Abstract
This review summarizes recent literature regarding corneal imaging in human subjects using in vivo confocal microscopy and corneal immune cells, nerves, and tear cytokine levels in ocular surface diseases as well as corneal immune privilege. The significance of interactions between corneal immune cells and nerves in health, neurotrophic keratopathy, and infectious keratitis are discussed. Furthermore, bilateral alterations of immune cells and nerves in clinically unilateral corneal diseases and the link to changes of tear cytokines or neuropeptide levels in contralateral eyes are described. Recent studies reported increased density and morphologic changes of corneal dendritic cells in ocular surface disease that correlated with a decrease in sub-basal nerve corneal nerves, suggesting potential interactions between the immune and nervous systems in the cornea. Although the relevance of tear cytokines is poorly understood, tear cytokines might have an important role in the pathogenesis of ocular surface diseases. In humans and experimental animal models, alterations in immune cells, cytokines and immunomodulatory neuropeptide levels in contralateral eyes might mediate the incidence of bilateral infectious keratitis and loss of immune privilege of the cornea in bilateral corneal transplantation or neurotrophic keratopathy cases. The discovery of bilateral alterations of immune cells and nerves in ocular surface diseases is considered the missing link between the immune and nervous systems in the cornea, and demonstrates how studies of animal models and human patients aid our understanding of human corneal disease phenomena.
Keywords: cornea, dendritic cell, nerve, tear cytokine, in vivo imaging
INTRODUCTION
The cornea is the most innervated tissue in the human body with a nerve density of 300 to 600 times that of the skin.1, 2 The corneal nerves are supplied by ciliary nerves from branches of the ophthalmic division of the trigeminal nerves. Corneal nerves penetrate the peripheral corneal stroma and form the sub-basal nerve plexus between Bowman’s layer and the basal epithelium in a radial distribution pattern.2 Corneal innervation regulates corneal sensation, provides protective and trophic functions and promotes epithelial integrity, proliferation and wound healing.3, 4 Intact innervation is necessary for the maintenance of corneal structure and function.5, 6
Many corneal neurological diseases may result in neurotrophic keratopathy (NTK), albeit with various degrees of severity. These include, but are not limited to ocular infections,7–9 herpetic eye disease,10, 11 dry eye syndrome,12, 13 corneal transplantation,14, 15 diabetes,16 and intracranial lesions17. NTK caused by these diseases manifests as dry eye, impaired blink reflex, persistent corneal epithelial defects, inflammation, corneal melting and potential corneal perforation, possibly leading to permanent vision loss or blindness.18, 19 Furthermore, although corneal perforation or corneal scarring as a result of NTK may require corneal transplantation, these transplants often have difficulty with epithelial wound healing and have a very high rate of graft rejection.20 Several recent studies using animal models have shown an association between innervation, corneal inflammation21 and corneal stem cell homeostasis.22 Furthermore, recent studies on humans suggest an interaction between inflammation and denervation.7, 23
Until recently, it has been a widely accepted dogma that immune cells are absent in the central cornea; a putative lack of passenger leukocytes has been cited as a critical facet of the immune privilege of the cornea.24, 25 However, this paradigm was revised when Hamrah and Liu et al demonstrated that the cornea is endowed with immature resident dendritic cells (DCs) that lack the expression of major histocompatibility complex (MHC) class II, and which undergo maturation after inflammation or transplantation and then migrate into draining lymph nodes.26–28 DCs are potent antigen-presenting cells and mediate both innate and adaptive immune responses by stimulating T cells. In uninflamed conditions, MHC class II-negative DCs are present in the epithelium and anterior stroma of the central cornea, whereas MHC class II-positive DCs infiltrate the whole cornea during inflammation.28 Given their strategic location in the corneal epithelium and anterior stroma, they may be ready to respond to invading pathogens in the cornea and ocular surface.
The important role of immune cells in the cornea has been well-delineated in human clinical reports under normal and pathological conditions, including graft rejection after penetrating keratoplasty, and infectious and non-infectious keratitis;29–31 however, immune cells have not been directly observed in patients in vivo. Currently, slit-lamp microscopy examination is the gold standard for the detection and evaluation of abnormal findings of the cornea, such as corneal infiltration, edema, scarring, and opacity. Recently, in vivo confocal microscopy (IVCM) has greatly advanced the microscopic evaluation of ocular structures. The use of this noninvasive in vivo imaging technique provides a resolution of images comparable to that using ex vivo histochemical methods. IVCM allows the systematic study of corneal epithelial, stroma and endothelial cells and enables the quantification of nerve morphology and density, as well as the study of immune cells such as corneal epithelial DCs in human subjects.
Alterations of corneal DCs have been reported in patients with dry eye,32, 33 infectious keratitis,7 anterior uveitis,34 peripheral ulcerative keratitis35 and pterygium.36 Regarding the corneal nerve alteration in human subjects, specific changes in corneal sub-basal nerve density and morphology after infectious keratitis,7, 23 keratoconus,37, 38 corneal surgery,14, 39, 40 dry eye41, 42 and diabetes43 have been increasingly studied. Moreover, investigation of corneal DC and nerves using IVCM has clinical relevance because it provides valuable information to support clinical diagnosis, disease severity and treatment responses.11, 34, 41 However, despite many reports of corneal immune cells and nerves, there have been few reports using IVCM to study the correlation between the immune and nervous systems in the cornea. Interestingly, some studies reported bilateral findings in corneal DCs and nerves in unilateral corneal disease,11, 23, 37, 44 although the magnitudes of the alterations were minimal in the contralateral eye.
In this review, we highlight the connection between inflammation and the nervous system in the cornea and bilateral alterations and corneal cellular changes detected by IVCM, focusing on the clinical relevance of these findings. We also focus on the bilateral biochemical changes in the tear cytokines, which might mediate corneal inflammation and nerve reductions in bilateral corneas in unilateral corneal disease.
CONNECTION BETWEEN THE NERVOUS AND IMMUNE SYSTEMS IN THE CORNEA
Recent studies have revealed that the peripheral nervous system regulates innate immune reactions against pathogens via hormonal and neuronal routes.45 Furthermore, dysfunction of the peripheral nervous system may result in proinflammatory immunological responses, termed “neurogenic inflammation.”46–49 In addition, the motility and migration of immune cells were shown to be influenced by innervation of peripheral tissues such as the skin, lung and gut.50–52 Adrenergic sympathetic nerves regulate the recruitment of leukocyte to and within tissues.53, 54. Damage to the peripheral nerves can result in inflammatory diseases such as atopic dermatitis, colitis, collagen-induced arthritis and others, which can be suppressed by neuropeptides and neurotransmitters.55–57 Thus, the peripheral nervous system is thought to regulate the activation, deployment and homeostasis of the immune system and the priming of adaptive immunity.58
Regarding corneal nerve damage induced by various pathologies such as herpetic keratitis and diabetes, NTK is sometimes a vision-threatening condition, because there can be refractory inflammation, stromal melting and corneal perforation when NTK is complicated with inflammatory necrotizing stromal keratitis. Although several treatment options are available including transplantation of the amniotic membrane, tarsorrhaphy and various eye drops, the management of NTK is extremely challenging because of the lack of monitoring tools and definitive treatments for advanced stage disease.20 Although the mechanism of necrotizing stromal keratitis has remained elusive to date, cellular infiltrates and stromal inflammation in NTK consist of macrophages, Langerhans cells (LCs), lymphocytes, polymorphonuclear cells and plasma cells.20 To clinically manage NTK properly in daily clinics, an understanding of how refractory inflammation develops in severe types of NTK is crucial. Recently, the bidirectional interaction between the nervous and immune systems in the cornea was proposed (neurogenic inflammation) in other tissues.46–49 Cruzat et al evaluated the density of DCs and corneal nerves in eyes with infectious keratitis including bacterial, fungal and Acanthamoeba keratitis using IVCM.7 They found a concomitant increase of DC density and severe decrease of corneal nerve density, and reduced DC infiltration was observed in eyes with moderate corneal nerve loss. They concluded that an increased density of DC correlated with decreased corneal nerve density, suggesting a potential interaction between the immune and nervous systems in infectious keratitis. In humans, the corneal nerve density decreases after herpes simplex keratitis (HSK).11 Shtein et al reported that 84% of patients with clinically quiet HSK without apparent corneal inflammation for more than 6 months had histopathologically visible inflammation and a high rejection rate of 43.5%.29 This suggested a loss of immune privilege caused by HSK infection.59 In an experimental model of HSK, Hu et al reported that HSK leads to decreased nerve density and that corneal DCs play a pivotal role in local corneal defense against viral keratitis.60
BILATERAL ALTERATIONS OF NERVOUS AND IMMUNE SYSTEMS IN UNILATERAL CORNEAL DISEASES
Hamrah et al first reported the bilateral alteration of the corneal nerve density in eyes with unilateral corneal disease.11 They evaluated the corneal sensation and sub-basal nerve alteration in eyes with HSK using IVCM and found a diminishment of the sub-basal nerve plexus in eyes with HSK and a significant decrease in the contralateral unaffected eyes of patients, compared with healthy controls. They also demonstrated that reduced nerve density was correlated with the loss of corneal sensation in eyes with HSK. After their article on HSK,11 they reported contralateral nerve alterations in other corneal pathologies such as herpes zoster ophthalmicus, bacterial keratitis and in animal models.44, 61, 62 Cruzat et al evaluated the bilateral corneal nerve and DC density in eyes with unilateral bacterial keratitis and found a bilateral reduction of corneal nerve density and increase of DC density.44 Furthermore, they demonstrated a strong correlation between the reduction in corneal nerves and increase in DCs in both eyes of patients with unilateral bacterial keratitis, suggesting that a connection between the immune and nervous systems caused the contralateral responses. Recently, Postole et al evaluated DCs in various types of anterior uveitis (herpetic uveitis, juvenile idiopathic uveitis, and Fuchs uveitis syndrome) and reported increased corneal DC density in the contralateral eyes of cases with herpetic uveitis and juvenile idiopathic uveitis.34
Contralateral sub-basal nerve reduction might theoretically reflect asymmetric bilateral disease without clinically evident manifestations. Contralateral alterations after unilateral diseases or in experimental models have been previously reported.11, 23, 34, 61, 63, 64 Keijer et al and Simard-Lebrum et al evaluated bilateral tear production in patients with unilateral HSK and found no difference between the tear secretion of affected and unaffected eyes in these patients, although both eyes demonstrated significantly lower secretion rates than normal subjects.63, 64 Furthermore, in a capsaicin-induced neurogenic inflammation model, aqueous humor protein levels were altered at 30 minutes after treatment, but both treated and contralateral eyes showed similar levels at 5 hours after treatment.65
The cornea is an immune privileged tissue in the body and Streilein et al reported that the ocular immune privilege was under neural control.66 Aqueous humor contains immunosuppressive neuropeptides. When corneal nerves are severed, the tissues surrounding the anterior chamber cease secreting these immunosuppressive factors and anterior chamber-associated immune deviation (ACAID) fails until the nerves regrow.66 In the clinical setting, denervation of grafted cornea is well recognized, and it takes years for sensation to be restored in healthy grafts. In animal experiments, ACAID was not observed until 12 weeks after corneal transplantation, when the corneal nerves regenerate into the graft.66 Even though the exact mechanisms involved in the contralateral changes of the corneal nerve are unclear, bilateral alterations of nerves are likely to be important because there are many patients who require bilateral corneal transplantation. Paunicka et al reported the presence of bilateral sympathetic effects of unilateral nerve damage on the loss of immune privilege,67 and further reported that a circular corneal incision in contralateral eyes induced a marked upregulation of neuropeptides such as substance P, calcitonin gene-related peptide and vasoactive intestinal peptide,67 suggesting the sympathetic regulation of neuropeptides in the cornea. In experimental models, axotomy of the ciliary nerve, a branch of trigeminal nerves, caused an immediate decrease in sub-basal nerve density in the center of the contralateral cornea.61 Corneal nerve damage in the contralateral eyes abolished the immune privilege of corneal allografts in the ipsilateral eyes via neuropeptides, which might explain the increased incidence of corneal allograft rejection in hosts receiving corneal transplantation in both eyes.67, 68
BILATERAL ALTERATION IN TEAR CYTOKINES
Recent studies have suggested that proinflammatory cytokines in tears may play a key role in the pathogenesis of several corneal diseases, including dry eye disease,69 keratoconus,70, 71 graft versus host disease (GVHD)72 and conjunctivitis,73 as well as in the development of corneal neovascularization.74 Although Jun et al reported an imbalance of pro- and anti-inflammatory cytokines in patients with keratoconus,17 Lema et al reported an increased expression of interleukin (IL)-6, tumor necrosis factor-α and matrix metalloproteinase-9 that were associated with the severity of keratoconus.71 In addition, Riemens et al reported increased IL-6 and interferon-γ in patients with GVHD.72 Furthermore, Zakaria et al reported elevated IL-6, IL-8, and vascular endothelial growth factor levels in eyes with corneal neovascularization, but no correlation was found between the cytokine levels and the severity of neovascularization.74 Villani et al evaluated tear cytokine levels and IVCM data in patients with rheumatoid arthritis and reported both a decrease in tear IL-1 and IL-6 levels, as well as DC density after systemic therapy.75 We hypothesized that the tear cytokine levels would alter bilaterally in eyes with unilateral bacterial keratitis, by assessing the correlation between the tear cytokine levels and microscopic cellular changes of the nervous and immune systems in the cornea detected by IVCM.23
Regarding the tear cytokine levels, IL-1β, IL-6, and IL-8 were only significantly elevated in affected eyes, compared with healthy controls, and CCL-2, IL-10 and IL-17A were only elevated in contralateral eyes, compared with controls. Triggering of receptors expressed on myeloid cells (TREM)-1 and the density of DCs were significantly elevated in both affected and contralateral unaffected eyes, compared with controls (Figure 1).23 IL-1β, IL-6 and IL-8 were significantly correlated with DC density and nerve density. Thus, we demonstrated that increased proinflammatory tear cytokines correlated with increased corneal DC density and size.23
FIGURE 1. Bilateral alterations in nervous and immune systems in unilateral corneal disease.
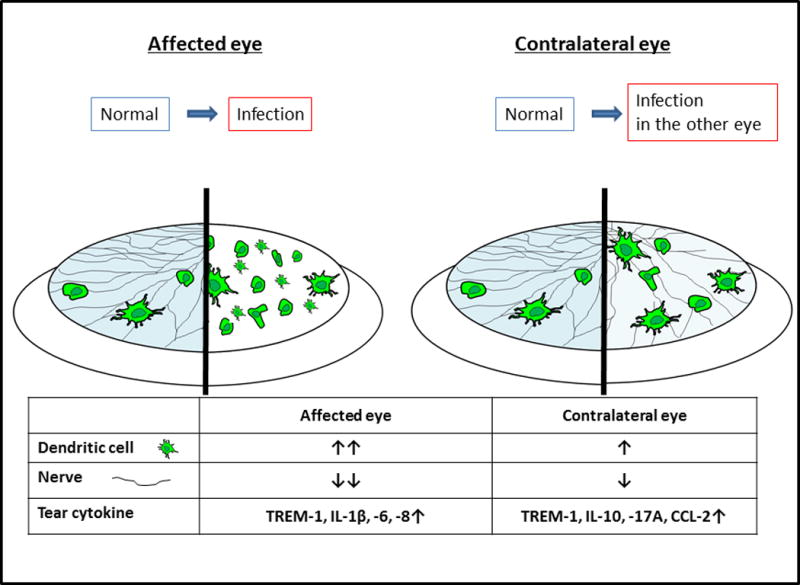
(Left) affected eye, (Right) contralateral (unaffected) eye. IL-1β, IL-6, and IL-8 were significantly elevated above healthy controls in affected eyes, but not in contralateral eyes. CCL-2, IL-10 and IL-17A were elevated in contralateral eyes, but not in affected eyes. Significantly elevated TREM-1 levels were observed in both eyes.
TREM-1, triggering of receptors expressed on myeloid cells
The tear concentrations of IL-1β, IL-6, and IL-8 increased only in affected eyes with bacterial keratitis, but not in contralateral clinically unaffected eyes. IL-1β production, which increased in our study, induced the production of additional proinflammatory mediators, such as IL-6, IL-8, fibroblast growth factor-2, prostaglandin E2 and cyclooxgenase-2.76–78 Furthermore, IL-1β enhanced host defense against infections by upregulating the antimicrobial function of macrophages and initiating T helper (Th) 1 and Th17 adaptive immune responses.79 IL-6, which is also elevated in the tears of patients with unilateral bacterial keratitis, is a major proinflammatory cytokine in infectious keratitis,80, 81 epithelial wound healing in the skin and cornea,82–85 and corneal transplantation models.86, 87 In response to inflammation, IL-6 stimulates the maturation and trafficking of DCs, promotes IL-2 production by T cells, the differentiation of B cells and mediates immune responses against pathogens.88, 89 Both IL-1β and IL-6 are important mediators of fever induced by lipopolysaccharide from gram-negative bacteria90 and are produced by epithelial cells.91 IL-8 is also an important inflammatory mediator during infection,92 and is increased in corneal epithelial cells in response to lipopolysaccharide stimulation.93 Santacruz et al reported that IL-1β, IL-6 and IL-8 were elevated in tears from eyes with infectious keratitis, compared with those in the control contralateral eyes.94
While the changes in tear proinflammatory cytokines and corneal DC density can be explained by infection-induced inflammation in affected eyes, alteration of cytokine concentrations in the contralateral clinically unaffected eyes is intriguing. Interestingly, IL-17A was only significantly elevated in the contralateral eyes, but not the affected eyes with bacterial keratitis when compared with controls. IL-17A produced by γδ T cells regulates prophylactic host defense against infection via Th17 cell responses, which improves the mucocutaneous barrier function.51, 95 IL-17 also stimulates the release of antimicrobial peptides and chemokines for neutrophil recruitment.79 Recently, a population of neutrophils was shown to produce IL-17A in response to IL-6 stimulation in an autocrine manner to activate fibroblasts and epithelial cells to produce chemokines and proinflammatory cytokines, leading to enhanced reactive oxygen species and antifungal activity.96 Clinically, the incidence of bilateral corneal infection is relatively rare, ranging from 1–3%.97, 98 It is tempting to speculate that the elevation of IL-17A in contralateral eyes in this study may potentially be due to prophylactic defense mechanisms in these eyes to prevent infection in the fellow eye.
In our previous study on unilateral bacterial keratitis, TREM-1 was elevated in both eyes.99 TREM-1 is expressed at high levels on macrophages and monocytes, to amplify inflammation in tissues infected by bacteria or fungi. TREM-1 upregulates the production of proinflammatory cytokines, and stimulates rapid neutrophil degranulation and oxidative burst.99 In contrast, TREM-2 regulates DCs, microglia and osteoclasts during inflammation in the central nervous system and in rheumatoid arthritis. TREM-1 is not upregulated in noninfectious diseases.99 In an experimental model of infectious keratitis, TREM-1 was upregulated by Pseudomonas aeruginosa or lipopolysaccharides.100 Although there were no correlations between TREM-1 levels and DC parameters in our previous study,23 the TREM-1 concentration was inversely correlated with corneal nerve density. The bilateral alteration of bilateral DCs and corneal nerves are mediated by tear cytokine levels.
CONCLUSIONS
In vivo confocal microscopy allows corneal cellular imaging in corneas from patients with various ocular surface diseases, such as NTK, dry eye, infectious keratitis and those that have undergone corneal transplantation. Important clinical studies have reported novel findings regarding the interactions between the nervous and immune systems and a connection between tear cytokines and corneal DCs. These interactions and connections should help increase our understanding of the mechanisms involved in corneal homeostasis and the pathophysiology of corneal diseases.
Acknowledgments
Source of Funding: Part of this work was supported by grants from the Uehara Memorial Foundation and the Ohyama Health Foundation.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rozsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 2.Muller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Gallar J, Pozo MA, Rebollo I, et al. Effects of capsaicin on corneal wound healing. Invest Ophthalmol Vis Sci. 1990;31:1968–1974. [PubMed] [Google Scholar]
- 7.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurbanyan K, Hoesl LM, Schrems WA, et al. Corneal nerve alterations in acute Acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye (Lond) 2012;26:126–132. doi: 10.1038/eye.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi A, Yokogawa H, Higashide T, et al. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol. 2012;153:445–453. doi: 10.1016/j.ajo.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg ME, Tervo TM, Muller LJ, et al. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21:265–269. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 13.Villani E, Galimberti D, Viola F, et al. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48:2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 14.Patel SV, Erie JC, McLaren JW, et al. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and in late endothelial failure. Arch Ophthalmol. 2007;125:1693–1698. doi: 10.1001/archopht.125.12.1693. [DOI] [PubMed] [Google Scholar]
- 15.Niederer RL, Perumal D, Sherwin T, et al. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–626. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 16.De Cilla S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50:5155–5158. doi: 10.1167/iovs.09-3384. [DOI] [PubMed] [Google Scholar]
- 17.Bekar A, Kocaeli H, Yilmaz E, et al. Trigeminal neuralgia caused by a pontine abscess: case report. Neurosurgery. 2004;55:1434. [PubMed] [Google Scholar]
- 18.Epstein DL, Paton D. Keratitis from misuse of corneal anesthetics. N Engl J Med. 1968;279:396–399. doi: 10.1056/NEJM196808222790802. [DOI] [PubMed] [Google Scholar]
- 19.Lambiase A, Rama P, Aloe L, et al. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10:270–276. doi: 10.1097/00055735-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah P, Pavan-Langston D, Dana R. Herpes simplex keratitis and dendritic cells at the crossroads: lessons from the past and a view into the future. Int Ophthalmol Clin. 2009;49:53–62. doi: 10.1097/IIO.0b013e3181924dd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari G, Chauhan SK, Ueno H, et al. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011;52:2532–2539. doi: 10.1167/iovs.10-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno H, Ferrari G, Hattori T, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53:867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Calvacanti BM, Cruzat A, et al. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:7457–7466. doi: 10.1167/iovs.14-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282:326–327. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- 25.Jager MJ. Corneal Langerhans cells and ocular immunology. Reg Immunol. 1992;4:186–195. [PubMed] [Google Scholar]
- 26.Hamrah P, Liu Y, Zhang Q, et al. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Hamrah P, Zhang Q, et al. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamrah P, Zhang Q, Liu Y, et al. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–646. [PubMed] [Google Scholar]
- 29.Shtein RM, Garcia DD, Musch DC, et al. Herpes simplex virus keratitis: histopathologic inflammation and corneal allograft rejection. Ophthalmology. 2009;116:1301–1305. doi: 10.1016/j.ophtha.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinomiya K, Ueta M, Sotozono C, et al. Immunohistochemical analysis of inflammatory limbal conjunctiva adjacent to Mooren’s ulcer. Br J Ophthalmol. 2013;97:362–366. doi: 10.1136/bjophthalmol-2012-302631. [DOI] [PubMed] [Google Scholar]
- 31.Mathers W, Stevens G, Jr, Rodrigues M, et al. Immunopathology and electron microscopy of Acanthamoeba keratitis. Am J Ophthalmol. 1987;103:626–635. doi: 10.1016/s0002-9394(14)74321-1. [DOI] [PubMed] [Google Scholar]
- 32.Lin H, Li W, Dong N, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51:122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 33.Kheirkhah A, Rahimi Darabad R, Cruzat A, et al. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Invest Ophthalmol Vis Sci. 2015;56:7179–7185. doi: 10.1167/iovs.15-17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postole AS, Knoll AB, Auffarth GU, et al. In vivo confocal microscopy of inflammatory cells in the corneal subbasal nerve plexus in patients with different subtypes of anterior uveitis. Br J Ophthalmol. 2016 doi: 10.1136/bjoophthalmol-2015-307429. pii: bjoophthalmol-2015-307429. [DOI] [PubMed] [Google Scholar]
- 35.Hatou S, Dogru M, Ibrahim OM, et al. The application of in vivo confocal scanning laser microscopy in the diagnosis and evaluation of treatment responses in Mooren’s ulcer. Invest Ophthalmol Vis Sci. 2011;52:6680–6689. doi: 10.1167/iovs.10-5906. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zhao F, Zhu W, et al. In vivo confocal microscopic evaluation of morphologic changes and dendritic cell distribution in pterygium. Am J Ophthalmol. 2010;150:650–655 e1. doi: 10.1016/j.ajo.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Pahuja NK, Shetty R, Nuijts RM, et al. An In Vivo Confocal Microscopic Study of Corneal Nerve Morphology in Unilateral Keratoconus. Biomed Res Int. 2016;2016:5067853. doi: 10.1155/2016/5067853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederer RL, Perumal D, Sherwin T, et al. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest Ophthalmol Vis Sci. 2008;49:2964–2970. doi: 10.1167/iovs.07-0968. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed-Noriega K, Riau AK, et al. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Invest Ophthalmol Vis Sci. 2014;55:1823–1834. doi: 10.1167/iovs.13-13324. [DOI] [PubMed] [Google Scholar]
- 40.Vestergaard AH, Gronbech KT, Grauslund J, et al. Subbasal nerve morphology, corneal sensation, and tear film evaluation after refractive femtosecond laser lenticule extraction. Graefes Arch Clin Exp Ophthalmol. 2013;251:2591–2600. doi: 10.1007/s00417-013-2400-x. [DOI] [PubMed] [Google Scholar]
- 41.Kheirkhah A, Dohlman TH, Amparo F, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122:662–668. doi: 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labbe A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 43.Tavakoli M, Boulton AJ, Efron N, et al. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011;34:7–11. doi: 10.1016/j.clae.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruzat A, Schrems WA, Schrems-Hoesl LM, et al. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Invest Ophthalmol Vis Sci. 2015;56:6612–6620. doi: 10.1167/iovs.15-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 48.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 49.Rabin BS, Cohen S, Ganguli R, et al. Bidirectional interaction between the central nervous system and the immune system. Crit Rev Immunol. 1989;9:279–312. [PubMed] [Google Scholar]
- 50.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riol-Blanco L, Ordovas-Montanes J, Perro M, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veres TZ, Rochlitzer S, Shevchenko M, et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. Am J Respir Cell Mol Biol. 2007;37:553–561. doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- 53.Straub RH, Mayer M, Kreutz M, et al. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- 54.Nakai A, Hayano Y, Furuta F, et al. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straub RH, Rauch L, Fassold A, et al. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-gamma secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 2008;58:3450–3460. doi: 10.1002/art.24030. [DOI] [PubMed] [Google Scholar]
- 56.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 58.Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, et al. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015;36:578–604. doi: 10.1016/j.it.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lepisto AJ, Frank GM, Hendricks RL. How herpes simplex virus type 1 rescinds corneal privilege. Chem Immunol Allergy. 2007;92:203–212. doi: 10.1159/000099271. [DOI] [PubMed] [Google Scholar]
- 60.Hu K, Harris DL, Yamaguchi T, et al. A Dual Role for Corneal Dendritic Cells in Herpes Simplex Keratitis: Local Suppression of Corneal Damage and Promotion of Systemic Viral Dissemination. PLoS One. 2015;10:e0137123. doi: 10.1371/journal.pone.0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi T, Turhan A, Harris DL, et al. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One. 2013;8:e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simard-Lebrun A, Boisjoly H, Al-Saadi A, et al. Association between unilateral quiescent stromal herpetic keratitis and bilateral dry eyes. Cornea. 2010;29:1291–1295. doi: 10.1097/ICO.0b013e3181cbf9f5. [DOI] [PubMed] [Google Scholar]
- 64.Keijser S, van Best JA, Van der Lelij A, et al. Reflex and steady state tears in patients with latent stromal herpetic keratitis. Invest Ophthalmol Vis Sci. 2002;43:87–91. [PubMed] [Google Scholar]
- 65.Gonzalez GG, Garcia de la Rubia P, Gallar J, et al. Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Invest Ophthalmol Vis Sci. 1993;34:3329–3335. [PubMed] [Google Scholar]
- 66.Streilein JW, Okamoto S, Sano Y, et al. Neural control of ocular immune privilege. Ann N Y Acad Sci. 2000;917:297–306. doi: 10.1111/j.1749-6632.2000.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 67.Paunicka KJ, Mellon J, Robertson D, et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112–1122. doi: 10.1016/j.ajo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 69.VanDerMeid KR, Su SP, Krenzer KL, et al. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–1063. [PMC free article] [PubMed] [Google Scholar]
- 70.Jun AS, Cope L, Speck C, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437. doi: 10.1371/journal.pone.0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lema I, Duran JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112:654–659. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 72.Riemens A, Stoyanova E, Rothova A, et al. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 73.Leonardi A, Curnow SJ, Zhan H, et al. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–784. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 74.Zakaria N, Van Grasdorff S, Wouters K, et al. Human tears reveal insights into corneal neovascularization. PLoS One. 2012;7:e36451. doi: 10.1371/journal.pone.0036451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villani E, Galimberti D, Del Papa N, et al. Inflammation in dry eye associated with rheumatoid arthritis: Cytokine and in vivo confocal microscopy study. Innate Immun. 2013;19:420–427. doi: 10.1177/1753425912471692. [DOI] [PubMed] [Google Scholar]
- 76.Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF-2 signaling induced by IL-1beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:2067–2076. doi: 10.1167/iovs.08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YA, Choi HM, Lee SH, et al. Synergy between adiponectin and interleukin-1beta on the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in fibroblast-like synoviocytes. Exp Mol Med. 2012;44:440–447. doi: 10.3858/emm.2012.44.7.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HT, Lee JG, Na M, et al. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279:32325–32332. doi: 10.1074/jbc.M405208200. [DOI] [PubMed] [Google Scholar]
- 79.van de Veerdonk FL, Netea MG, Dinarello CA, et al. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Arranz-Valsero I, Schulze U, Contreras-Ruiz L, et al. Involvement of corneal epithelial cells in the Th17 response in an in vitro bacterial inflammation model. Mol Vis. 2013;19:85–99. [PMC free article] [PubMed] [Google Scholar]
- 81.Duan F, Liao J, Huang Q, et al. HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro. Clin Dev Immunol. 2012;2012:192791. doi: 10.1155/2012/192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 83.Gallucci RM, Sugawara T, Yucesoy B, et al. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- 84.McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 85.Ebihara N, Matsuda A, Nakamura S, et al. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Invest Ophthalmol Vis Sci. 2011;52:8549–8557. doi: 10.1167/iovs.11-7956. [DOI] [PubMed] [Google Scholar]
- 86.Funding M, Vorum H, Nexo E, et al. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol Scand. 2005;83:234–239. doi: 10.1111/j.1600-0420.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 87.Nosov M, Wilk M, Morcos M, et al. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am J Transplant. 2012;12:1313–1322. doi: 10.1111/j.1600-6143.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 88.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 89.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004;18:1439–1441. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 90.Romanovsky AA, Almeida MC, Aronoff DM, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 91.Sugaya S, Sakimoto T, Shoji J, et al. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55:277–282. doi: 10.1007/s10384-011-0002-x. [DOI] [PubMed] [Google Scholar]
- 92.Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–398. [PubMed] [Google Scholar]
- 93.Erdinest N, Shmueli O, Grossman Y, et al. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53:4396–4406. doi: 10.1167/iovs.12-9724. [DOI] [PubMed] [Google Scholar]
- 94.Santacruz C, Linares M, Garfias Y, et al. Expression of IL-8, IL-6 and IL-1beta in tears as a main characteristic of the immune response in human microbial keratitis. Int J Mol Sci. 2015;16:4850–4864. doi: 10.3390/ijms16034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacLeod AS, Hemmers S, Garijo O, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest. 2013;123:4364–4374. doi: 10.1172/JCI70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor PR, Roy S, Leal SM, Jr, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bharathi MJ, Ramakrishnan R, Shivakumar C, et al. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J Ophthalmol. 2010;58:497–507. doi: 10.4103/0301-4738.71678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 100.Wu M, Peng A, Sun M, et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating Toll-like receptor signaling and Th1/Th2-type immune responses. Infect Immun. 2011;79:2709–2716. doi: 10.1128/IAI.00144-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


