Abstract
Sporothrix globosa is the main causative agent of sporotrichosis, a common mycosis that usually affects the skin, in China. Despite increasing efforts in the molecular identification of this fungal pathogen, its modes of transmission and epidemiology remain poorly understood. The goals of this study were to assess the genetic diversity of S. globosa using amplified fragment length polymorphism (AFLP) analysis and to assess the correlation of AFLP profiles with the geographic origins, growth rates, clinical forms, and antifungal susceptibilities of S. globosa isolates. AFLP analysis of 225 clinical S. globosa isolates from eight provinces or municipalities in China identified eight distinct clustering groups (I–VIII), with groups I, II and IV being the most common. The AFLP genotypes showed distinct distribution patterns among different regions within Jilin Province and between northern and southern China, but there was no obvious association between the AFLP genotypes and the growth rates, clinical forms or antifungal susceptibilities of the S. globosa isolates. These results expand our understanding of the genetic variation of S. globosa and suggest that AFLP analysis is a potentially useful tool for studying the epidemiology of this fungal pathogen.
Keywords: amplified fragment length polymorphism, genotyping, sporotrichosis, Sporothrix globosa
INTRODUCTION
Sporotrichosis is a common chronic deep mycosis caused by the dimorphic fungus Sporothrix schenckii.1 Based on its clinical manifestations, sporotrichosis can be classified into fixed cutaneous, lymphocutaneous, disseminated cutaneous and extracutaneous forms. Sporotrichosis was first described in 1898 in the United States2 and has since been reported worldwide, with a high prevalence in tropical and subtropical areas. While S. schenckii has long been considered a single species, increasing numbers of phenotypic and molecular studies suggest that the pathogenic Sporothrix species comprises at least four closely related but clearly distinct species, including S. schenckii sensu stricto, S. globosa, S. brasiliensis and S. luriei.3, 4 Among them, S. globosa is perhaps the most extensively studied, with reports from North, Central, and South America, Europe and Asia.5, 6
Since the first report of sporotrichosis in China in 1916, the incidence of the disease has continued to increase. Several outbreaks have been reported, particularly in Jilin Province, where the largest number of sporotrichosis cases in China have been recorded.7, 8 Many molecular studies have demonstrated that S. globosa is the most prevalent etiologic agent of sporotrichosis in China.8, 9, 10, 11, 12 Several retrospective studies of clinical S. schenckii isolates from China using random amplified polymorphic DNA analysis13, 14 and restriction fragment length polymorphism analysis15 have shown a correlation among the genotypes, clinical forms, and geographic origins of isolates, although S. schenckii was not differentiated from S. globosa or other Sporothrix species.
Amplified fragment length polymorphism (AFLP) analysis is a highly sensitive method for detecting DNA polymorphisms and has been widely used for genetic variation and linkage analysis of bacteria, plants, and animals as well as fungi. When this technique was used to examine the genetic diversity of S. schenckii isolates in Peru, two distinct clusters were noted, although there was no correlation between these AFLP genotypes and the geographical origins or clinical manifestations of the disease.16 Recently, Zhang et al.17 applied AFLP analysis to 20 S. globosa isolates of diverse geographic origins, including nine isolates from China, and found that all isolates were tightly clustered into the same group. However, the study gave no detail on the genetic diversity of the nine isolates from China, and any geographic and phenotypic associations were not reported.
In the present study, we used AFLP analysis to examine 225 clinical S. globosa isolates from eight provinces or municipalities in China with the aim of identifying any correlations between AFLP profiles and the geographic origins, growth rates, clinical characteristics and antifungal susceptibilities of the isolates.
MATERIALS AND METHODS
Fungal isolates and cultivation
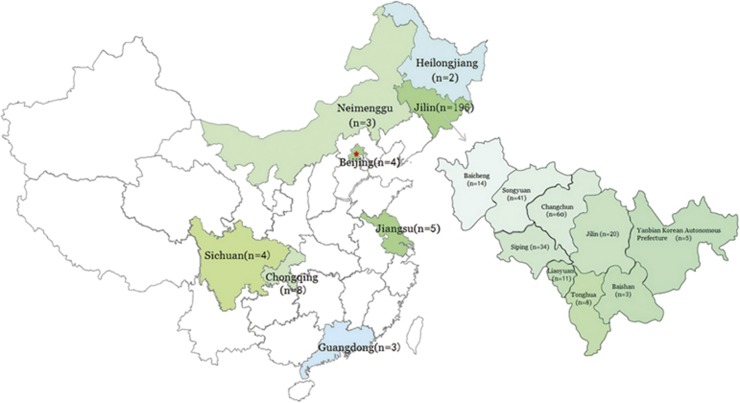
A total of 225 clinical S. globosa isolates from China were included in this study. The isolates were collected between 2009 and 2013 from patients with sporotrichosis (one isolate per patient). The samples were collected at six hospitals, with the patients originating from eight different provinces or municipalities in China (Figure 1 and Table 1). Twenty four of the isolates had been previously identified as S. globosa based on sequence analysis of the calmodulin gene (CAL; Shiying, 2015, unpublished data). These sequences are available from the GenBank database using the accession numbers listed in Table 1. Demographic information and clinical manifestation data for each patient were provided by the investigators at each hospital (Table 1). Aspergillus fumigatus strain IFM40808 (wild-type) and three S. globosa isolates (ATCC MYA-4911, ATCC MYA-4912, and ATCC MYA-4914) were used as controls for AFLP analysis. These strains were provided as a gift by the Chinese Academy of Medical Sciences and Peking Union Medical College. All isolates were inoculated onto potato dextrose agar slants and cultured at 28 °C for 7 days.
Figure 1.
Geographical origins of the Sporothrix globosa isolates studied in this work.
Table 1. Clinical isolates of Sporothrix globosa included in the study.
| Location | Isolate IDa | Year of identification | Gender (M/F) | Age (year) | Clinical form (D, F, L, E)b | GenBank Accession code | |
|---|---|---|---|---|---|---|---|
| Jilin Province | Baicheng City | FHJU09033102c | 2009 | M | 4 | F | KY349948 |
| FHJU09060201 | 2009 | F | 56 | L | KY349946 | ||
| FHJU09072902 | 2009 | F | 42 | F | KY349944 | ||
| FHJU10042702 | 2010 | M | 41 | L | KY350105 | ||
| FHJU11021404 | 2011 | F | 11 | L | KY349942 | ||
| FHJU11030301 | 2011 | F | 49 | L | KY349947 | ||
| FHJU11051803 | 2011 | M | 0.25 | F | KY350125 | ||
| FHJU11053102c | 2011 | F | 57 | D | KY350126 | ||
| FHJU11070405 | 2011 | F | 3 | F | KY349943 | ||
| FHJU11110301 | 2011 | F | 43 | F | KY349940 | ||
| FHJU11122805 | 2011 | M | 34 | F | KY349945 | ||
| FHJU12022005 | 2012 | F | 15 | L | KY349934 | ||
| FHJU12061704 | 2012 | F | 44 | F | KY349939 | ||
| FHJU13041904 | 2013 | F | 53 | F | KY349941 | ||
| Baishan City | FHJU09042601 | 2009 | F | 54 | F | KY349965 | |
| FHJU10122702c | 2010 | F | 24 | F | KY350091 | ||
| FHJU13031801c | 2013 | F | 51 | F | KY349978 | ||
| Changchun City | FHJU09030702 | 2009 | M | 9 | F | KY350078 | |
| FHJU09032101 | 2009 | M | 57 | F | KY350061 | ||
| FHJU09041501 | 2009 | M | 13 | F | KY350075 | ||
| FHJU09112501 | 2009 | F | 56 | L | KY350073 | ||
| FHJU09120401 | 2009 | F | 53 | L | KY350068 | ||
| FHJU09121901 | 2009 | F | 29 | F | KY350046 | ||
| FHJU10041001 | 2010 | F | 9 | F | KY350123 | ||
| FHJU10041302 | 2010 | F | 73 | L | KY350085 | ||
| FHJU10042601 | 2010 | F | 38 | L | KY350116 | ||
| FHJU10042701 | 2010 | F | 27 | L | KY350110 | ||
| FHJU10052601 | 2010 | F | 17 | L | KY350109 | ||
| FHJU10122201 | 2010 | M | 5 | F | KY350088 | ||
| FHJU10122703 | 2010 | F | 2 | F | KY350090 | ||
| FHJU10122903c | 2010 | F | 74 | L | KY350101 | ||
| FHJU10122904 | 2010 | F | 59 | F | KY350086 | ||
| FHJU10123003 | 2010 | F | 4 | F | KY350120 | ||
| FHJU10123103 | 2010 | M | 5 | F | KY350092 | ||
| FHJU10082301c | 2011 | F | 73 | E | KY350117 | ||
| FHJU11010402 | 2011 | F | 60 | F | KY350054 | ||
| FHJU11021105 | 2011 | F | 65 | F | KY350060 | ||
| FHJU11021802 | 2011 | M | 58 | F | KY350064 | ||
| FHJU11022504 | 2011 | F | 48 | F | KY350058 | ||
| FHJU11030102 | 2011 | F | 53 | L | KY350071 | ||
| FHJU11050205 | 2011 | F | 62 | L | KY350133 | ||
| FHJU11050502c | 2011 | M | 0.25 | F | KY350094 | ||
| FHJU11050703 | 2011 | F | 4 | L | KY350080 | ||
| FHJU11051301 | 2011 | F | 22 | F | KY350098 | ||
| FHJU11051306 | 2011 | F | 47 | L | KY350134 | ||
| FHJU11051601c | 2011 | F | 77 | L | KY350074 | ||
| FHJU11060901 | 2011 | M | 9 | L | KY350097 | ||
| FHJU11061001 | 2011 | F | 28 | F | KY350041 | ||
| FHJU11062001c | 2011 | F | 56 | D | KY350056 | ||
| FHJU11101901c | 2011 | M | 78 | F | KY350044 | ||
| FHJU11102601c | 2011 | F | 59 | F | KY350076 | ||
| FHJU11112102 | 2011 | F | 40 | F | KY350072 | ||
| FHJU11121203 | 2011 | F | 39 | L | KY350055 | ||
| FHJU12010903 | 2012 | F | 65 | F | KY350059 | ||
| FHJU12012901 | 2012 | F | 45 | F | KY350057 | ||
| FHJU12013003 | 2012 | F | 68 | L | KY350077 | ||
| FHJU12020401 | 2012 | F | 42 | F | KY350082 | ||
| FHJU12021201 | 2012 | F | 51 | L | KY350084 | ||
| FHJU12021506 | 2012 | F | 54 | L | KY350093 | ||
| FHJU12030802 | 2012 | F | 9 | F | KY350040 | ||
| FHJU12031901 | 2012 | F | 46 | F | KY350069 | ||
| FHJU12031903 | 2012 | M | 33 | F | KY350042 | ||
| FHJU12032401 | 2012 | F | 60 | L | KY350062 | ||
| FHJU12040603 | 2012 | M | 43 | F | KY350095 | ||
| FHJU12041601 | 2012 | F | 62 | L | KY350096 | ||
| FHJU12042901 | 2012 | F | 3 | F | KY350081 | ||
| FHJU12050305 | 2012 | F | 48 | F | KY350065 | ||
| FHJU12050403 | 2012 | F | 9 | L | KY350039 | ||
| FHJU12052202 | 2012 | F | 79 | F | KY350063 | ||
| FHJU12052302 | 2012 | F | 62 | L | KY350079 | ||
| FHJU12060402 | 2012 | F | 45 | L | KY350066 | ||
| FHJU12060702 | 2012 | F | 50 | F | KY350043 | ||
| FHJU12061503 | 2012 | F | 47 | F | KY350067 | ||
| FHJU12061601 | 2012 | F | 37 | F | KY350083 | ||
| FHJU12062301 | 2012 | F | 6 | F | KY350045 | ||
| FHJU12082002 | 2012 | F | 64 | F | KY350070 | ||
| FHJU13032302 | 2013 | M | 17 | F | KY350001 | ||
| Jilin City | FHJU09041602 | 2009 | F | 45 | L | KY349981 | |
| FHJU09041603c | 2009 | M | 0.3 | F | KY349980 | ||
| FHJU09042002 | 2009 | M | 44 | L | KY349989 | ||
| FHJU09042801 | 2009 | F | 52 | L | KY349967 | ||
| FHJU10051701c | 2010 | F | 67 | F | KY350112 | ||
| FHJU11030601 | 2011 | M | 9 | F | KY349991 | ||
| FHJU11030803 | 2011 | F | 75 | L | KY350129 | ||
| FHJU11052001 | 2011 | M | 4 | F | KY349993 | ||
| FHJU11102001c | 2011 | M | 4 | F | KY349972 | ||
| FHJU12010402 | 2012 | F | 68 | L | KY349982 | ||
| FHJU12010502 | 2012 | F | 59 | L | KY349986 | ||
| FHJU12021602 | 2012 | F | 44 | L | KY349987 | ||
| FHJU12021603 | 2012 | F | 12 | F | KY349974 | ||
| FHJU12022003 | 2012 | F | 76 | L | KY349985 | ||
| FHJU12030604 | 2012 | F | 5 | F | KY349988 | ||
| FHJU12031206 | 2012 | M | 60 | F | KY349983 | ||
| FHJU12032601 | 2012 | M | 6 | F | KY349992 | ||
| FHJU12040304 | 2012 | M | 12 | F | KY349990 | ||
| FHJU12062602 | 2012 | F | 54 | L | KY349984 | ||
| FHJU12091101 | 2012 | F | 59 | L | KY349973 | ||
| Liaoyuan City | FHJU09020301 | 2009 | F | 59 | L | KY350008 | |
| FHJU09052601 | 2009 | M | 61 | L | KY350007 | ||
| FHJU10020301c | 2010 | F | 55 | L | KY350113 | ||
| FHJU10060901 | 2010 | M | 51 | F | KY350099 | ||
| FHJU11021301 | 2011 | F | 46 | F | KY349979 | ||
| FHJU11021806c | 2011 | M | 48 | D | KY349976 | ||
| FHJU11061302c | 2011 | F | 70 | L | KY350006 | ||
| FHJU11070404c | 2011 | M | 59 | L | KY350005 | ||
| FHJU11120503 | 2011 | M | 1.5 | F | KY349994 | ||
| FHJU12061702 | 2012 | F | 28 | L | KY350004 | ||
| FHJU13041102 | 2013 | F | 42 | F | KY349975 | ||
| Siping City | FHJU09022702 | 2009 | F | 44 | F | KY350127 | |
| FHJU09030501 | 2009 | F | 7 | F | KY350131 | ||
| FHJU09033105 | 2009 | M | 1 | L | KY350011 | ||
| FHJU09090101 | 2009 | F | 59 | F | KY350015 | ||
| FHJU09112601 | 2009 | F | 4 | F | KY350020 | ||
| FHJU10042303c | 2010 | M | 29 | F | KY350121 | ||
| FHJU10051101 | 2010 | M | 2 | F | KY350103 | ||
| FHJU10052401 | 2010 | F | 50 | F | KY350107 | ||
| FHJU10080101 | 2010 | F | 36 | F | KY350100 | ||
| FHJU10121501 | 2010 | M | 1 | F | KY350087 | ||
| FHJU10123101 | 2010 | F | 41 | F | KY350102 | ||
| FHJU11011004 | 2011 | M | 3 | F | KY349996 | ||
| FHJU11011202c | 2011 | F | 47 | L | KY350034 | ||
| FHJU11021107 | 2011 | F | 6 | L | KY350012 | ||
| FHJU11022201 | 2011 | M | 48 | L | KY349998 | ||
| FHJU11022802 | 2011 | F | 67 | L | KY350018 | ||
| FHJU11030403 | 2011 | F | 60 | F | KY350014 | ||
| FHJU11042502 | 2011 | F | 19 | L | KY350016 | ||
| FHJU11050201 | 2011 | F | 30 | L | KY349999 | ||
| FHJU11060202 | 2011 | M | 28 | F | KY350013 | ||
| FHJU11061501 | 2011 | F | 63 | L | KY350017 | ||
| FHJU11082601 | 2011 | F | 60 | F | KY350024 | ||
| FHJU11120601 | 2011 | M | 7 | L | KY350021 | ||
| FHJU11120602 | 2011 | M | 7 | L | KY350038 | ||
| FHJU12020201 | 2012 | F | 55 | F | KY350023 | ||
| FHJU12022803 | 2012 | M | 2 | F | KY350037 | ||
| FHJU12033001c | 2012 | M | 82 | L | KY350009 | ||
| FHJU12040302c | 2012 | F | 70 | F | KY349995 | ||
| FHJU12041004 | 2012 | F | 44 | F | KY350130 | ||
| FHJU12050903 | 2012 | M | 55 | L | KY350022 | ||
| FHJU12051605 | 2012 | F | 24 | F | KY350010 | ||
| FHJU12062901 | 2012 | F | 4 | F | KY350000 | ||
| FHJU12080901 | 2012 | F | 54 | F | KY349997 | ||
| FHJU13032301 | 2013 | M | 14 | L | KY350019 | ||
| Songyuan City | FHJU10020401 | 2010 | F | 47 | L | KY350108 | |
| FHJU10042001c | 2010 | M | 3 | F | KY350111 | ||
| FHJU10051501 | 2010 | M | 3 | F | KY350104 | ||
| FHJU10060202c | 2010 | M | 0.5 | F | KY350106 | ||
| FHJU10122401 | 2010 | M | 5 | F | KY350089 | ||
| FHJU11021402c | 2011 | F | 52 | D | KY349968 | ||
| FHJU11021405c | 2011 | F | 48 | D | KY349950 | ||
| FHJU11021701 | 2011 | M | 39 | L | KY349954 | ||
| FHJU11022103 | 2011 | M | 4 | F | KY349953 | ||
| FHJU11022402 | 2011 | M | 6 | L | KY349952 | ||
| FHJU11022602c | 2011 | F | 6 | F | KY349955 | ||
| FHJU11030101 | 2011 | M | 7 | L | KY349936 | ||
| FHJU11030304 | 2011 | F | 24 | F | KY349949 | ||
| FHJU11030702 | 2011 | F | 54 | F | KY349956 | ||
| FHJU11050903 | 2011 | M | 11 | F | KY349938 | ||
| FHJU11051702 | 2011 | F | 62 | L | KY350122 | ||
| FHJU11052002 | 2011 | F | 27 | L | KY350032 | ||
| FHJU11052402 | 2011 | F | 62 | L | KY350124 | ||
| FHJU11052501 | 2011 | M | 43 | L | KY349958 | ||
| FHJU11061605 | 2011 | M | 48 | L | KY349963 | ||
| FHJU11062006 | 2011 | F | 50 | F | KY350028 | ||
| FHJU11081502c | 2011 | M | 25 | F | KY349970 | ||
| FHJU11090602 | 2011 | F | 58 | F | KY349957 | ||
| FHJU11112801c | 2011 | M | 55 | F | KY349969 | ||
| FHJU11120502 | 2011 | F | 75 | F | KY350132 | ||
| FHJU11121201 | 2011 | F | 29 | L | KY349959 | ||
| FHJU12013102 | 2012 | M | 64 | F | KY350036 | ||
| FHJU12021402 | 2012 | F | 62 | L | KY350029 | ||
| FHJU12022102 | 2012 | F | 54 | L | KY349971 | ||
| FHJU12030101c | 2012 | F | 58 | L | KY349964 | ||
| FHJU12030203c | 2012 | M | 50 | D | KY350030 | ||
| FHJU12030901 | 2012 | M | 43 | F | KY350035 | ||
| FHJU12032602 | 2012 | F | 58 | F | KY350031 | ||
| FHJU12032804 | 2012 | F | 5 | F | KY349935 | ||
| FHJU12040301c | 2012 | F | 53 | L | KY349960 | ||
| FHJU12041002c | 2012 | F | 45 | F | KY349951 | ||
| FHJU12041902 | 2012 | M | 55 | F | KY350033 | ||
| FHJU12051002 | 2012 | F | 64 | F | KY349966 | ||
| FHJU12082201 | 2012 | F | 55 | L | KY349937 | ||
| FHJU13031803c | 2013 | F | 46 | F | KY349962 | ||
| FHJU13040501 | 2013 | M | 10 | F | KY349961 | ||
| Tonghua City | FHJU11021702 | 2011 | M | 36 | F | KY350026 | |
| FHJU11062005 | 2011 | F | 51 | F | KY350128 | ||
| FHJU11081801c | 2011 | F | 9 | F | KY350053 | ||
| FHJU11111102 | 2011 | M | 44 | L | KY350050 | ||
| FHJU11122402 | 2011 | F | 50 | F | KY350027 | ||
| FHJU12053001 | 2012 | F | 49 | F | KY350025 | ||
| FHJU13022601 | 2013 | F | 58 | L | KY350003 | ||
| FHJU13051103c | 2013 | F | 7 | F | KY350002 | ||
| Yanbian Korean Autonomous Prefecture | FHJU11102401c | 2011 | F | 3 | F | KY350047 | |
| FHJU11121301c | 2011 | F | 77 | L | KY350049 | ||
| FHJU11122602 | 2011 | M | 7 | L | KY350118 | ||
| FHJU12031204 | 2012 | F | 68 | L | KY350119 | ||
| FHJU12040702c | 2012 | M | 65 | F | KY350115 | ||
| Neimenggu Autonomous Region | Tongliao City | FHJU11021401c | 2011 | F | 41 | F | KY350052 |
| FHJU12032202c | 2012 | F | 60 | L | KY350051 | ||
| FHJU12041101 | 2012 | M | 4 | F | KY350048 | ||
| Heilongjiang Province | Wuchang City | FHJU11050101c | 2011 | M | 7 | L | KY349977 |
| Hegang City | FHJU12071101c | 2012 | M | 64 | F | KY350114 | |
| Jiangsu | CMCC1 | KR075722d | |||||
| Jiangsu | CMCC2 | KR075723d | |||||
| Jiangsu | CMCC3 | KR075724d | |||||
| Jiangsu | CMCC4 | KR075725d | |||||
| Jiangsu | CMCC5 | KR075726d | |||||
| Chongqing | CQMU11 | KR075728d | |||||
| Chongqing | CQMU2 | KR075729d | |||||
| Chongqing | CQMU3 | KR075730d | |||||
| Chongqing | CQMU4 | KR075731d | |||||
| Chongqing | CQMU5 | KR075762d | |||||
| Chongqing | CQMU6 | KR075732d | |||||
| Chongqing | CQMU7 | KR075763d | |||||
| Chongqing | CQMU8 | KR075733d | |||||
| Beijing | FHPU3 | KR075744d | |||||
| Beijing | FHPU4 | KR075745d | |||||
| Beijing | FHPU5 | KR075746d | |||||
| Beijing | FHPU7 | KR075748d | |||||
| Guangdong | SHZU2 | KR075750d | |||||
| Guangdong | SHZU5 | KR075753d | |||||
| Guangdong | SHZU6 | KR075754d | |||||
| Sichuan | WHSU1 | KR075758d | |||||
| Sichuan | WHSU3 | KR075759d | |||||
| Sichuan | WHSU4 | KR075760d | |||||
| Sichuan | WHSU5 | KR075761d |
Abbreviations: female, F; male, M.
The first four alphabet letters in the isolate ID represent abbreviations for the following hospitals: FHJU, The First Hospital of Jilin University; CCMC, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College; CQMU, the First Affiliated Hospital of Chongqing Medical University; FHPU, Peking University First Hospital; SHZU, Second Affiliated Hospital of Zhongshan University; WHSU, West China Hospital of Sichuan University.
F—fixed cutaneous; L—lymphocutaneous; D—disseminated cutaneous; E—extracutaneous.
Isolates which performed the antifungal susceptibility.
These 24 isolates were previously identified as S. globosa by Shiying (2015, unpubil. data).
Morphological and physiological studies
Samples of mycelia (~1 mm diameter) from each culture slant were subcultured on fresh potato dextrose agar plates and incubated at various temperatures (30 °C, 35 °C or 37 °C) for 21 days. Colony diameters were measured after 7, 14 and 21 days of incubation. Assimilation of carbon sources, including sucrose and raffinose, was examined according to previously described methods.18 All isolates were assayed in 96-well microplates, and each plate contained positive controls with glucose and negative controls with no carbon source. Conidial viability in the presence of the different carbon sources was determined following incubation for 5 days at 25 °C.
DNA extraction and sequencing
Total genomic DNA of all isolates was extracted using an alkaline lysis extraction method.19, 20 Briefly, fungal pellets were resuspended sequentially in Solution I (0.9% w/v glucose, 25 mmol/L Tris, 6 mmol/L ethylenediaminetetraacetic acid, pH 8.0) and Solution II (1% SDS, 0.2 mol/L sodium hydroxide), followed by precipitation with Solution III (12% sodium acetate, 12% acetic acid). The supernatant was collected and successively treated with phenol:chloroform:isoamyl alcohol (25:24:1) and chloroform:isoamyl alcohol (24:1). Nucleic acids were precipitated with ice-cold isoamyl alcohol at −20 °C. DNA pellets were washed with 70% ethanol, dissolved in 40 μL of ddH2O, and stored at −20 °C until use.
All DNA samples were quantified using an ultraviolet spectrophotometer, and then the quality was checked using 0.8% (w/v) agarose gel electrophoresis. To confirm that an isolate was S. globosa, we performed a PCR assay to amplify the S. globosa CAL gene using the previously reported primers CL1 (5′-GA(GA)T(AT)CAAGGAGGCCTTCTC-3′) and CL2A (5′-TTT TTG CAT CAT GAG TTG GAC-3′).21 The thermocycling conditions included an initial denaturation at 94 °C for 1 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min, and a final extension of 72 °C for 5 min. The PCR products were examined by electrophoresis in 0.8% (w/v) agarose gels. In addition, all products were purified and subjected to Sanger sequencing by Sangon Biotech (Shanghai, China).
AFLP analysis
The AFLP procedure was carried out essentially as described by Vos et al.22 with some modifications. All primers and adapters22 were synthesized by Sangon Biotech. Briefly, 1 μg of genomic DNA was digested with FastDigest EcoRI (1 μL) and FastDigest SaqAI (1 μL; an isoschizomer of MseI) in a 20- μL reaction mixture at 37 °C for 5 min. The digested products were then ligated to their respective adapters (EcoRI adapter, 5′-CTCGTAGACTGCGTACC-3′ SaqAI adapter, 5′-GACGATGAGTCCTGAG-3′) using T4 DNA Ligase (Invitrogen, Carlsbad, CA, USA) at 25 °C for 1 h. The quality and quantity of the digested and ligated products were examined by agarose gel electrophoresis.
Preamplification was performed in a total volume of 20 μL, containing 5 μL of diluted (1:20) ligation products, 2 mM magnesium chloride, 0.2 mM of each dNTP, 2 μL of 10 × PCR buffer, 1 U Taq DNA polymerase (Takara, Otsu, Japan), and 1 μL of each primer (EcoRI-A, 5′-GTA GAC TGC GTA CCA ATT CA-3′ SaqAI-C, 5′-GAC GAT GAG TCC TGA GTA AC-3′ 10 μM). The thermocycling conditions were: 94 °C for 2 min, followed by 25 cycles of 94 °C for 30 s, 56 °C for 1 min, and 72 °C for 1 min, and a final extension of 72 °C for 10 min.
Selective amplification was carried out in a 20- μL reaction volume consisting of 5 μL of diluted (1:20) preamplification products, 1 mM magnesium chloride, 0.15 mM of each dNTP, 2 μL of 10 × PCR buffer, 1.5 U Taq DNA polymerase (Takara), and 0.6 μL of each primer (EcoRI-ACT, 5′-GAC TGC GTA CCA ATT CAC T-3′ SaqAI-CAA, 5′-GAT GAG TCC TGA GTA ACA A-3′ 10 μM). The thermocycling conditions were as follows: 94 °C for 4 min, followed by 12 cycles of 94 °C for 30 s, 65 °C (with a 0.7 °C decrease per cycle) for 1 min, and 72 °C for 1 min, and then 23 cycles of 94 °C for 30 s, 56 °C for 1 min, and 72 °C for 1 min. The products of the selective amplification were separated by 6% (w/v) denaturing polyacrylamide gel in 1 × TBE buffer for ~1.5 h at 50 W. Following staining with 2% (w/v) silver nitrate, the gels were scanned with a Bio-Rad gel imaging system (Hercules, CA, USA), and the DNA bands were manually scored as present (1) or absent (0) and compiled into a binary matrix. The raw data were analyzed using the unweighted pair-group method with arithmetic average and the Dice coefficient, as implemented in NTSYS-pc version 2.10 (Exeter Software Co., Setauket, NY, USA).
Antifungal agents and antifungal susceptibility testing
We assessed the susceptibility of 43 of the S. globosa isolates (Table 1), which represented each of the different AFLP genotypes, to eight antifungal agents, including amphotericin B (AMB; Bio Basic Inc., Markham, ON, Canada), terbinafine (TRB), itraconazole (ICZ), fluconazole (FCZ), voriconazole (VCZ), posaconazole (POS), albaconazole (ALB) and caspofungin (CAS). TRB, ICZ, FCZ and VCZ were purchased from Tokyo Chemical Industry, Tokyo, Japan. POS, ALB, and CAS were purchased from Toronto Research Chemicals, Toronto, ON, Canada. All susceptibility assays were carried out in RPMI 1640 medium buffered to pH 7 with 0.165 mol/L morpholinepropanesulfonic acid (MOPS). The S. globosa isolates were cultured in microplates, which were prepared as described by the Clinical and Laboratory Standards Institute (standard M38-A2).23 Final drug concentrations ranged from 0.125–64 μg/mL for FCZ, and from 0.03–16 μg/mL for the other drugs. Each inoculum was prepared by adding 5 mL of sterile saline to the agar plate and then removing the colony surface by gentle scraping. The resulting suspensions were diluted, and the numbers of conidia in the suspensions were adjusted to twice the desired final concentration ((1–5) × 104 colony-forming units/mL). The microplates were incubated at 30 °C and read after 72 h. Minimum inhibitory concentrations (MIC) were determined as per the guidelines of the Clinical and Laboratory Standards Institute (standard M38-A2).23 Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality control strains in the antifungal susceptibility testing assays.
Statistical analysis
Analysis of variance (ANOVA) and Dunnett’s T3 Test were used to evaluate the differences in the growth rates, colony sizes, and MIC values of isolates grown under different conditions, and the relationship between clinical manifestation and colony size. The chi-square test and Fisher’s exact test were used to evaluate the relationships between AFLP profiles and the geographic origin, sex and age of patients, clinical manifestation, and year of identification. All statistical analyses were performed using SPSS software version 21 (IBM SPSS Statistics, Somers, NY, USA). A value of P<0.05 was considered statistically significant. The reliability of AFLP clustering analysis was evaluated using a high cophenetic correlation coefficient after 1000 permutations (r=0.772).
RESULTS
Morphological and physiological analyses
All isolates demonstrated good growth by 21 days of cultivation on potato dextrose agar at 30 °C and 35 °C. All isolates initially produced cream-colored colonies, some of which gradually deepened in color to brown or black. Most colonies were oval or round in shape, with a wrinkled surface and a milky membranous edge. The colony diameters were 16–42 mm at 30 °C and 3–15 mm at 35 °C. When the culture temperature was increased to 37 °C, most isolates showed very limited growth, with colony sizes ranging from 1.5–5.5 mm in diameter. Seven isolates showed no growth at this temperature (FHJU12030101, FHJU12010502, FHJU12010402, FHJU11050201, FHJU12062301, FHJU11102601 and CCMC1). All growth data are summarized in Table 2. ANOVA and Dunnett’s T3 Test results showed that the average colony size of AFLP group IV isolates was significantly different from those of group I and group II isolates, while no significant difference in the average colony size was observed between any other AFLP groups. There were no significant differences (P>0.05) in the average colony size between isolates grown at different temperatures or between isolates obtained from patients with different clinical forms of sporotrichosis. All isolates assimilated glucose and sucrose, but none could assimilate raffinose.
Table 2. Morphological characteristics and AFLP genotypes of Sporothrix globosa isolates in China.
| Group by AFLP | Number of isolates | Mean colony diameter (mm)±SD | Growth rate (mm/week)±SD | ||||
|---|---|---|---|---|---|---|---|
| 30 °C | 35 °C | 37 °C | 30 °C | 35 °C | 37 °C | ||
| I | 52 | 29.89±5.02a | 6.48±2.16a | 3.12±1.17a | 9.96±1.67a | 2.16±0.27a | 1.04±0.39a |
| II | 93 | 31.86±5.02a | 8.58±2.20b | 2.54±0.63b | 10.62±1.67a | 2.86±0.73b | 0.85±0.21b |
| III | 2 | 32.00±2.83a,b | 9.50±0.71a,b | 4.95±0.07c | 10.67±0.94a,b | 3.17±0.24a,b | 1.65±0.02c |
| IV | 49 | 34.53±3.25b | 9.21±2.34b | 2.56±0.72a,b | 11.51±1.08b | 3.07±0.78b | 0.85±0.24a,b |
| V | 12 | 32.73±6.04a,b | 9.67±3.22b | 3.20±1.15a,b | 10.91±2.01a,b | 3.22±1.07b | 1.07±0.38a,b |
| VI | 2 | 34.25±1.06a,b | 8.25±2.47a,b | 2.40±0.28a,b,c | 11.42±0.35a,b | 2.75±0.82a,b | 0.80±0.09a,b,c |
| VII | 3 | 33.83±5.80a,b | 10.3±0.58a,b | 3.23±0.84a,b,c | 11.28±1.93a,b | 3.44±0.19a,b | 1.08±0.28a,b,c |
| VIII | 3 | 25.83±3.75a,b | 6.17±0.29a,b | 2.97±0.35a,b | 8.61±1.25a,b | 2.06±0.10a,b | 0.99±0.12a,b |
| total | 216 | 31.9±4.97 | 8.26±2.49 | 2.76±0.90 | 10.67±1.66 | 2.75±0.83 | 0.92±0.30 |
α=0.05; a, b, c=Groups; Nine isolates were excluded since they were not clustered into the AFLP groups.
Sequencing of the S. globosa CAL gene
High-quality DNA (OD260/OD280 ratio values of 1.8–2.0) was extracted from all isolates, and the CAL gene was successfully amplified in all cases. The resulting ~770 bp amplicons were sequenced and subjected to BLAST analysis against the GenBank database. All sequences showed 99%–100% nucleotide sequence identity to CAL from S. globosa type strain CBS 120340, confirming that all isolates were S. globosa. The sequences generated in this study have been deposited in GenBank under the accession numbers shown in Table 1.
AFLP profile and correlation analysis
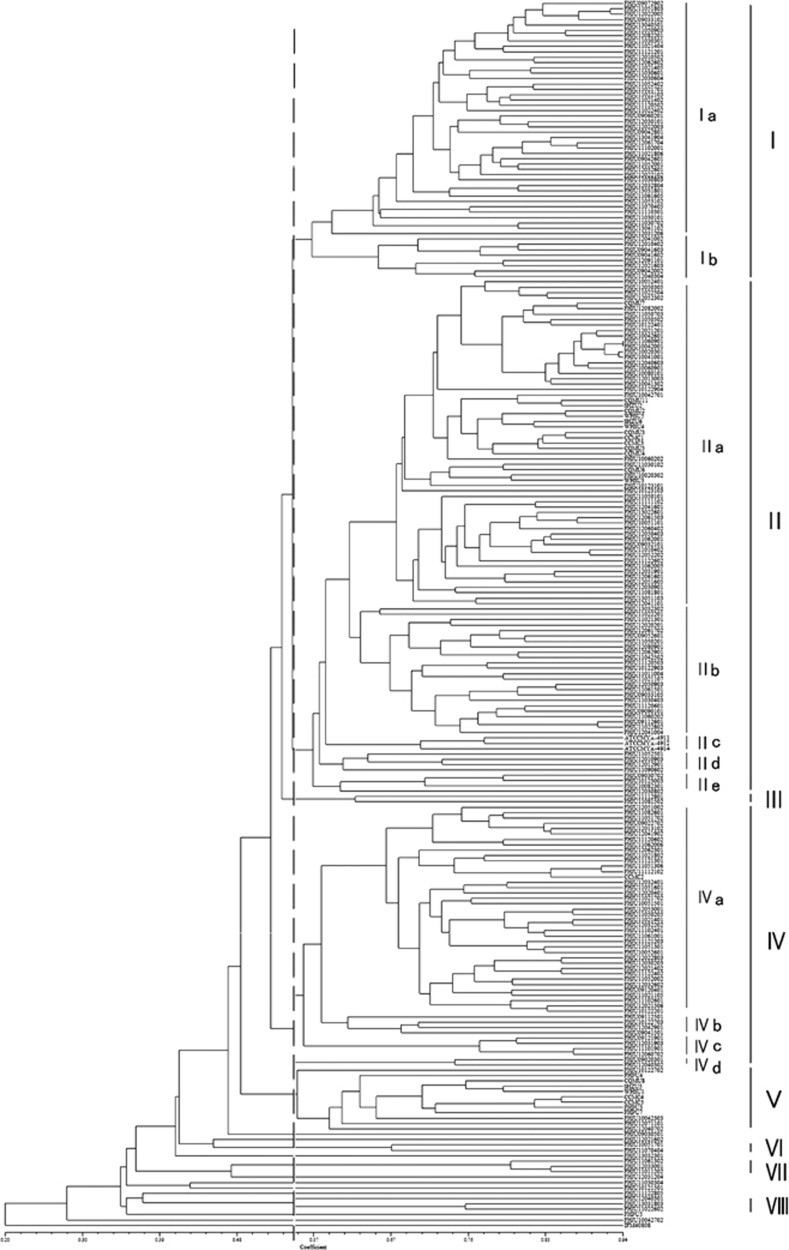
The AFLP profiles of the 225 S. globosa isolates and four reference strains are shown in Figure 2. A total of eight main clustering groups (designated I–VIII) were identified at a cophenetic correlation coefficient of 0.55. Nine isolates (FHJU11122805, FHJU12021602, FHJU13032301, FHJU12031204, FHJU11030304, FHJU10121501, FHJU10042702, FHJU09030501 and FHPU5) failed to form clusters and were well separated from the eight main clustering groups. Groups I, II, and IV could each be divided into a further two or five subgroups (Figure 2). The AFLP profile similarity levels among these 229 isolates ranged from 0.20 to 0.94. Group II was the most prevalent group, accounting for 42% of all isolates, followed by group I (23%) and group IV (21%). Three Sporothrix globosa reference isolates were clustered into Group II, and the A. fumigatus control isolate deviated from all S. globosa isolates. All the remaining groups were much less prevalent (no more than 5%).
Figure 2.
Clustering dendrogram of the 225 Sporothrix globosa isolates based on amplified fragment length polymorphism profiles generated using the unweighted pair-group method with arithmetic mean and the Dice coefficient. Eight major groups (designated I–VIII) were obtained at a coefficient of 0.55.
The majority of the isolates involved in this study originated from nine different regions within Jilin Province (n=196; 86%). As shown in Table 3, isolates from Changchun (n=60) belonged to AFLP groups II (35/60) and IV (25/60). The Siping isolates mainly belonged to group II (23/31), while the Baicheng isolates (n=12) and most of the isolates from Jilin City (18/19) were clustered together into group I. Groups III and VIII consisted entirely of isolates from Songyuan. A significant association was found between AFLP profiles and geographic origins within Jilin Province (χ2-test, P=0.000, Table 3).
Table 3. Distribution of Sporothrix globosa AFLP genotypes among different geographic origins in China.
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ia | Ib | IIa | IIb | IId | IIe | IVa | IVb | IVc | IVd | |||||||
| Jilin Province | ||||||||||||||||
| Changchun | 27 | 2 | 2 | 4 | 17 | 4 | 4 | 60 | ||||||||
| Songyuan | 17 | 1 | 5 | 2 | 2 | 10 | 3 | 40 | ||||||||
| Siping | 5 | 18 | 4 | 1 | 1 | 2 | 31 | |||||||||
| Jilin | 11 | 7 | 1 | 19 | ||||||||||||
| Baicheng | 12 | 12 | ||||||||||||||
| Liaoyuan | 2 | 2 | 4 | 1 | 1 | 1 | 11 | |||||||||
| Tonghua | 5 | 3 | 8 | |||||||||||||
| Baishan | 2 | 1 | 3 | |||||||||||||
| Yanbian | 1 | 2 | 1 | 4 | ||||||||||||
| Heilongjiang | 1 | 1 | 2 | |||||||||||||
| Neimenggu | 1 | 2 | 3 | |||||||||||||
| Beijing | 3 | 3 | ||||||||||||||
| Jiangsu | 2 | 1 | 2 | 5 | ||||||||||||
| Sichuan | 3 | 1 | 4 | |||||||||||||
| Chongqing | 7 | 1 | 8 | |||||||||||||
| Guangdong | 2 | 1 | 3 | |||||||||||||
| Total | 44 | 8 | 61 | 24 | 4 | 4 | 2 | 39 | 4 | 4 | 2 | 12 | 2 | 3 | 3 | 216 |
| 52 | 93 | 49 | ||||||||||||||
The remaining 28 isolates originated from seven other provinces or municipalities, and most (25/28, 89%) clustered into groups IIa and V. When comparing isolates from northern China (including Jilin, Heilongjiang, Neimenggu and Beijing) and southern China (including Jiangsu, Sichuan, Chongqing and Guangdong), the isolates from northern China primarily clustered in groups I, II and IV (52/196, 26.5% 79/196, 40.3% and 48/196, 24.5%, respectively), while the isolates from southern China mostly clustered in groups IIa, IVa and V (14/20, 70% 1/20, 5% and 5/20, 25%, respectively). Statistical analysis showed a significant difference in the distribution of the AFLP genotypes between northern and southern China (χ2-test, P=0.000, Table 3).
We attempted to correlate the AFLP profiles with the clinical forms of sporotrichosis (Table 4) but observed no significant correlation (P=0.251). In addition, Fisher’s exact test showed no significant association between the AFLP profiles and the sex (P=0.159) or age (P=0.565) of the patients or the sampling dates (P=0.052).
Table 4. Relationship between AFLP genotypes and different clinical forms.
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical formsa | |||||||||
| F | 26 | 46 | 2 | 31 | 4 | 1 | 2 | 112 | |
| L | 22 | 31 | 16 | 1 | 3 | 1 | 74 | ||
| D | 4 | 1 | 1 | 6 | |||||
| E | 1 | 1 | |||||||
F—fixed cutaneous; L—lymphocutaneous; D—disseminated cutaneous; E—extracutaneous.
Antifungal susceptibility testing
Antifungal susceptibility testing results are presented in Table 5. Of the eight drugs tested, TRB showed the strongest anti- S. globosa activity, with MIC values ranging from 0.03 to 8 μg/mL (geometric mean, 0.05 μg/mL), followed by POS, which produced MIC values ranging from 0.5 to >16 μg/mL (geometric mean, 2.99 μg/mL). Moderate anti- S. globosa activity was observed for CAS, ALB and ICZ, with MIC values ranging from 0.25 to >16 μg/mL, 4 to 16 μg/mL, and 1 to >16 μg/mL, respectively. FCZ, VCZ and AMB showed poor activity against S. globosa.
Table 5. Susceptibility testing results in μg/mL of Sporothrix globosa isolates.
| Group by AFLP | MIC | FCZ | ICZ | VCZ | TRB | AMB | POS | CAS | ALB |
|---|---|---|---|---|---|---|---|---|---|
| I (n=10) | Range | 64–>64 | 2–>16 | 8–>16 | 0.03–8 | >16 | 0.5–>16 | 0.5–>16 | 4–16 |
| GM | >64 | 12.12 | >16 | 0.06 | >16 | 3.03 | 6.06 | 7.46 | |
| II (n=10) | Range | >64 | 2–>16 | 8–>16 | 0.03–0.06 | >16 | 1–>16 | 8–>16 | 4–16 |
| GM | >64 | 9.19 | >16 | 0.03 | >16 | 2.14 | >16 | 8 | |
| III (n=2) | Range | >64 | 8–>16 | 16–>16 | 0.03 | >16 | 1–4 | 0.25–16 | 4–16 |
| GM | — | — | — | — | — | — | — | — | |
| IV (n=9) | Range | >64 | 2–>16 | >16 | 0.03–0.5 | >16 | 1–16 | 8–>16 | 4–16 |
| GM | >64 | 12.70 | >16 | 0.07 | >16 | 2 | >16 | 8.64 | |
| V (n=4) | Range | >64 | 2–>16 | 16–>16 | 0.03–0.06 | >16 | 1–>16 | 1–16 | 8–16 |
| GM | >64 | 9.51 | >16 | 0.04 | >16 | 6.72 | 4.76 | 11.31 | |
| VI (n=2) | Range | >64 | >16 | >16 | 0.03–0.25 | >16 | >16 | 4–>16 | 8–16 |
| GM | — | — | — | — | — | — | — | — | |
| VII (n=3) | Range | >64 | 16–>16 | 16–>16 | 0.03–0.06 | >16 | 1–>16 | 1–16 | 8–16 |
| GM | >64 | >16 | >16 | 0.04 | >16 | 4 | 2.52 | 10.08 | |
| VIII (n=3) | Range | >64 | 1–16 | 16–>16 | 0.03 | >16 | 1–8 | 1–>16 | 4–8 |
| GM | >64 | 6.35 | >16 | 0.03 | >16 | 2 | 8 | 5.04 | |
| Total (n=43) | Range | >64 | 1–>16 | 8–>16 | 0.03–8 | >16 | 0.5–>16 | 0.25–>16 | 4–16 |
| GM | >64 | 11.78 | >16 | 0.05 | >16 | 2.99 | 9.55 | 8.26 |
Abbreviations: amplified fragment length polymorphism, AFLP; albaconazole, ALB; amphotericin B, AMB; caspofungin, CAS; fluconazole, FCZ; genometric mean, GM; itraconazole, ICZ; minimum inhibitory concentrations, MIC; posaconazole, POS; terbinafine, TRB.
Statistical analysis showed that the observed MIC values were not associated with the AFLP genotypes, the origins of the isolates, or the clinical manifestations of the infection (ANOVA and Dunnett’s T3 Test, P>0.05, Table 5).
DISCUSSION
In the present study, we examined the growth characteristics of 225 S. globosa isolates from sporotrichosis patients originating from eight provinces or municipalities in China. Using AFLP analysis, we categorized the isolates into eight distinct clustering groups. We also examined whether there was any correlation between the AFLP profiles and the in vitro growth characteristics, antifungal susceptibility, geographic origins and clinical forms of sporotrichosis.
AFLP analysis of the 225 S. globosa isolates in the current study showed that the AFLP genotypes had certain associations with the geographical origins of the isolates, especially those from Jilin Province. In particular, the isolates from Baicheng and Jilin City mostly clustered into group I, while isolates from Siping mainly clustered in group II. Isolates from Changchun were clustered into two groups: those from the central area clustered into group II, and isolates from areas near the border were identified as group IV. However, isolates from other regions, such as Songyuan and Liaoyuan, showed a great variety of genotypes. The reason for this genetic variation is unclear, but we suspect that it may be related to the frequent migration of the people in these regions. In addition, in contrast to the isolates from northern China, which were primarily clustered in groups I, II and IV, the isolates from southern China mainly clustered in groups IIa, V and IVa. While further studies are needed using a larger number of samples (especially from southern China), this observation agrees with Zhang et al.,15 who found a significant difference in the restriction fragment length polymorphism and Southern blotting band patterns of S. globosa isolates from southern and northern China. In contrast, Zhang et al.17 recently reported an AFLP analysis of 20 S. globosa isolates from wide geographic origins, wherein all of the isolates showed an identical AFLP pattern. The reasons for these discrepancies may include different experimental conditions or differences in the sample sizes. Different restriction endonucleases and different numbers of selective bases and primer pairs will influence the observed genetic diversity. For example, Neyra et al.16 used the restriction endonucleases EcoRI and MseI, along with a combination of six primers, in their AFLP analysis of Peruvian strains of S. schenckii, identifying two stable populations. Zhang et al.17 used the same restriction enzymes but only one selective primer pair to divide 122 strains of Sporothrix into 13 groups. In the present study, we chose the same restriction endonucleases and one pair of primers (out of 64 primer pairs screened) and achieved reproducible results with a relatively high number of polymorphic bands.
In the current study, the AFLP genotypes appeared to be differentially distributed among different years. For example, AFLP group I accounted for the largest proportion of isolates in 2009 (8/20, 40%) and 2013 (4/8, 50%) but was absent in 2010, while group II was dominant in 2010 (18/25, 72%) but decreased in prevalence in 2011 (27/77, 35.1%) and 2012 (25/63, 39.7%). However, these differences did not reach statistical significance. Further studies are needed to confirm this observation using a larger number of isolates over a longer period of time.
All of the S. globosa isolates in the current study grew well at both 30 °C and 35 °C, with 97% (218/225) of isolates showing some growth at 37 °C, although the average growth rate and colony size were decreased compared with those at the lower temperatures (Table 2). Only seven isolates could not grow at 37 °C. These observations are consistent with the report of Yu et al.10 but contradict the report of Marimon et al.,18 who found that S. globosa did not grow at 37 °C, with the exception of four strains that produced colonies of 2 mm in diameter. There have been conflicting reports regarding the relationship between temperature sensitivity and clinical forms of Sporothrix infection. Kwon-Chung24 reported that isolates causing lymphocutaneous and extracutaneous sporotrichosis grew well at both 35 °C and 37 °C, whereas isolates causing fixed cutaneous sporotrichosis grew well at 35 °C but failed to grow at 37 °C. However, Yu et al.10 found that isolates obtained from the three cutaneous forms of sporotrichosis were able to grow at 37 °C. In our study, all but seven of the 225 isolates showed growth at 37 °C. The seven isolates that did not grow included four isolates associated with lymphocutaneous sporotrichosis, two from fixed cutaneous sporotrichosis, and one (CCMC1) from an undefined form of sporotrichosis. We found no significant association between the clinical form of the disease and the AFLP genotype or growth rate of S. globosa. Hence, the clinical manifestation of sporotrichosis is most likely related to the immune status of the patient rather than the thermotolerance or AFLP genotype of the causative S. globosa strain.
In the present study, we examined the drug susceptibilities of 43 S. globosa isolates representing each of the AFLP genotypes and did not detect any significant association between the in vitro antifungal drug susceptibility and the AFLP genotype. Nevertheless, our data indicated that all S. globosa isolates are highly sensitive to TRB, consistent with previous studies.25, 26 Although only two of the examined isolates belonged to group VI, both were more sensitive to TRB than to any of the other drugs, including ICZ. Ten isolates (23%) showed resistance to POS, with MIC values of ⩾16 μg/mL. Moreover, AMB showed poor activity against all isolates, which is in contrast to previous studies.12, 25 These findings suggest a need to determine the antifungal susceptibility of S. globosa isolates in China on a larger scale to optimize the treatment of sporotrichosis.
In summary, the current AFLP analysis revealed significant genetic diversity among S. globosa isolates in China. The AFLP profiles of the isolates are associated with their geographic origins, but not with other phenotypic properties of the isolates. This study suggests that AFLP analysis is a potentially useful tool for studying the epidemiology of S. globosa. Further studies using a larger number of S. globosa isolates from patients from wider geographic origins and suffering from more diverse well-defined clinical forms of sporotrichosis are required to better understand the implications of the high degree of AFLP variation in the epidemiology of sporotrichosis.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Projects NO 81573060) and the Science and Technology Project Foundation of Jilin Province (Projects NO 20160101047JC and 20150520039JH). We thank Weida Liu (Department of Mycology, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, 210042), Ruoyu Li (Department of Dermatology, Peking University First Hospital, 100034), Yuping Ran (Department of Dermatovenereology, West China Hospital of Sichuan University, 610041), Liyan Xi (Department of Dermatology and Venerology, The Second Affiliated Hospital of Zhongshan University, 510120), and Xun Zhou (Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, 400016) for supplying the clinical strains used in this study. We also thank Tamsin Sheen, PhD, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript.
References
- Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 2011; 24: 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the Sporotricha. Bull Johns Hopkins Hosp 1898; 9: 286–290. [Google Scholar]
- de Beer ZW, Duong TA, Wingfield MJ. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 2016; 83: 165–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis 2015; 9: e0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid H, Cano J, Gené J et al. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev Iberoam Micol 2009; 26: 218–222. [DOI] [PubMed] [Google Scholar]
- Oliveira MM, Maifrede SB, Ribeiro MA et al. Molecular identification of Sporothrix species involved in the first familial outbreak of sporotrichosis in the state of Espírito Santo, southeastern Brazil. Mem Inst Oswaldo Cruz 2013; 108: 936–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Liu HS, Zheng H et al. [Clinical analysis of 585 cases of cutaneous sporotrichosis.] Chin J Dermatol 2011; 44: 161–164. Chinese. [Google Scholar]
- Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC et al. Global epidemiology of sporotrichosis. Med Mycol 2015; 53: 3–14. [DOI] [PubMed] [Google Scholar]
- Tan JW, Liu W, Wan Z et al. [Reclassification of 33 clinical strains of Sporothrix from northern China based on phenotypic and molecular characters.] Mycosystema 2013; 32: 161–167. Chinese. [Google Scholar]
- Yu X, Wan Z, Zhang Z et al. Phenotypic and molecular identification of Sporothrix isolates of clinical origin in Northeast China. Mycopathologia 2013; 176: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Zhang K, Zhou X. Molecular identification of Sporothrix clinical isolates in China. J Zhejiang Univ Sci B 2014; 15: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MD, Zhou X, Liu TT et al. Morphological and physiological comparison of taxa comprising the Sporothrix schenckii complex. J Zhejiang Univ Sci B 2015; 16: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian CH, Jin LJ, Liu XM et al. [Characterization of Sporothrix schenckii by random amplification of polymorphic DNA assay.] Chin J Dermatol 2001; 34: 355–357. Chinese. [PubMed] [Google Scholar]
- Kong X, Xiao T, Lin J et al. Relationships among genotypes, virulence and clinical forms of Sporothrix schenckii infection. Clin Microbiol Infect 2006; 12: 1077–1081. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Yang G et al. Genotyping of Sporothrix schenckii by analysis of ribosomal DNA regions. Mycoses 2006; 49: 305–310. [DOI] [PubMed] [Google Scholar]
- Neyra E, Fonteyne PA, Swinne D et al. Epidemiology of human sporotrichosis investigated by amplified fragment length polymorphism. J Clin Microbiol 2005; 43: 1348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hagen F, Stielow B et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 2015; 35: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimon R, Cano J, Gené J et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 2007; 45: 3198–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschapalda K, Streitner N, Voss C et al. Generation of chromosomal DNA during alkaline lysis and removal by reverse micellar extraction. Appl Microbiol Biotechnol 2009; 84: 199–204. [DOI] [PubMed] [Google Scholar]
- Du YX, Sha W, Zhang MJ. [Using Alkaline Lysis Method for Recombine Plasmid DNA Extraction and PCR Verification.] Biotechnology 2009; 19: 35–37. Chinese. [Google Scholar]
- O’Donnell K, Nirenberg H, Aoki T et al. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 2000; 41: 61–78. [Google Scholar]
- Vos P, Hogers R, Bleeker M et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 1995; 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards InstituteReference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, Document M38-A2. Wayne, PA, USA: CLSI, 2008. Available at http://shop.clsi.org/site/Sample_pdf/M38A2_sample.pdf. [Google Scholar]
- Kwon-Chung KJ. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J Infect Dis 1979; 139: 424–431. [DOI] [PubMed] [Google Scholar]
- Marimon R, Serena C, Gené J et al. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother 2008; 52: 732–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JW, Liu W, Liu WX et al. [In vitro interactions of itraconazole with caspofungin or terbinafine against Sporothrixglobosa.] Chin J Mycol 2013; 8: 20–25. Chinese. [Google Scholar]