator of T lymphocytes and natural killer (NK) cells, whose increased level is observed in bronchoalveolar lavage cells in asthmatic patients, particularly those with steroid-resistant asthma (10,11). The IL-15 gene is localized on chromosome 4q31 (12). IL-15 mRNA expression occurs in numerous cells, including fibroblasts, epithelial cells, monocytes and dendritic cells (11). Unlike IL-2, whose expression is only detected in activated T lymphocytes, IL-15 mRNA is subject to low-level constitutive expression that increases in response to infections, particularly in macrophages exposed to lipopolysaccharides and mycobacteria (13,14). Although IL-15 itself is not downregulated in the presence of IL-4, IL-13 or TGF-β, its mRNA level has been reported to increase following stimulation by IL-10, which also inhibits other monokines (13).
IL-15 is a growth and differentiation factor for NK cells (15), which also affects the proliferation of B lymphocytes and the production of antibodies (16). It has been demonstrated to have pro-inflammatory properties in rheumatoid arthritis and sarcoidosis (17). However, the role of IL-15 in asthma remains unclear. Certain authors claim that while it does not play any role in the inflammatory process in asthma, it influences the course of pulmonary inflammatory diseases in which the inflammation is mediated by Th1 lymphocytes (18). The results of other studies indicate that IL-15 inhibits eosinophil apoptosis (19), induces IL-5 synthesis through Th cell activity (20) and suppresses the Th2-dependent response by promoting the Th1 response in asthmatic mice (21). Experiments on cell lines and the examination of bronchoalveolar lavage fluid have revealed that IL-15 expression in macrophages is increased in Rhinovirus infections, and that this mechanism is impaired in asthmatic patients (22). Moreover, IL-15 expression measured in induced expectorated matter and sputum cell culture has been shown to increase following the administration of glucocorticoid therapy (23).
As IL-15 appears to play a significant role in the pathogenesis of asthma, the aim of the present study was to evaluate the potential association between IL-15 and TGF-β1, as well as various clinical parameters, by measuring the expression of IL-15 mRNA in asthmatic patients.
Materials and methods
Patients and controls
The study was approved by the bioethics committee at the Medical University of Lodz (Lodz, Poland; nos. RNN/133/09/KE and RNN/31/14/KE). Prior to the study, written informed consent was provided by all participants. The study comprised 221 asthmatic and healthy volunteers, all of Caucasian ethnicity, who were recruited in Norbert Barlicki University Clinical Hospital No. 1 (Lodz, Poland) between October 2010 and June 2015.
The asthmatic patient group comprised 130 people diagnosed in compliance with Global Initiative for Asthma (GINA) guidelines (24), on the basis of clinical symptoms and lung functional activity. In addition to providing written consent and having a diagnosis of asthma according to GINA guidelines, inclusion on the study also required a good quality spirometry result and Asthma Control Test (ACT™) results. Of the 130 patients, 26 (20%) had mild asthma, 61 (46.92%) moderate asthma and 43 (33.08%) severe asthma according to the GINA criteria. The exclusion criteria comprised the following: Symptoms of a viral infection, either systemic and of the respiratory tract, or an ongoing episode of clinically significant exacerbation, defined as a progressive increase in symptoms sufficient to require a change of treatment.
The control group consisted of 91 healthy volunteers fulfilling the following criteria: No history of asthma, symptoms of asthma or other pulmonary diseases, no history or symptoms of allergy, no history or symptoms of atopic dermatitis, no history or symptoms of hyperreactivity to aspirin, and no symptoms of infection in the previous 3 months.
The mean ± standard deviation age of the 221 volunteers was 49.16±15.79 years (range, 20–82 years). The asthmatic patient group consisted of 82 (63.1%) females and 48 (36.9%) males, with a mean age of 50.10±15.11 years (range, 20–80 years). Of the 91 volunteers from the control group, 56 (61.5%) were female and 35 (38.5%) male, with a mean age of 47.82±16.72 years (range, 21–80 years).
Assessment of obturation and the degree of asthma
Following the guidelines of the Polish Society of Lung Diseases (25), obturation and the degree of asthma were evaluated on the basis of the three optimum spirometry measurements, as defined below. A statistician analyzed the forced expiratory volume in 1 sec (FEV1) expressed in liters, FEV1% (measured FEV as a percentage of the expected value) and the FEV1/forced vital capacity (FVC) index (FEV1 to FVC ratio, expressed as a percentage). The spirometric examination was performed at a hospital outpatient clinic, in compliance with European Respiratory Society/American Thoracic Society standards (26): At least three FVC manoeuvers which met within-manoeuver (good start; extrapolated volume <5% of FVC or 0.15 liters, whichever is greater; lack of artefacts such as cough or leak; exhalation time ≥6 sec, or plateau in the volume-time curve) and between-manoeuver (difference between largest and next largest FVC and FEV1 are <0.15 liters) acceptability criteria.
In the asthmatic patient group, the mean value of FEV1 was 2.28±0.89 liters; the mean value of FEV1% was 75.53±20.28%; and FEV1/FVC was 67.98±11.88%. In the control group, the mean value of FEV1 was 2.94±0.83 liters; the mean value of FEV1% was 96.14±12.15%; and FEV1/FVC was 79.43±6.62%. Table I presents the baseline characteristics of the two groups.
Table I.
Baseline characteristics of the study population including epidemiological data and pulmonary function.
| A, Healthy control (n=91) | |||||
|---|---|---|---|---|---|
| Parameter | Mean | Median | Minimum | Maximum | Standard deviation |
| Age, years | 47.82 | 50.00 | 21 | 80 | 16.72 |
| BMI | 26.08 | 25.30 | 18.30 | 42.00 | 4.41 |
| FEV1, 1 | 2.94 | 2.97 | 1.40 | 4.85 | 0.83 |
| FEV1, % | 96.14 | 95.00 | 66.00 | 128.00 | 12.15 |
| FVC, l | 3.71 | 3.48 | 1.87 | 1.87 | 1.87 |
| FVC, % | 101.59 | 101.00 | 71.00 | 150.00 | 14.39 |
| FEV1/FVC, % | 79.43 | 78.38 | 64.15 | 95.15 | 6.62 |
| B, Asthmatic patients (n=130) | |||||
| Age, years | 50.10 | 52.00 | 20 | 80 | 15.11 |
| BMI | 26.93 | 26.40 | 17.80 | 43.00 | 5.32 |
| FEV1, l | 2.28 | 2.25 | 0.79 | 4.68 | 0.89 |
| FEV1, % | 75.53 | 76.00 | 25.00 | 125.00 | 20.28 |
| FVC, 1 | 3.34 | 3.19 | 1.54 | 6.48 | 1.10 |
| FVC, % | 92.87 | 94.00 | 38.00 | 134.00 | 17.45 |
| FEV1/FVC, % | 67.78 | 71.43 | 42.92 | 90.76 | 11.88 |
FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity, FEV1/FVC, ratio of FEV1 as to FVC.
The degree of asthma control was evaluated with the application of the ACT™, a five-question questionnaire, prepared by Nathan et al (27). The following criteria were adopted: 0–19 points, uncontrolled; 20–24 points, partly controlled; ≥25 points, well-controlled.
In addition, participants were asked to complete a Borg Scale questionnaire, used for the psychosocial evaluation of perceived exertion (28). Information on drug treatments received was obtained from the patient's disease history.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Venous blood was collected from the participants in test tubes filled with ethylenediaminetetraacetate (Sarstedt AG, Nümbrecht, Germany). RNA was then isolated from peripheral blood lymphocytes with the application of TRI Reagent® Solution (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the standard acid guanidinium-phenol-chloroform procedure (29,30). The quality of the isolated RNA was evaluated with spectrometry at 260 nm using a Nanodrop ND-1000 analyzer (Thermo Fisher Scientific, Inc.).
Following this, 1 µg RNA was reversely transcribed using the ImProm-II™ Reverse Transcription system (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol (annealing at 25°C for 5 min, first-strand synthesis reaction at 42°C for 60 min, inactivation of reverse transcriptase at 70°C for 15 min). The products were subjected to qPCR using primers specific for the analyzed genes with the application of Phusion High Fidelity DNA Polymerase (Thermo Fisher Scientific, Inc.) and EvaGreen® dye (Biotum, Inc., Freemont, CA, USA) and a Stratagene Mx3000P thermocycler (Agilent Technologies, Inc., Santa Clara, CA, USA). The following gene-specific primers were used: TGF-β1, forward 5′-GGTACCTGAACCCGTGTTGCT-3′ and reverse 5′-TGTTGCTGTATTTCTGGTACAGCTC-3′ (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); IL-15, forward 5′-GGAATGTAACAGAATCTGGATG-3′ and reverse 5-GTT ATGTCTAAGCAGCAGAG-3′ (Sigma-Aldrich; Merck KGaA); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-AGCCACATCGCTGAGACA-3′ and reverse 5′-GCCCAATACGACCAAATCC-3′ (Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland).
The studied genes were amplified using a two-step process, with standard temperature profiles. The primer annealing temperature for TGF-β1 was 61°C and the primer annealing time was 20 sec. For IL-15, the primer annealing temperature was 61°C and the primer annealing time was 15 sec. For each sample, Cq was calculated using Mx-Pro v4.10 software (Agilent Technologies, Inc.). mRNA expression in each sample was measured three times and the mean value was calculated. The values were then compared with those of GAPDH, used as a reference gene. The ∆Cq value was calculated for use in the 2−∆Cq analysis of the results as only the peripheral blood mononuclear cells (PMBCs) from the patients were subjected to RNA analysis (31,32).
Statistical analysis
Statistical analysis was performed with STATISTICA 12 (StatSoft Polska Sp. z o.o., Kraków, Poland) licensed software. P<0.05 was considered to indicate a statistically significant difference. The choice of tests was based mainly on the distribution of variables and their types. The main descriptive statistics used to characterize the individual continuous variables were the mean and median, the SD, and minimum and maximum values. Due to the relatively large size of the test groups, Student's t-test was used for comparing two groups, while analysis of variance with Fisher's post hoc test was used for comparing multiple independent groups. To evaluate the association between two continuous variables, Spearman's correlation coefficient was used.
Results
IL-15 and TGF-β1 mRNA expression
Analysis of the RT-qPCR results revealed a significantly higher level of IL-15 mRNA expression in the patient group compared with the control group (P=0.0016; Fig. 1). TGF-β1 mRNA expression was also significantly higher in asthmatic patients compared with the control group (P=0.033), as shown in Fig. 2. Table II presents the expression levels of these two cytokines in the two groups.
Figure 1.

Comparison of the 2−ΔCq value of IL-15 mRNA expression in the control group (no asthma, n=91) and asthmatic patients (asthma, n=131). IL-15, interleukin 15; M, mean; SE, standard error; 95% CI, 95% confidence interval.
Figure 2.
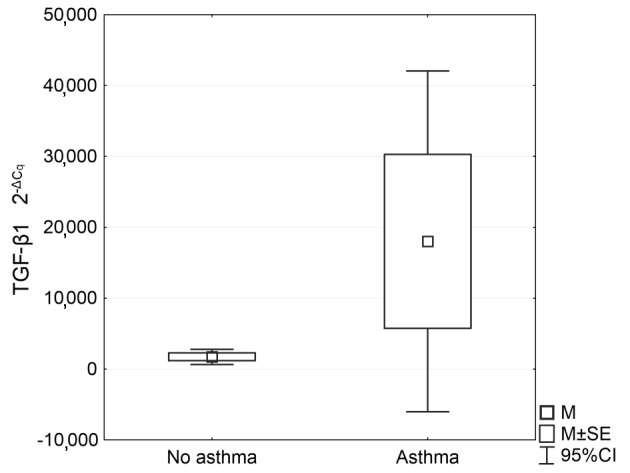
Comparison of the 2−ΔCq value of TGF-β1 mRNA expression in the control group (no asthma, n=48) and asthmatic patients (asthma, n=40). TGF-β1, transforming growth factor-β; M, mean; SE, standard error; 95% CI, 95% confidence interval.
Table II.
Expression levels of IL-15 and TGF-β1 mRNA (ΔCq) in the healthy control group and asthmatic patients.
| A, Healthy control | ||||||
|---|---|---|---|---|---|---|
| Parameter | n | Mean | Median | Minimum | Maximum | Standard deviation |
| IL-15 | 91 | 0.73 | 0.60 | −1.24 | 4.27 | 0.94 |
| TGF-β1 | 48 | −8.70 | −9.04 | −14.15 | −0.45 | 2.77 |
| B, Asthmatic patients | ||||||
| IL-15 | 131 | 0.542 | 0.27 | −1.51 | 4.57 | 0.97 |
| TGF-β1 | 40 | −10.28 | −10.29 | −18.46 | −3.04 | 3.17 |
IL-15, interleukin-15; TGF-β1, transforming growth factor-β1.
IL-15 correlation with asthma severity and spirometry results
No significant correlation was identified between IL-15 mRNA expression and the degree of asthma severity according to the GINA criteria. However, a significant correlation was observed between IL-15 expression and TGF-β1 expression (R=0.41, P=0.0005), as presented in Fig. 3.
Figure 3.
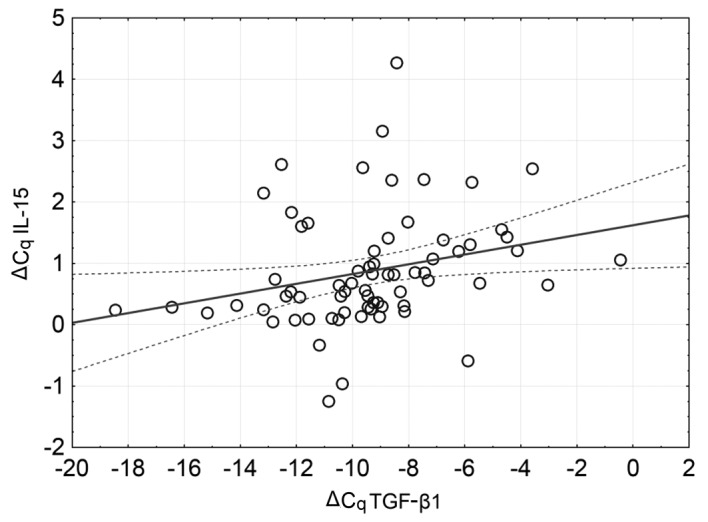
Correlation of the mRNA expression of IL-15 and TGF-β1. Dotted lines represent the 95% confidence interval. IL-15, interleukin 15; TGF-β1, transforming growth factor-β.
No significant correlation was observed between IL-15 expression and the results of the spirometric examination (FEV1, FEV1% and FEV1/FVC) in the group of asthmatic patients.
Association of IL-15 expression with various drug treatments
Patients who had been treated, according to the GINA guidelines available at the time of treatment, with inhaled glucocorticosteroids (iGCs: Budesonide, ciclesonide, beclomethasone and fluticasone; all doses were converted to the budesonide equivalent dose) demonstrated higher IL-15 expression (2−∆Cq, 1.00±0.59) compared with the controls (2−∆Cq, 0.70±0.41) (P=0.024). In addition, no clear association was observed between IL-15 expression and the dose of iGC as an equivalent of budesonide. The expression levels of IL-15 mRNA in patients according to the iGC dose they received are presented in Table III.
Table III.
Mean and SD values of IL-15 mRNA (ΔCq) expression and the dose of iGCs as an equivalent of budesonide in asthmatic patients.
| iGC dose (µg) | Patients (n) | Mean | SD |
|---|---|---|---|
| 0 | 13 | 0.91 | 1.36 |
| 1–1,000 | 68 | 0.24 | 0.87 |
| >1,000 | 49 | 0.61 | 0.95 |
IL-15, interleukin-15; iGC, inhaled glucocorticosteroid; SD, standard deviation.
IL-15 mRNA expression did not differ according to whether patients were treated or not treated for the last 3 months with systemic glucocorticosteroids, or with short- and long-acting betamimetics, tiotropium bromide or ipratropium bromide. In addition, no association was detected between the level of IL-15 mRNA expression and the use of antileukotrienes or histamine H1 receptor antagonists. However, in patients who were treated with methyloxanthines (MXTs), IL-15 expression was clearly lower (2−∆Cq, 0.60±0.04) compared with that in the control group (2−∆Cq, 0.82±0.05) (P<0.001). Detailed results of the IL-15 mRNA levels for patients treated or not treated with the various types of drug are presented in Table IV.
Table IV.
IL-15 mRNA (ΔCq) expression in groups taking and not taking various drugs.
| Drugs | Not treated (n) | IL-15 mRNA expression | Treated (n) | IL-15 mRNA expression | P-value |
|---|---|---|---|---|---|
| SABA | 55 | 0.46±0.93 | 75 | 0.44±1.02 | 0.86 |
| iGC | 13 | 0.91±1.36 | 117 | 0.39±0.92 | 0.02 |
| MXT | 112 | 0.54±0.95 | 18 | 0.95±1.02 | <0.001 |
| LABA | 27 | 0.64±1.00 | 103 | 1.00±0.51 | 0.10 |
| Ipratriopium bromide | 112 | 0.44±1.01 | 18 | 0.47±0.79 | 0.63 |
| Tiotropium bromide | 121 | 0.57±0.98 | 9 | 0.98±0.87 | 0.65 |
| Anti-LT | 83 | 0.59±0.97 | 47 | 0.97±0.52 | 0.78 |
| Anti-H1 | 75 | 0.64±1.03 | 55 | 1.03±0.4 | 0.46 |
| sGC | 92 | 0.59±1.00 | 38 | 1.00±0.53 | 0.91 |
SABA, short acting β-adrenoreceptor agonist; iGC, inhaled glucocorticosteroid; MXT, methyloxanthine; LABA, long acting β-adrenoreceptor agonist; anti-LT, antileukotriene; anti-H1, H1-receptor antagonist; sGC, systemic glucocorticosteroid.
Table V presents information concerning the IL-15 mRNA expression levels in patients treated with and without iGCs and/or MXTs. IL-15 mRNA expression was lowest among the patients who were not receiving MTXs or iGCs (2−∆Cq, 0.24). It was higher in the group of patients treated with medium and high doses of iGCs, and those who had been treated with MTXs at the same time. Mean IL-15 mRNA expression was highest among patients who had been treated with MTX and iGC at the medium dose (2−∆Cq, 0.88), although these results were not observed to be statistically significant.
Table V.
IL-15 mRNA (ΔCq) expression in groups taking and not taking MTXs with consideration of the dose of iGC as an equivalent of budesonide.
| MTX | Without MTX | |||
|---|---|---|---|---|
| iGC dose (µg) | Mean | 95% CI | Mean | 95% CI |
| 0 | 0.56 | −0.09 to 1.22 | 2.04 | −3.43 to 7.51 |
| 1–1,000 | 0.19 | −0.01 to 0.38 | 1.37 | −2.92 to 5.66 |
| >1,000 | 0.59 | 0.24 to 0.95 | 0.68 | 0.36 to 1.00 |
IL-15, interleukin-15; MTX, methyloxanthine; iGC, inhaled glucocorticosteroid; 95% CI, 95% confidence interval.
Association of IL-15 expression with asthma exacerbations, ACT results and Borg scale scores
IL-15 mRNA expression did not contribute to more frequent exacerbations of asthma, occurring at least two times a year (P>0.05). In addition, no correlation was detected between IL-15 expression and the ACT results (P>0.05).
Healthy volunteers from the control group demonstrated lower values (1.42±1.30) on the Borg Scale compared with asthmatic patients (3.9±2.9) (P=0.0001); however, this was not found to be associated with IL-15 expression (P>0.05).
Discussion
TGF-β1 is a cytokine essential for the pathogenesis of inflammatory obstructive diseases of the respiratory system, and the degree of its expression correlates with indices of airway remodelling, the severity of asthma and asthma control measured with the use of ACT (33,34). Identification of pathways through which it affects the inflammatory process occurring in lungs and consequently further bronchial remodelling are notable areas of study. One of the cytokines that could potentially have a role in this process is IL-15. Its role in asthma has not yet been fully explained; the results of studies conducted so far are equivocal and do not give clear evidence with regard to the etiopathogenesis of asthma.
Attempts to identify the association between TGF-β1 and IL-15 have provided particularly promising results. Unlike the role of IL-15, the role of genetic polymorphisms connected with the TGF-β pathway in asthma is quite well known (35,36). Studies concerning polymorphic forms of the IL-15 gene conducted on a Danish population did not confirm an occurrence of any significant polymorphism of the IL-15 gene in the etiology of asthma (37). Although one study, conducted using a German population, identified a weak association between IL-15 and childhood asthma, further studies did not confirm any relationship between IL-15 gene polymorphisms and asthma or atopic diseases (38–40).
The positive correlation between IL-15 mRNA and TGF-β1 mRNA expression observed in the present study is noteworthy since these cytokines are considered to act in opposition (41). As of yet, there have been no studies on the mutual association of these cytokines in asthma. It is known that in celiac disease, IL-15 inhibits the SMAD3-dependent TGF-β1 signalling pathway in T lymphocytes (42). The results of the present study indicate that IL-15 mRNA expression is elevated in asthma. TGF-β1 is not likely to directly regulate IL-15 expression (13), but is one of the main factors in airway remodelling, which is associated with an increased number of fibroblasts and epithelial cells, a source of IL-15 (14), which might explain the association identified between IL-15 mRNA and TGF-β1 mRNA expression levels.
Infection exacerbations in asthma are an important problem. It has been revealed that deficits of IL-15 in asthmatic patients result from an impaired response to Rhinovirus infection in vitro, which possible indicates that the cytokine has a protective effect against such infections (22). The respiratory syncytial virus was found to contribute to the upregulation of IL-15 in a study on bronchial and alveolar epithelial cell lines (43). Moreover, there is evidence that IL-15 is a significant factor responsible for the production of CD8+ memory T cells in response to viral infection (44). However, the results of the present study do not indicate any association between IL-15 expression and the frequency of exacerbations. This might result from the fact that this analysis did not take any etiological factors into consideration, did not classify exacerbations into bacterial and viral, and did not analyze samples from patients with active infection; this was due to a lack of appropriate patients being available.
Experiments conducted on ovalbumin-immunized transgenic mice revealed that IL-15 disrupts the Th2 lymphocyte-dependent response and the production of immunoglobulin E (IgE) antibodies, possibly by the production of interferon (IFN)-γ by cytotoxic CD8+ lymphocytes (21), which impairs the mechanisms responsible for the development of allergic inflammation. This suggests that a high level of IL-15 expression may be a protective factor in patients with allergic asthma. This hypothesis was confirmed in a study conducted by Esen et al (45), who reported a reduction in the number of IgE-producing cells and IgE itself under the influence of IL-15. This information remains highly important when considering the potential of IgE in airway remodelling, even in the absence of exposure to an allergen. The remodelling process is inhibited by omalizumab (46). Moreover, the local synthesis of IgE has been suggested as a potential mechanism of inflammation observed in non-allergic asthma (47).
It is possible that in asthmatic patients, an increase in TGF-β1 expression may increase IL-15 expression, causing a shift of the Th1/Th2 balance in favour of Th1. This assumption is supported by the results of studies implying that the progress of asthma is accompanied by a weaker response of patients to antihistamines and antileukotrienes; reports on the activity of omalizumab in IgE non-dependent asthma (48) and its inhibitory effect on CD3+, CD4+, CD8+ cells and B lymphocytes (49) confirm the weaker response.
A previous study revealed significantly higher levels of IL-15 in the induced sputum of patients taking GCs compared with patients who had not been administered any therapy or normal controls (23). Also, the in vitro culture of sputum white cells, isolated from asthmatic patients treated with GCs, showed significantly higher levels of IL-15 synthesis in comparison with the control group (23). These results highlight the considerable immunomodulating role of IL-15 in asthma, which involves the induction of changes in the inflammation profile, from a profile connected with Th2 lymphocytes to one connected with Th1 lymphocytes. A consequence of such a shift would be an increased level of IFN-γ, which might delay the remodelling of airways (50). Mechanisms responsible for an increase in IL-15 mRNA expression following the administration of GCs have not yet been elucidated. However, the in vitro inhibition of IL-15-induced NK cell apoptosis by GCs has been reported (51). GCs inhibit IL-2 synthesis, at the levels of transcription and translation (52). IL-2 is structurally similar to IL-15, as their structures possess a common g chain (53). In addition, GCs increase the expression of the IL-2 receptor and receptors for other cytokines (52). In PBMCs, IL-2 and IL-4 maintain an inhibited response to GCs associated with a lowered affinity of GCs for the GC receptor (54). The results of the present study indicate that IL-15 mRNA expression is elevated in asthmatic patients taking iGCs. Considering the key role of GCs in asthma therapy, it is advisable to performed a more extensive analysis of the role of IL-15 in future studies.
MXTs are bronchial vasodilators that are characterized by anti-inflammatory properties. They act through the non-selective blockage of phosphodiesterase (PDE), and are antagonists for the adenosine receptor and activators of histone deacetylase (HDAC) (1). Experiments on human fetal lung fibroblasts (HFL-1 cell line) confirmed that MXTs have an inhibitory effect on the release of TGF-β1 and the metalloproteinases MMP-2 and MMP-9 from fibroblasts induced by peroxynitrite (55). It has also been noted that the PDE4 inhibitor rolipram inhibits the polarization, migration and activation of T lymphocytes induced by IL-15 (56). Adenosine is known to inhibit the release of IL-15 by bone marrow-derived cells. This process can be reversed by the adenosine receptor inhibitor (57). Moreover, it has been hypothesized that IL-15 acts synergistically with HDAC inhibitors in hematological diseases (58).
Prior to the present study, the direct effect of MXTs on IL-15 expression was unclear. To the best of our knowledge, the present study is the first to address this uncertainty. In our opinion, the lower level of IL-15 mRNA expression observed in patients treated with MXTs results from the fact that such patients suffer from severe asthma with frequent exacerbations, and such disease is impossible to control only with GCs. Another important fact is that MXTs also act as adenosine receptor inhibitors, which may contribute to a reduction in IL-15 expression.
In conclusion, the results of the present study confirm the presence of a significant correlation between TGF-β1 expression and IL-15 expression in asthmatic patients, and show that IL-15 mRNA expression and TGF-β1 expression are significantly higher in patients with asthma than in participants from the control group. Greater IL-15 mRNA expression was observed in the PBMCs of patients who were treated with iGCs than those who were not, but IL-15 mRNA expression was reduced in patients treated with MXTs. No clinical implications were associated with higher IL-15 mRNA expression; the attempt to identify an association between IL-15 and a degree of asthma severity, degree of asthma control or number of exacerbation episodes was unsuccessful. The level of IL-15 expression was not observed to significantly affect the results of spirometry. In addition, a higher degree of dyspnoea evaluated with the application of the Borg Scale in asthmatic patients was not found to have an association with IL-15 expression. However, there are limitations to the study, as there is some evidence contradicting our findings and the IL-15 mRNA level in PMBCs might not directly translate into the IL-15 protein level in the lungs. These limitations are acknowledged, and assessment of the serum protein levels of studied genes is suggested as the next step in the evaluation of the findings of the present study. New patients will be recruited to achieve this goal. As the nature of the correlation between IL-15 expression and TGF-β1 expression remains unknown, further studies on the role of IL-15 in asthma, its regulation and its connection with the TGF-β1 signalling pathway are required for the purpose of identifying their potential implications in the treatment of asthma.
Acknowledgements
The authors thank Joanna Molińska for assisting in administrative work, and offer acknowledgements to all of those who provided support in any respect during the completion of this project. This study was financially supported by grant no. 503/1-095-03/503-01 from the Department of Internal Diseases, Asthma and Allergy, II Chair of Internal Diseases, Medical University of Lodz and grant no. 502-03/1-095-03/502-14-083 from the Medical University of Lodz, Poland, for completing the research within the financial framework for the development of young scientists and PhD students.
References
- 1.Droszcz W. Wydawnictwo Lekarskie PZWL. Warszawa: 2007. Astma. [Google Scholar]
- 2.Reddel HK, Levy ML. The GINA asthma strategy report: What's new for primary care? Primary Care Respiratory Med. 2015;25:15050. doi: 10.1038/npjpcrm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Moustakas A. Smad signalling network. J Cell Sci. 2002;115:3355–3356. doi: 10.1242/jcs.115.17.3355. [DOI] [PubMed] [Google Scholar]
- 5.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 6.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 8.Letterio JJ, Roberts AB. TGF-beta: A critical modulator of immune cell function. Clin Immunol Immunopathol. 1997;84:244–250. doi: 10.1006/clin.1997.4409. [DOI] [PubMed] [Google Scholar]
- 9.Duvernelle C, Freund V, Frossard N. Transforming growth factor-beta and its role in asthma. Pulm Pharmacol Ther. 2003;16:181–196. doi: 10.1016/S1094-5539(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 10.Mehta AA, Mahajan S. Role of cytokines in pathophysiology of asthma. Iranian J Pharmacol Therapeut. 2006;5:1–14. [Google Scholar]
- 11.McInnes IB, Gracie JA. Interleukin-15: A new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DM, Johnson L, Glaccum MB, Copeland NG, Gilbert DJ, Jenkins NA, Valentine V, Kirstein MN, Shapiro DN, Morris SW, et al. Chromosomal assignment and genomic structure of II15. Genomics. 1995;25:701–706. doi: 10.1016/0888-7543(95)80013-C. [DOI] [PubMed] [Google Scholar]
- 13.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 14.Kennedy MK, Park LS. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J Clin Immunol. 1996;16:134–143. doi: 10.1007/BF01540911. [DOI] [PubMed] [Google Scholar]
- 15.Leclercq G, Debacker V, de Smedt M, Plum J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J Exp Med. 1996;184:325–336. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 17.Kirman I, Vainer B, Nielsen OH. Interleukin-15 and its role in chronic inflammatory diseases. Inflamm Res. 1998;47:285–289. doi: 10.1007/s000110050331. [DOI] [PubMed] [Google Scholar]
- 18.Muro S, Taha R, Tsicopoulos A, Olivenstein R, Tonnel AB, Christodoulopoulos P, Wallaert B, Hamid Q. Expression of IL-15 in inflammatory pulmonary diseases. J Allergy Clin Immunol. 2001;108:970–975. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 19.Hoontrakoon R, Chu HW, Gardai SJ, Wenzel SE, McDonald P, Fadok VA, Henson PM, Bratton DL. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-kappaB activation. Am J Respir Cell Mol Biol. 2002;26:404–412. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- 20.Mori A, Suko M, Kaminuma O, Inoue S, Ohmura T, Nishizaki Y, Nagahori T, Asakura Y, Hoshino A, Okumura Y, et al. IL-15 promotes cytokine production of human T helper cells. J Immunol. 1996;156:2400–2405. [PubMed] [Google Scholar]
- 21.Ishimitsu R, Nishimura H, Yajima T, Watase T, Kawauchi H, Yoshikai Y. Overexpression of IL-15 in vivo enhances Tc1 response, which inhibits allergic inflammation in a murine model of asthma. J Immunol. 2001;166:1991–2001. doi: 10.4049/jimmunol.166.3.1991. [DOI] [PubMed] [Google Scholar]
- 22.Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, Kebadze T, Kon OM, Mallia P, Stanciu LA, Johnston SL. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog. 2011;7:e1002114. doi: 10.1371/journal.ppat.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komai-Koma M, McKay A, Thomson L, McSharry C, Chalmers GW, Liew FY, Thomson NC. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy. 2001;31:1441–1448. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 24.http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention, Updated. 2017 [Google Scholar]
- 25.Zalecenia Polskiego Towarzystwa Ftyzjopneumonologicznego dotyczące wykonywania badań spirometrycznych. PPneumonologia i Alergologia Polska. 2006;74(Suppl 1) [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 33.Panek M, Pietras T, Szemraj J, Fabijan A, Kuna P. Identification and association of TGFβ-1 expression in patients with asthma in a Polish population-Lodz metropolitan area study. Int J Biochem Mol Biol. 2013;4:67–74. [PMC free article] [PubMed] [Google Scholar]
- 34.Koćwin M, Jonakowski M, Przemęcka M, Zioło J, Panek M, Kuna P. The role of the TGF-SMAD signalling pathway in the etiopathogenesis of severe asthma. Pneumonol Alergol Pol. 2016;84:290–301. doi: 10.5603/PiAP.2016.0037. [DOI] [PubMed] [Google Scholar]
- 35.Panek M, Pietras T, Fabijan A, Zioło J, Wieteska L, Małachowska B, Fendler W, Szemraj J, Kuna P. Identification and association of the single nucleotide polymorphisms, C-509T, C+466T and T+869C, of the TGF-β1 gene in patients with asthma and their influence on the mRNA expression level of TGF-β1. Int J Mol Med. 2014;34:975–986. doi: 10.3892/ijmm.2014.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panek M, Pietras T, Fabijan A, Zioło J, Wieteska Ł, Małachowska B, Fendler W, Szemraj J, Kuna P. The NR3C1 glucocorticoid receptor gene polymorphisms may modulate the TGF-beta mRNA expression in asthma patients. Inflammation. 2015;38:1479–1492. doi: 10.1007/s10753-015-0123-3. [DOI] [PubMed] [Google Scholar]
- 37.Christensen U, Haagerup A, Binderup HG, Vestbo J, Kruse TA, Børglum AD. Family based association analysis of the IL2 and IL15 genes in allergic disorders. Eur J Hum Genet. 2005;14:227–235. doi: 10.1038/sj.ejhg.5201541. [DOI] [PubMed] [Google Scholar]
- 38.Pinto LA, Depner M, Steudemann L, Klopp N, Illig T, von Mutius E, Kabesch M. IL15 gene variants are not associated with asthma and atopy. Allergy. 2009;64:643–646. doi: 10.1111/j.1398-9995.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 39.Bierbaum S, Nickel R, Zitnik S, Ahlert I, Lau S, Deichmann KA, Wahn U, Heinzmann A. Confirmation of association of IL-15 with pediatric asthma and comparison of different controls. Allergy. 2006;61:576–580. doi: 10.1111/j.1398-9995.2006.01059.x. [DOI] [PubMed] [Google Scholar]
- 40.Jachnik M, Szczepankiewicz A, Bręborowicz A. Influence of IL15 gene polymorphism on the course of bronchial asthma in children. Alergia Astma Immunologia. 2011;16:200–204. [Google Scholar]
- 41.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benahmed M, Meresse B, Arnulf B, Barbe U, Mention JJ, Verkarre V, Allez M, Cellier C, Hermine O, Cerf-Bensussan N. Inhibition of TGF-beta signaling by IL-15: A new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132:994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Zdrenghea MT, Telcian AG, Laza-Stanca V, Bellettato CM, Edwards MR, Nikonova A, Khaitov MR, Azimi N, Groh V, Mallia P, et al. RSV infection modulates IL-15 production and MICA levels in respiratory epithelial cells. Eur Respir J. 2012;39:712–720. doi: 10.1183/09031936.00099811. [DOI] [PubMed] [Google Scholar]
- 44.Azimi N, Shiramizu KM, Tagaya Y, Mariner J, Waldmann TA. Viral activation of interleukin-15 (IL-15): Characterization of a virus-inducible element in the IL-15 promoter region. J Virol. 2000;74:7338–7348. doi: 10.1128/JVI.74.16.7338-7348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esen M, Forster J, Ajua A, Spänkuch I, Paparoupa M, Mordmüller B, Kremsner PG. Effect of IL-15 on IgG versus IgE antibody-secreting cells in vitro. J Immunol Methods. 2012;375:7–13. doi: 10.1016/j.jim.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Roth M, Zhao F, Zhong J, Lardinois D, Tamm M. Serum IgE induced airway smooth muscle cell remodeling is independent of allergens and is prevented by omalizumab. PLoS One. 2015;10:e0136549. doi: 10.1371/journal.pone.0136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domingo C. Omalizumab for severe asthma: Efficacy beyond the atopic patient? Drugs. 2014;74:521–533. doi: 10.1007/s40265-014-0203-y. [DOI] [PubMed] [Google Scholar]
- 48.Menzella F, Piro R, Facciolongo N, Castagnetti C, Simonazzi A, Zucchi L. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol. 2011;7:9. doi: 10.1186/1710-1492-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupryś-Lipińska I, Kuna P. Omalizumab, recombinant humanized monoclonal antibody anti-IgE-new fields of studies on the therapeutic indications. Pneumonol Alergol Pol. 2009;77:43–51. (In Polish) [PubMed] [Google Scholar]
- 50.Yan L, Xiao-Ling S, Zheng-Yan C, Guo-Ping L, Sen Z, Zhuang C. HSP70/CD80 DNA vaccine inhibits airway remodeling by regulating the transcription factors T-bet and GATA-3 in a murine model of chronic asthma. Archives Med Sci. 2013;9:906–915. doi: 10.5114/aoms.2013.33180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moustaki A, Argyropoulos KV, Baxevanis CN, Papamichail M, Perez SA. Effect of the simultaneous administration of glucocorticoids and IL-15 on human NK cell phenotype, proliferation and function. Cancer Immunol Immunother. 2011;60:1683–1695. doi: 10.1007/s00262-011-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiegers GJ, Reul JM. Induction of cytokine receptors by glucocorticoids: Functional and pathological significance. Trends Pharmacol Sci. 1998;19:317–321. doi: 10.1016/S0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 53.Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol. 2012;13:1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sher ER, Leung DY, Surs W, Kam JC, Zieg G, Kamada AK, Szefler SJ. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest. 1994;93:33–39. doi: 10.1172/JCI116963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiura H, Kawabata H, Ichikawa T, Koarai A, Yanagisawa S, Kikuchi T, Minakata Y, Matsunaga K, Nakanishi M, Hirano T, et al. Inhibitory effects of theophylline on the peroxynitrite-augmented release of matrix metalloproteinases by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2012;302:L764–L774. doi: 10.1152/ajplung.00342.2011. [DOI] [PubMed] [Google Scholar]
- 56.Layseca-Espinosa E, Sanchez-Madrid F, Gonzalez-Amaro R. Phosphodiesterase inhibitors as immunomodulatory drugs. Inmunología. 2003;22:39–52. [Google Scholar]
- 57.Cohen Y, Eini H, Chaimovitz C, Douvdevani A. 53: Inhibition of interleukin-15 dependent cytotoxic T-cell proliferation by the action of adenosine on dendritic cell. Cytokine. 2013;63:255–256. doi: 10.1016/j.cyto.2013.06.056. [DOI] [Google Scholar]
- 58.Zdrenghea MT. Could interleukin-15 potentiate histone deacetylase inhibitor effects in haematological malignancy? Med Hypotheses. 2013;81:311–315. doi: 10.1016/j.mehy.2013.04.021. [DOI] [PubMed] [Google Scholar]


