Abstract
Candidemia is a growing concern worldwide, and its species distribution has shifted toward non-albicans Candida in recent decades, especially in patients with malignancy. This study aimed to update the epidemiology and antifungal susceptibility of non-albicans candidemia isolates from the cancer patients. Adult cancer patients with non-albicans candidemia were recruited, and clinical data were retrospectively collected from five medical centers in Taiwan from 1 July 2011 to 30 June 2014. In vitro susceptibility was determined by the broth dilution method using a Sensititre YeastOne system and interpreted according to the guidelines of the Clinical and Laboratory Standards Institute. A total of 346 episodes of non-albicans candidemia were identified in cancer patients. Candida tropicalis was the most common species (n=145, 41.9%) and had the highest resistance rate to fluconazole (n=17, 13.9%) among all the preserved isolates, including C. tropicalis, Candida glabrata and Candida parapsilosis. A higher Charlson comorbidity index, non-albicans candidemia due to C. tropicalis, neutropenia and septic shock were independent predictors of 28-day mortality. In conclusion, the species distribution and antifungal susceptibility of non-albicans candidemia isolates in our study differed from those in Western countries, providing useful information about local epidemiology for the selection of empirical antifungal agents for cancer patients.
Keywords: cancer, epidemiology, non-albicans candidemia, susceptibility
INTRODUCTION
Candida species have become increasingly important pathogens in healthcare settings worldwide. In 2004, candidemia was reported as the fourth to sixth leading cause of nosocomial bloodstream infections in Western countries, including in the United States and Switzerland.1, 2 A more recent multicenter study in 2014 that enrolled 183 hospitals in the United States showed that candidemia was the most common etiology of healthcare-associated bloodstream infections.3 In Taiwan, the incidence of candidemia has also shown an increasing trend, from 0.8 patients per 10 000 discharges in 1981 to 28.8 patients per 10 000 discharges in 2000.4 Furthermore, the species distribution of candidemia varies geographically and has changed in recent decades. The proportion of different species causing candidemia has shifted toward non-albicans Candida species; Candida tropicalis and Candida parapsilosis are prominent in Taiwan and China, respectively,5, 6, 7 and Candida glabrata is prominent in the United States.8, 9
Malignancy is a major underlying comorbidity of patients with candidemia. For cancer patients, candidemia is associated with a high mortality rate (30%–50%) that results in substantial healthcare costs and prolonged hospital stays.10, 11, 12, 13 Recent literature has shown that despite the introduction of novel antifungal agents, candidemia in cancer patients remains a severe disease with a high mortality rate of 31–68%.6, 14, 15, 16 Candidemia caused by fluconazole-resistant strains, which are composed exclusively of non-albicans Candida species, has been shown to significantly increase mortality among patients with solid organ or hematological malignancies.14 Furthermore, previous studies have reported that the contribution of non-albicans Candida species to candidemia in cancer patients is increasing.16, 17, 18 Hence, an understanding of the epidemiology of non-albicans candidemia and the antifungal susceptibilities of these isolates is currently imperative to guide optimal empirical treatment strategies for affected patients.
In this report, we conducted a multicenter study to determine the clinical characteristics and antifungal susceptibilities of non-albicans candidemia in cancer patients and to identify the potential risk factors associated with 28-day mortality.
MATERIALS AND METHODS
Setting and study design
This multicenter study was conducted at the Taipei Veterans General Hospital with 2900 beds in northern Taiwan, Chi Mei Hospital with 900 beds in southern Taiwan, Kaohsiung Medical University Hospital with 1600 beds in southern Taiwan, China Medical University Hospital with 2111 beds in central Taiwan and Changhua Christian Hospital with 1487 beds in central Taiwan. All adult cancer patients older than 20 years of age, with a bloodstream infection due to non-albicans Candida, and who were admitted to any of the above-mentioned five medical centers between 1 July 2011 and 30 June 2014 were enrolled in the study. In the case of patients who suffered two or more episodes of non-albicans candidemia during the study period, only the first episode was included in our analysis. Patients with a bloodstream infection caused by two or more Candida species were excluded. Non-albicans Candida isolates obtained from patients at the enrolled hospitals were analyzed for antifungal susceptibility at a medical center.
The medical records of all enrolled patients were reviewed by infectious disease specialists, and the variables collected from medical charts were as follows: age, gender, Charlson comorbidity index, underlying comorbidities, cancer types, origin of non-albicans candidemia, underlying conditions (use of a central venous catheter, recently received chemotherapy, total parenteral nutrition, steroid, proton-pump inhibitor and abdominal surgery, and prior exposure to broad-spectrum antibiotics), severity of illness (neutropenia and septic shock), concomitant bacterial infections, antifungal therapy and 28-day outcome. This study was approved by the Medical Ethics Committees of the five enrolled hospitals.
Definitions
Patients infected with non-albicans candidemia were confirmed by at least one set of positive blood cultures and compatible clinical symptoms and signs.19 The origin of the candidemia was identified as the site from which the same non-albicans Candida species was isolated. Catheter-related bloodstream infection (CRBSI) was defined by semi-quantitative tip culture as the growth of ≥15 colonies that were identical to the species identified from the peripheral blood culture.20 Primary candidemia was defined as a non-albicans candidemia occurring in the absence of an apparent portal of entry. Healthcare-associated infection was determined according to the criteria provided by the Centers for Disease Control and Prevention, USA.21 Recent abdominal surgery, the recent use of steroids (at least 10 mg of prednisolone or an equivalent daily dosage for more than 7 days), broad-spectrum antibiotics or proton-pump inhibitors were defined as undergoing interventions or agents when they occurred within 30 days before the positive blood culture. Chemotherapy received within 90 days before the infection was defined as ‘recently received chemotherapy’. Neutropenia was indicated by an absolute neutrophil count of <500 × 106 cells per liter, and septic shock was defined by a systolic pressure below 90 mm Hg and a need for vasopressors. Antifungal therapy was determined based on the predominant agent, which was used for most of the duration of the treatment course. The 28-day mortality values were identified based on the medical records of the five enrolled hospitals, including the discharge day before 28 days from the onset of non-albicans candidemia.
Microbiological analysis of non-albicans Candida isolates
All preserved, available non-albicans Candida isolates were identified by morphology analysis on CHROMagar (Creative Life Science, New Taipei City, Taiwan) and biochemical methods using a Vitek 2 system with the YST Card (bioMérieux, Durham, NC, USA). The broth microdilution method using a Sensititre YeastOne system (Trek Diagnostic Systems, East Grinstead, UK) was performed to determine the minimum inhibitory concentration (MIC) according to the manufacturer’s instructions. C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as quality control strains. The MICs of amphotericin B, caspofungin, micafungin, anidulafungin, fluconazole, voriconazole, itraconazole, posaconazole and flucytosine were determined, and interpretation criteria were based on the MIC breakpoints of non-albicans Candida species, as recommended by the Clinical and Laboratory Standards Institute.22
Statistical analyses
Statistical analyses were performed using SPSS software version 17 (SPSS, Chicago, IL, USA). Categorical data were compared using χ2 or Fisher’s exact tests. Continuous data were analyzed using Student t-test or Mann–Whitney U test. Statistical significances were determined using two-tailed tests, and a value of P<0.05 was considered significant. Logistic regression analysis was used to identify the independent predictors of 28-day mortality. All biologically plausible variables with values of P<0.10 in univariate testing were included in the model.
RESULTS
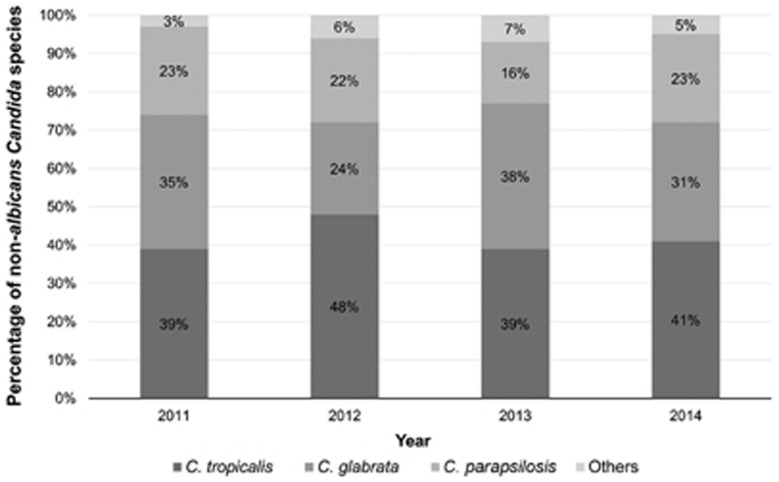
During the study period, 346 episodes of candidemia caused by non-albicans Candida were identified in a study population of 346 patients. No significant differences were observed in the proportions of candidemia caused by different non-albicans Candida species each year (P=0.489). In addition, Figure 1 shows the species ratio trends of non-albicans candidemia cases from 2011 to 2014. Table 1 summarizes the demographics and clinical data of the patients with non-albicans candidemia enrolled in the study. The study population included 221 (63.9%) male and 125 (36.1%) female participants, and the mean age of all patients enrolled was 63.9±15.4 years old. Gastrointestinal cancer was the most common malignancy in patients with non-albicans candidemia enrolled in the study (160 patients, 46.2%). Apart from malignancy, 168 (48.6%) patients had other underlying comorbidities; diabetes mellitus (107 patients, 30.9%) was the most frequent disease among these conditions. CRBSI (108 patients, 31.2%) accounted for the most commonly identified origin of non-albicans candidemia, and 136 (39.3%) episodes were classified as primary infection. The presence of a central venous catheter (294 patients, 85%) and prior exposure to broad-spectrum antibiotics (306 patients, 88.4%) were the most common risk factors associated with non-albicans candidemia in this study. The majority of predominant antifungal agents were triazoles (192 patients, 55.5%). Across the study population, only 29 (8.4%) and 83 (24%) patients ever received prophylactic or empirical antifungal therapies, respectively. In addition to the patients undergoing prophylactic or no treatment, the median (interquartile range) duration of prescribed antifungal agents after collecting index blood cultures was two (0.20–3.18) days.
Figure 1.
Trend in species distribution of non-albicans candidemia from 2011 to 2014.
Table 1. Demographics and clinical characteristics of cancer patients with non-albicans candidemia.
| Characteristic | Number of patients (%) |
|---|---|
| Gender, male | 221 (63.9) |
| Age, years | 63.9±15.4 |
| Age ≥65 years | 165 (47.7) |
| Charlson comorbidity index | 5.6±2.8 |
| Underlying comorbidities | |
| Diabetes mellitus | 107 (30.9) |
| Cerebrovascular accident | 24 (6.9) |
| Congestive heart failure | 18 (5.2) |
| End-stage renal disease | 14 (4) |
| Liver cirrhosis | 26 (7.5) |
| Chronic lung disease | 36 (10.4) |
| Type of cancer | |
| Head and neck cancer | 58 (16.8) |
| Lung cancer | 22 (6.4) |
| Gastrointestinal cancer | 160 (46.2) |
| Genitourinary cancer | 40 (11.6) |
| Breast cancer | 15 (4.3) |
| Hematological malignancy | 46 (13.3) |
| Othersa | 7 (2) |
| Non-albicans Candida species | |
| C. tropicalis | 145 (41.9) |
| C. glabrata | 111 (32.1) |
| C. parapsilosis | 70 (20.2) |
| C. krusei | 7 (2) |
| C. guilliermondii | 5 (1.4) |
| C. sake | 2 (0.6) |
| C. dubliniensis | 2 (0.6) |
| C. lipolytica | 1 (0.3) |
| C. lusitaniae | 1 (0.3) |
| C. norvegensis | 1 (0.3) |
| C. famata | 1 (0.3) |
| Origin of non-albicans candidemia | |
| Primary infection | 136 (39.3) |
| Catheter-related infection | 108 (31.2) |
| Urinary tract infection | 29 (8.4) |
| Respiratory tract infection | 42 (12.1) |
| Intra-abdominal infection | 29 (8.4) |
| Skin and soft tissue infection | 2 (0.6) |
| Underlying conditions | |
| Presence of central venous catheter | 294 (85) |
| Presence of Foley catheter | 127 (36.7) |
| Chemotherapy | 168 (48.6) |
| Total parental nutrition | 96 (27.7) |
| Steroid | 119 (34.4) |
| Proton-pump inhibitor | 145 (41.9) |
| Abdominal surgery | 58 (16.8) |
| Prior exposure to broad-spectrum antibiotics | 306 (88.4) |
| Neutropenia | 21 (6.1) |
| Septic shock | 124 (35.8) |
| Concomitant bacterial infections | 127 (36.7) |
| Antifungal therapy | |
| Amphotericin B | 5 (1.4) |
| Triazole | 192 (55.5) |
| Echinocandin | 102 (29.5) |
| None | 47 (13.6) |
| Length of hospital stay after infection (days) | 22.1±20.4 |
| 28-day mortality | 154 (44.5) |
Data are presented as mean±standard deviation (s.d.) or frequency with percentage (%).
Others included glioblastoma multiforme, peritoneal mesothelioma, retroperitoneal carcinoma, skin cancer and malignancy of unknown origin.
Table 2 shows the distribution and frequency of non-albicans Candida species isolated from patients with different types of cancers. C. tropicalis was the major cause of non-albicans candidemia in all patients, regardless of cancer type. However, no significant differences were observed in the distribution of non-albicans Candida species among the five major cancer categories analyzed. Notably, when patients with solid organ malignancies were compared with those with hematological cancers, non-albicans candidemia caused by C. tropicalis was significantly higher in patients with hematological malignancies (58.7% versus 39.3%, P=0.020).
Table 2. Distribution and frequency of non-albicans Candida species in patients with different types of cancers.
| Species | Number of isolates (%) | ||||
|---|---|---|---|---|---|
| Head and neck cancer | Gastrointestinal tract cancer | Lung cancer | Genitourinary cancer | Hematological cancer | |
| C. tropicalis | 26 (44.8) | 62 (38.8) | 10 (45.5) | 16 (40) | 27 (58.7) |
| C. glabrata | 14 (24.1) | 60 (37.5) | 6 (27.3) | 14 (35) | 10 (21.7) |
| C. parapsilosis | 17 (29.3) | 29 (18.1) | 6 (27.3) | 8 (20) | 4 (8.7) |
| C. krusei | 0 (0) | 3 (1.9) | 0 (0) | 0 (0) | 3 (6.5) |
| C. guilliermondii | 0 (0) | 3 (1.9) | 0 (0) | 2 (5) | 0 (0) |
| Othersa | 1 (1.7) | 3 (1.9) | 0 (0) | 0 (0) | 2 (4.3) |
Data are presented as frequency with percentage (%).
Other species included C. sake, C. lusitaniae, C. lipolytica, C. norvegensis, C. famata and C. dubliniensis.
A total of 251 non-albicans candidemia isolates were available for the antifungal susceptibility test, as shown in Table 3. Of these non-albicans candidemia-causative isolates, 22 (8.8%) were resistant to in vitro fluconazole, including 17 C. tropicalis, 3 C. glabrata and 2 C. parapsilosis isolates. Although C. tropicalis showed the highest rate of resistance to fluconazole (13.9%) among the preserved isolates, C. glabrata exhibited the highest fluconazole MIC50 and MIC90 (16 mg/L and 32 mg/L, respectively) among all non-albicans Candida species. The overall susceptibility rates for echinocandins were high (>90%), except for caspofungin in C. glabrata (83.3%) isolates. The echinocandin MIC50 and MIC90 of C. parapsilosis (0.5–1 mg/L and 0.5–2 mg/L) were higher than other non-albicans Candida species, but all C. parapsilosis isolates were susceptible to three echinocandins.
Table 3. In vitro activities of antifungal agents tested against 251 non-albicans Candida isolates obtained from patients with candidemia according to the Clinical and Laboratory Standards Institute recommendations.
| Non-albicans Candida species | MIC range (mg/L) | MIC50(mg/L) | MIC90 (mg/L) | Number of isolates (%) | |||
|---|---|---|---|---|---|---|---|
| S | SDD | I | R | ||||
| C. tropicalis (n=122) | |||||||
| Amphotericin B | 0.25–2 | 0.5 | 1 | — | — | — | — |
| Fluconazole | 0.25–128 | 2 | 8 | 87 (71.3) | 18 (14.8) | — | 17 (13.9) |
| Voriconazole | 0.015–4 | 0.25 | 1 | 60 (49.2) | 49 (40.2) | — | 13 (10.7) |
| Posaconazole | 0.015–2 | 0.25 | 0.5 | — | — | — | — |
| Itraconazole | 0.06–1 | 0.25 | 0.5 | — | — | — | — |
| Caspofungin | 0.015–>8 | 0.06 | 0.25 | 118 (96.7) | — | 1 (0.8) | 3 (2.5) |
| Micafungin | 0.015–2 | 0.03 | 0.03 | 118 (96.7) | — | 1 (0.8) | 3 (2.5) |
| Anidulafungin | 0.03–1 | 0.12 | 0.25 | 119 (97.5) | — | 1 (0.8) | 2 (1.6) |
| Flucytosine | ≤0.06–>64 | ≤0.06 | 0.12 | — | — | — | — |
| C. glabrata (n=96) | |||||||
| Amphotericin B | ≤0.12–4 | 0.5 | 1 | — | — | — | — |
| Fluconazole | 0.5–>256 | 16 | 32 | — | 93 (96.7) | — | 3 (3.1) |
| Voriconazole | ≤0.008–4 | 0.5 | 1 | — | — | — | — |
| Posaconazole | 0.015–>8 | 1 | 2 | — | — | — | — |
| Itraconazole | 0.03–2 | 0.5 | 1 | — | — | — | — |
| Caspofungin | 0.03–1 | 0.12 | 0.25 | 80 (83.3) | — | 14 (14.6) | 2 (2.1) |
| Micafungin | ≤0.008–1 | 0.015 | 0.015 | 90 (93.8) | — | 1 (1) | 5 (5.2) |
| Anidulafungin | ≤0.015–1 | 0.06 | 0.12 | 90 (93.8) | — | 1 (1) | 5 (5.2) |
| Flucytosine | ≤0.06–0.5 | ≤0.06 | 0.12 | — | — | — | — |
| C. parapsilosis (n=33) | |||||||
| Amphotericin B | 0.25–1 | 0.5 | 0.5 | — | — | — | — |
| Fluconazole | 0.25–8 | 1 | 2 | 30 (90.9) | 1 (3) | — | 2 (6.1) |
| Voriconazole | ≤0.008–0.25 | 0.015 | 0.03 | 32 (97) | 1 (3) | — | 0 (0) |
| Posaconazole | 0.015–0.12 | 0.03 | 0.06 | — | — | — | — |
| Itraconazole | 0.03–0.25 | 0.06 | 0.12 | — | — | — | — |
| Caspofungin | 0.25–1 | 0.5 | 0.5 | 33 (100) | — | 0 (0) | 0 (0) |
| Micafungin | 0.25–2 | 1 | 2 | 33 (100) | — | 0 (0) | 0 (0) |
| Anidulafungin | 0.25–2 | 1 | 2 | 33 (100) | — | 0 (0) | 0 (0) |
| Flucytosine | ≤0.06–0.5 | 0.12 | 0.25 | — | — | — | — |
Abbreviations: intermediate, I; minimum inhibitory concentrations for 50% and 90% of the organisms, respectively, MIC50/90; susceptible, S; susceptible dose-dependent, SDD; resistant, R.
Data are presented as frequency with percentage (%).
The overall 28-day mortality rate in this study was 44.5% (154 patients). Moreover, the mortality rates among the three most common non-albicans Candida species, C. tropicalis, C. glabrata and C. parapsilosis, were 53.8% (78 out of 145 patients), 40.5% (45 out of 111 patients) and 31.4% (22 out of 70 patients), respectively. To identify the independent risk factors associated with 28-day mortality in cancer patients with non-albicans candidemia, we stratified the 346 patients into survival and death groups (Table 4). In the univariate analysis, patients with a higher Charlson comorbidity index (P=0.011), a non-albicans candidemia caused by C. tropicalis (P=0.003), a primary infection (P=0.006), neutropenia (P=0.005) or septic shock (P<0.001) had significantly higher 28-day mortality rates. The results of this multivariate analysis showed that a higher Charlson comorbidity index (odds ratio (OR): 1.18; 95% confidence interval (CI): 1.08–1.30; P<0.001), a non-albicans candidemia caused by C. tropicalis (OR: 1.91; 95% CI: 1.11–3.29; P=0.019), neutropenia (OR: 3.67; 95% CI: 1.07–12.55; P=0.038) and septic shock (OR: 2.29; 95% CI: 1.39–3.77; P=0.001) were independent predictors of 28-day mortality.
Table 4. Analysis of potential risk factors associated with 28-day mortality in patients with non-albicans candidemia.
| Variables | Survival n=192 Number (%) | Death n=154 Number (%) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|---|
| P-value | Odds ratio (95% CI) | P-value | |||
| Male | 122 (63.5) | 99 (64.3) | 0.886 | 0.79 (0.48–1.31) | 0.361 |
| Age in years | 63.8±15.3 | 64±15.5 | 0.923 | 0.99 (0.98–1.01) | 0.381 |
| Charlson comorbidity index | 5.2±2.6 | 6±3 | 0.011 | 1.18 (1.08–1.30) | <0.001 |
| Hematological malignancy | 20 (10.4) | 26 (16.9) | 0.081 | 0.80 (0.35–1.82) | 0.598 |
| Non-albicans Candida species | |||||
| C. tropicalis | 67 (34.9) | 78 (50.6) | 0.003 | 1.91 (1.11–3.29) | 0.019 |
| C. glabrata | 66 (34.4) | 45 (29.2) | 0.308 | ||
| C. parapsilosis | 48 (25) | 22 (14.3) | 0.015 | 0.94 (0.47–1.86) | 0.858 |
| C. krusei | 4 (2.1) | 3 (2) | 0.931 | ||
| C. guilliermondii | 3 (1.6) | 2 (1.3) | 0.839 | ||
| C. sake | 2 (1) | 0 (0) | 0.999 | ||
| C. dubliniensis | 0 (0) | 2 (1.3) | 0.999 | ||
| C. lipolytica | 0 (0) | 1 (0.7) | 1.000 | ||
| C. norvegensis | 0 (0) | 1 (0.7) | 1.000 | ||
| C. famata | 1 (0.5) | 0 (0) | 1.000 | ||
| Origin of non-albicans candidemia | |||||
| Primary infection | 63 (32.8) | 73 (47.4) | 0.006 | 1.37 (0.77–2.42) | 0.283 |
| Catheter-related infection | 76 (39.6) | 32 (20.8) | <0.001 | 0.58 (0.31–1.07) | 0.082 |
| Urinary tract infection | 15 (7.8) | 14 (9.1) | 0.670 | ||
| Respiratory tract infection | 23 (12) | 19 (12.3) | 0.919 | ||
| Intra-abdominal infection | 14 (7.3) | 15 (9.7) | 0.416 | ||
| Skin and soft tissue infection | 1 (0.5) | 1 (0.6) | 0.876 | ||
| Underlying conditions | |||||
| Presence of central venous catheter | 167 (87) | 127 (82.5) | 0.245 | ||
| Presence of Foley catheter | 66 (34.4) | 61 (39.6) | 0.316 | ||
| Chemotherapy | 94 (49) | 74 (48.1) | 0.867 | ||
| Total parental nutrition | 58 (30.2) | 38 (24.7) | 0.254 | ||
| Steroid | 58 (30.2) | 61 (39.6) | 0.068 | 1.61 (0.97–2.67) | 0.068 |
| Proton-pump inhibitor | 78 (40.6) | 67 (43.5) | 0.589 | ||
| Abdominal surgery | 35 (18.2) | 23 (14.9) | 0.503 | ||
| Prior exposure to broad-spectrum antibiotics | 169 (88) | 137 (89) | 0.786 | ||
| Neutropenia | 5 (2.6) | 16 (10.4) | 0.005 | 3.67 (1.07–12.55) | 0.038 |
| Septic shock | 50 (26) | 74 (48.1) | <0.001 | 2.29 (1.39–3.77) | 0.001 |
| Concomitant bacterial infections | 65 (33.9) | 62 (40.3) | 0.220 | ||
Data are presented as mean±standard deviation (s.d.) or frequency with percentage (%).
DISCUSSION
Candidemia, with a shift in species distribution towards non-albicans Candida species, remains a lethal disease. Moreover, malignancy is identified as the major comorbidity of patients with candidemia. Here, we report several relevant findings obtained from a large multicenter study that characterized non-albicans candidemia in cancer patients. In this study, C. tropicalis was the main cause of non-albicans candidemia in cancer patients. Most isolates of non-albicans Candida (>80%) were susceptible or susceptible dose-dependent to echinocandins and fluconazole. Non-albicans candidemia due to C. tropicalis, as well as a higher Charlson comorbidity index, neutropenia and septic shock, were independently associated with poor prognosis.
C. parapsilosis has been reported to be the most common cause of non-albicans candidemia among patients with malignancy in Western countries, including the United States, Australia and Portugal.13, 14, 23 In our study, the major non-albicans Candida species identified in cancer patients with candidemia was C. tropicalis, consistent with previous reports in Taiwan.6, 15 The clinical features of the non-albicans Candida species distribution of candidemia seemed more likely to be influenced by geography than by underlying comorbidities. Consequently, establishing the epidemiology at each research surveillance site is important, and C. tropicalis is one of the most important and predominant causes of non-albicans candidemia in cancer patients, especially in Taiwan. In fact, C. tropicalis has been identified as the leading cause of non-albicans candidemia in all patients with or without a diagnosed malignancy (49.4%–35.4%) in Taiwan since the year 2000,7, 20, 24, 25, 26 except in a study by Lin et al., which enrolled a small number of patients with non-albicans candidemia.27
In our study, C. tropicalis was not only the most common cause of non-albicans candidemia but also the species with the highest resistance rate to fluconazole among the preserved isolates of the three non-albicans Candida species (C. tropicalis, C. glabrata and C. parapsilosis) analyzed. In general, C. glabrata, with reduced susceptibility,28 and C. krusei, with intrinsic resistance,29 were two commonly indicated non-albicans Candida species for which increasing fluconazole resistance was observed. According to the definitions set by the Clinical Laboratory Standards Institute [M27-S4],22 national surveillance research in Brazil showed that none of the C. tropicalis isolates obtained were resistant to fluconazole, whereas 36.4% (4/11) of C. glabrata isolates were resistant to fluconazole. However, 96.7% (93/96) of C. glabrata isolates were susceptible dose-dependent to fluconazole in our study, and 86.1% (105/122) of C. tropicalis isolates were susceptible or susceptible dose-dependent to fluconazole. Tan et al.30 conducted a multicenter study that characterized clinical isolates from patients with candidemia in countries in the Asia-Pacific region and reported the fluconazole susceptibility rates for C. tropicalis (susceptible rate=75.8% susceptible dose-dependent rate=6.1%) and C. glabrata (susceptible dose-dependent rate=94.9%), which are in accordance with the results of our study. Furthermore, Yang et al.5 reported that of the C. glabrata isolates obtained from patients with candidemia, none were resistant to fluconazole, but 27.8% (5/18) of C. tropicalis isolates were resistant to fluconazole in China. A more recent study by Chen et al.31 reported that the resistance rate to fluconazole of C. tropicalis was 9.5% in patients with candidemia in Taiwan and that the resistance rates of C. glabrata and C. parapsilosis were 5.4% and 2%, respectively. Taken together, these studies imply that not only the species distribution of non-albicans candidemia but also the antifungal susceptibility of the causative agents differs from region to region and that C. tropicalis is the most fluconazole-resistant species among clinically common non-albicans Candida species causing candidemia around the Asia-Pacific region, including Taiwan.
Previous studies have reported that underlying conditions and the severity of illness, including advanced age, septic shock, neutropenia, acute respiratory failure and a high Acute Physiology and Chronic Health Evaluation (APACHE) II score, are significantly associated with mortality in cancer patients with non-albicans candidemia,6, 13, 15 consistent with the results presented in this study. We further found that non-albicans candidemia due to C. tropicalis was an independent predictor of 28-day mortality. Compared with other species of non-albicans Candida, patients with C. tropicalis candidemia tended to present a higher severity of illness, such as neutropenia (10.3% versus 3%, P=0.006) and septic shock (44.8% versus 29.4%, P=0.003). These results and the relatively high resistance rate to fluconazole of C. tropicalis, as mentioned previously, may contribute to the poor outcome of patients with non-albicans candidemia due to C. tropicalis. In the literature, C. tropicalis is shown as the highest fluconazole-resistant species among the three most clinically common non-albicans Candida (C. tropicalis, C. glabrata and C. parapsilosis) in Asian countries,5, 30, 31 but these studies did not investigate the impact of different non-albicans Candida species on survival in cancer patients. A population-based study in Spain conducted by Puig-Asensio et al.16 demonstrated that candidemia caused by C. krusei was an independent risk factor of higher 30-day mortality in cancer patients with the use of a central venous catheter (OR: 12.59; 95% CI: 2.46–64.48; P=0.002). This disagrees with results from our study, possibly due to different geographies and study populations. Further large-scale, intensive research on this issue in different regions is imperatively needed.
A few limitations of the present study should be noted. First, this study was inherently retrospective in design, and thus missing values and potential information bias may have arisen. Second, APACHE II scores from the enrolled patients were not available. Septic shock and neutropenia are possible confounding factors in APACHE II scores, and therefore the frequencies of these two variables were monitored in the study to strengthen the results. Third, information about catheter removal in patients with CRBSI was unavailable, and the influence of this action on survival could not be evaluated. Finally, in vitro susceptibility results were only available for the preserved isolates, and appropriate or inappropriate therapy could not be defined for all of the study population. As a result, the analysis of mortality did not include the use of antifungal agents. Further research is needed to investigate the impact of antifungal therapy and catheter removal on the prognosis of patients with non-albicans candidemia. Despite its limitations, this is a large, multicenter study that may allow for more generalizable findings and may provide useful information for the treatment of fungal infections in cancer patients.
In conclusion, the epidemiology of non-albicans candidemia in cancer patients is a growing concern that must be addressed. Among cancer patients in Taiwan, C. tropicalis is the leading species causing non-albicans candidemia and is the species with the highest resistance to fluconazole among the clinically major non-albicans Candida species. Continuous monitoring of the species distribution and antifungal susceptibility of candidemia cases is necessary.
Acknowledgments
This study was supported in part by a research grant from the Taipei Veterans General Hospital (VGH-105-C-051).
References
- Marchetti O, Bille J, Fluckiger U et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis 2004; 38: 311–320. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39: 309–317. [DOI] [PubMed] [Google Scholar]
- Magill SS, Edwards JR, Bamberg W et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh PR, Teng LJ, Yang PC et al. Emergence of nosocomial candidemia at a teaching hospital in Taiwan from 1981 to 2000: increased susceptibility of Candida species to fluconazole. Microb Drug Resist 2002; 8: 311–319. [DOI] [PubMed] [Google Scholar]
- Yang ZT, Wu L, Liu XY et al. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis 2014; 14: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HJ, Liu WL, Lin HL et al. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS One 2014; 9: e99103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii IM, Chang HL, Lin LC et al. Changing epidemiology of candidemia in a medical center in middle Taiwan. J Microbiol Immunol Infect 2015; 48: 306–315. [DOI] [PubMed] [Google Scholar]
- Cleveland AA, Harrison LH, Farley MM et al. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008-2013: results from population-based surveillance. PLoS One 2015; 10: e0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib R, Johnson LB, Fakih MG et al. Current trends in candidemia and species distribution among adults: Candida glabrata surpasses C. albicans in diabetic patients and abdominal sources. Mycoses 2016; 59: 781–786. [DOI] [PubMed] [Google Scholar]
- Tortorano AM, Kibbler C, Peman J et al. Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents 2006; 27: 359–366. [DOI] [PubMed] [Google Scholar]
- Wey SB, Mori M, Pfaller MA et al. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 1988; 148: 2642–2645. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Argon J, Chu J et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005; 41: 1232–1239. [DOI] [PubMed] [Google Scholar]
- Sipsas NV, Lewis RE, Tarrand J et al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009; 115: 4745–4752. [DOI] [PubMed] [Google Scholar]
- Slavin MA, Sorrell TC, Marriott D et al. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother 2010; 65: 1042–1051. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang SY, Tsay W et al. Clinical characteristics of candidaemia in adults with haematological malignancy, and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2001–2010. Int J Antimicrob Agents 2012; 40: 533–538. [DOI] [PubMed] [Google Scholar]
- Puig-Asensio M, Ruiz-Camps I, Fernandez-Ruiz M et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect 2015; 21: 491.e1–10. [DOI] [PubMed] [Google Scholar]
- Bassetti M, Righi E, Costa A et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 2006; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem R, Hanna H, Kontoyiannis D et al. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida kruseias the leading causes of candidemia in hematologic malignancy. Cancer 2008; 112: 2493–2499. [DOI] [PubMed] [Google Scholar]
- Horn DL, Neofytos D, Anaissie EJ et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48: 1695–1703. [DOI] [PubMed] [Google Scholar]
- Chen LY, Kuo SC, Wu HS et al. Associated clinical characteristics of patients with candidemia among different Candida species. J Microbiol Immunol Infect 2013; 46: 463–468. [DOI] [PubMed] [Google Scholar]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI)Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. (Approved Standard M27-S4), 4th edn. Wayne, PA, USA: National Committee for Clinical and Laboratory Standards. 2012. [Google Scholar]
- Sabino R, Verissimo C, Brandao J et al. Epidemiology of candidemia in oncology patients: a 6-year survey in a Portuguese central hospital. Med Mycol 2010; 48: 346–354. [DOI] [PubMed] [Google Scholar]
- Chen PY, Chuang YC, Wang JT et al. Comparison of epidemiology and treatment outcome of patients with candidemia at a teaching hospital in Northern Taiwan, in 2002 and 2010. J Microbiol Immunol Infect 2014; 47: 95–103. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Wang CC, Kuo HY et al. Adult candidemia at a medical center in northern Taiwan: a retrospective study. J Microbiol Immunol Infect 2008; 41: 414–421. [PubMed] [Google Scholar]
- Chen LY, Liao SY, Kuo SC et al. Changes in the incidence of candidaemia during 2000–2008 in a tertiary medical centre in northern Taiwan. J Hosp Infect 2011; 78: 50–53. [DOI] [PubMed] [Google Scholar]
- Chi HW, Yang YS, Shang ST et al. Candida albicans versus non-albicans bloodstream infections: the comparison of risk factors and outcome. J Microbiol Immunol Infect 2011; 44: 369–375. [DOI] [PubMed] [Google Scholar]
- Ruan SY, Chu CC, Hsueh PR. In vitro susceptibilities of invasive isolates of Candida species: rapid increase in rates of fluconazole susceptible-dose dependent Candida glabrata isolates. Antimicrob Agents Chemother 2008; 52: 2919–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Gibbs DL et al. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 2007; 45: 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TY, Hsu LY, Alejandria MM et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 2016; 54: 471–477. [DOI] [PubMed] [Google Scholar]
- Chen YC, Kuo SF, Chen FJ et al. Antifungal susceptibility of Candida species isolated from patients with candidemia in southern Taiwan, 2007–2012: impact of new antifungal breakpoints. Mycoses 2017; 60: 89–95. [DOI] [PubMed] [Google Scholar]