Abstract
Malignant brain tumors and brain metastases are highly aggressive diseases that are often resistant to treatments. Consequently, the current prognosis of patients with brain tumors and metastases is dismal. Activated microglia and macrophages are often observed in close proximity to or within the malignant tumor masses, suggesting that microglia/macrophages play an important role in brain tumor progression. Microglia, being resident macrophages of the central nervous system, form a major component of the brain immune system. They exhibit anti-tumor functions by phagocytosis and the release of cytotoxic factors. However, these microglia/macrophages can be polarized into becoming tumor-supportive and immunosuppressive cells by certain tumor-derived soluble factors, thereby promoting tumor maintenance and progression. The activated microglia/macrophages also participate in the process of tumor angiogenesis, metastasis, dormancy, and relapse. In this review, we discuss the recent literature on the dual roles of microglia/macrophages in brain tumor progression. We have also reviewed the effect of several well-known microglia/macrophages-derived molecules and signals on brain tumor progression and further discussed the potential therapeutic strategies for targeting the pro-tumor and metastatic functions of microglia/macrophages.
Keywords: Microglia, Macrophage, brain tumor, brain metastasis
2. INTRODUCTION
Malignant brain tumors and brain metastasis are often considered fatal in adults because of extremely poor prognosis and frequent tumor recurrence. Glioblastoma is the most common primary brain tumor in adults. In addition to glioblastoma, brain metastasis from other primary tumors including those from the lung, skin, and breast cancers also represent significant number of the central nervous system (CNS) tumors (1). It has been reported that 15–30 % of the patients with metastatic breast and lung cancers develop brain metastases (2). Even with treatments involving surgical intervention, irradiation, and chemotherapy, only a fraction of these patients with malignant brain tumor/metastasis survives longer than 2 years after diagnosis (1, 2). Recently, several drugs have shown to target neoplastic cells, which directly modulate the progression of brain tumor (3–5). Brain tumors develop a complex tumor microenvironment, which contributes to the development of drug resistance. In addition to tumor cells, non-neoplastic cells such as astrocytes, microglial cells, macrophages, endothelial cells, and lymphocytes are present in brain tumor microenvironment. Communications between the cancer cells and the non-neoplastic cells play critical roles in tumorigenesis and tumor invasion. Among the non-neoplastic cells, microglial cells and macrophages account for 30–50% of the total brain tumor mass (6, 7), suggesting that microglia/macrophages play a pivotal role in the tumorigenesis and metastasis. Most in vitro and in vivo studies have demonstrated that activated microglia/macrophages indeed accelerate growth and invasion of brain tumors (6, 8, 9). Although the depletion of microglia/macrophages does not significantly reduce the already existing tumors at the primary site in animal models (10, 11), a lack of microglia/macrophages has been observed to significantly affect metastatic spread of tumors (12–14), suggesting that these two types of cells play an essential role in the brain tumor invasion and metastasis. Therefore, understanding the mechanism of microglia/macrophage activation in the tumor microenvironment is essential for the development of novel anti-brain tumor therapies. In this review, we discuss the role and functions of microglia/macrophages in the maintenance and progression of malignant brain tumor.
3. ORIGINS OF MICROGLIA AND MACROPHAGES IN BRAIN TUMOR
Microglia, the resident macrophage of the CNS, is involved in immune surveillance and host defense against infectious agents and neoplastic tumors in the CNS. Under physiological conditions, microglia are in a resting state characterized by ramified morphology (15). After they have been exposed to infectious and traumatic stimuli, microglia rapidly change their morphology to “amoeboid” activated phenotype, alter gene expression and produce reactive oxygen species, and nitric oxide, pro-inflammatory cytokines and chemokines, which contribute to the clearance of pathogenic infections. However, prolonged and chronic microglial activation may result in pathological forms of inflammation that contribute to neurodegenerative and neoplastic diseases (16). Although activated microglia is believed to secrete cytotoxic factors that suppress or destroy pathogens and cancer cells, they are also capable of producing growth factors that promote cell survival, growth and enhance neuron function (17, 18).
Microglia was first characterized and reported in neural tumors by Rio-Hortegain in 1921 (6). However, later immunohistological studies have consistently revealed abundant infiltration of microglia/macrophages within the glioma and brain tumor tissues (19, 20). Moreover, the degree of activation of microglia/macrophages positively correlates with the grade of brain tumor (20), suggesting that the activated microglia/macrophages are associated with neoplastic progression.
Microglial cells and macrophages in the brain are derived from two different sources: 1) The parenchymal resident microglia and 2) Monocytes/macrophages that enter the brain from bone-marrow. Previous studies supposed that both the types of cells, microglial cells and macrophages, were myeloid-derived, based on the similarity in their surface markers and physiological functions. However, more recently, studies have demonstrated that microglia and macrophages are two distinct myeloid populations with different developmental origins (21–23). Animal studies showed that microglial cells originate from erythromyeloid progenitors that begin on embryonic day 7.5 (E7.5)–E8.0 in the blood islands of the yolk sac. Until E9.5, erythromyeloid progenitors migrate to the developing CNS and mature into microglia (21–23). These early microglial cells reside in the brain throughout life and are thought to sustain the local microglial population. In contrast, macrophages originate from the hematopoietic stem cells that start in the aorta–gonad–mesonephros region at E10.5, and then in the fetal liver at E12.5. After the postnatal stage, macrophages are produced from monocytes in the bone marrow (Fig. 1) (21–23). Recent studies demonstrate that microglial cells and macrophage have distinct and specific surface antigens too (24, 25). Studies on CX3CR1 (+/GFP)/CCR2 (+/RFP) knock-in fluorescent protein reporter mice demonstrated that microglial cells are CX3CR1+/CCR2−, while the macrophages are CX3CR1−/CCR2+ (24, 25), strongly supporting the notion that microglial cells and macrophages are from different populations.
Figure 1. Different origin and lineage of microglial cells and macrophages in the brain.
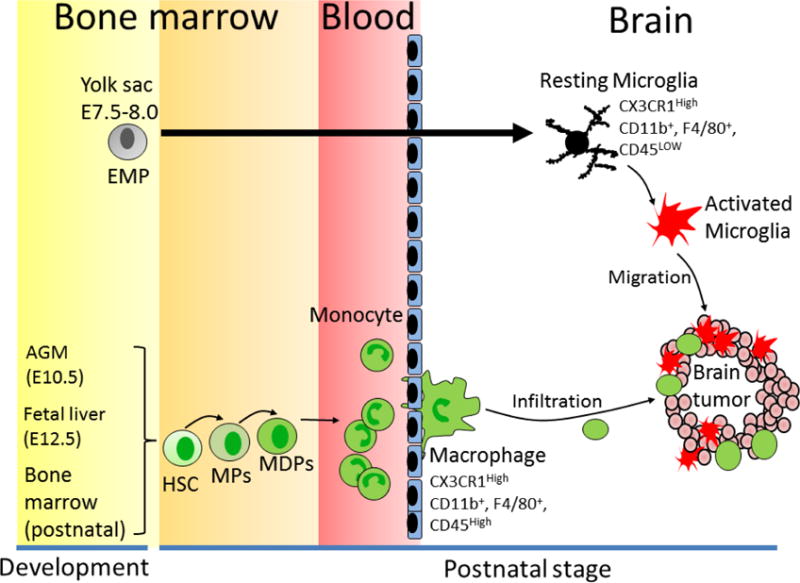
Microglial cells derive from erythromyeloid progenitors (EMPs) which locate in the yolk sac (embryonic day 7.5–8.0). In contrast, brain macrophages derive from hematopoietic stem cells (HSCs), which begin at embryonic day 10.5 in the aorta–gonad–mesonephros (AGM) region and at embryonic day 12.5 in the fetal liver. After postnatal stage, HSCs generate monocytes from myeloid precursors (MPs) and macrophage and/or dendritic cell progenitors (MDPs) in the bone marrow. The mature monocytes infiltrate into different oranges and differentiate to macrophage. In brain tumor microenvironment, tumor-released soluble factors recruit microglial cells and macrophages into the tumor site, which promotes tumor growth and metastasis.
In brain tumor and brain metastasis, microglial cells and macrophages are recruited either within or in close proximity to the tumor masses. These tumor-associated microglia/macrophages can be detected by several biomarkers, including CD11b/c, CD163, CD200, CD204, CD68, F4/80, and the lectin binding protein Iba-1 (22, 23). Because there are no definitive markers to distinguish between these two cells, many investigators use the more general term “microglia/macrophages” instead of microglia or macrophage alone to describe them in the brain tumors. However, microglia and macrophages can be distinguished by the differential expression levels of certain cell-surface markers (26). Microglial cells are defined as CD45low and macrophages as CD45high expressing cells (16, 26, 27). Based on this observation, several studies investigated distinct populations of microglial cells and macrophages in the glioma tissue. Badie et al. performed flow cytometric analysis and characterized the distribution of microglial cells and macrophages in experimental gliomas and found that microglial cells (CD11b/chigh, CD45low), mainly present at the site of tumor or tumor periphery, accounted for 13–34% of the tumor mass (27). By contrast, macrophages (CD11b/chigh, CD45high) were less prominent within the tumors or the tumor periphery and accounted for just 4.2–12% of the tumor mass (27). These results suggest that microglial cells play a key role in mediating the tumor-related inflammatory response.
Although the reactive microglia/macrophage are frequently found both in primary brain tumor and metastatic brain tumor (28), the ratio of microglia/macrophage in these tumors is different. Previous studies showed that microglia/macrophages account for 4–70% of all cells in human brain metastases, but 8–78% of cells in human gliomas (29, 30). Microglia are the main responders to primary brain tumors, inhibition of microglial activation has been shown to significantly reduce glioma proliferation (6). Microglia-derived enzymes, cytokines, growth factors have been shown to directly lead to tumor proliferation and invasion, immunosuppression and angiogenesis in primary brain tumor (6). In contrast to these cancer-promoting effects, however, microglia has also been reported to elicit cytotoxicity toward lung cancer brain metastases in the early phase by the production of NO (31). Moreover, activated microglial cells are observed at different phase of primary and metastatic brain tumor. Microglial activation often occurs in the middle-phase of the primary tumor, whereas reaction of microglia/macrophages to metastatic brain tumor cells is immediate (28). Activated microglial cells have been found accumulating around single metastatic cancer cells that just started to extravasate into the brain from the blood stream on day 7 after intra-carotid artery injection of breast cancer cells (28), suggesting that microglia participate metastatic cancer cell brain colonization in the earliest steps.
4. CLASSICAL (M1) OR ALTERNATIVE (M2) MICROGLIA/MACROPHAGES IN BRAIN TUMOR
Microglia/macrophages can be differentiated into classical (M1) or alternative (M2) phenotype by microenvironment stimuli (32). M1 cells are activated by type I cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), and lipoproteins (33, 34). M1 cells perform an anti-tumor immune function by producing pro-inflammatory cytokines and reactive oxygen species (ROS) and express signal transducer and activator of transcription 1 (STAT1). Upregulating STAT1 induces the production of inflammatory cytokines such as IL-1β, TNF-α, iNOS, IFN-γ, and IL-12 that alter the function of protein, DNA, or RNA, or induce lipid peroxidation which leading to inhibit tumor growth (16, 35, 36). The activated M1 microglia have antigen-presenting capabilities (37, 38) and are able to present antigen to Th1 cell to induce T-cell mediated cytolytic activity and induce cytotoxic T cell differentiation (38), resulting in tumor death. In nonmalignant or regressing tumors, the majority of microglia is M1-like, which increases pro-inflammatory activity to promote tumor lysis and tumor killing (39–41). These M1 microglia reduce the sphereforming ability of cancer stem cells and suppress glioma growth (42), resulting in tumor inhibition. Several surface markers such as major histocompatibility complex (MHC) II, CD11c, CD74+, and iNOS have been used to identify M1 microglia/macrophages (25). These M1 subtype cells also express interleukin (IL)-12high, IL-23high, and IL-10low(35).
In contrast, M2 cells are activated by type II cytokines such as IL-4, IL-10, IL-13, and transforming growth factor-β (TGF-β) (43, 44). Chemokine stimuli including chemokine (C-C motif) ligands (e.g., CCL2, CCL17, and CCL22) and macrophage-derived chemokines can also promote M2 polarization (43). These M2 cells have a pro-tumor immune response by producing immunosuppressive factors (e.g., IL-10 and TGF-β) and exhibit a high level of intracellular STAT3 (16). STAT3 activation has also been associated with promoting immunosuppression (45). Activated STAT3 decreases the expression of surface molecules in microglia that are necessary for antigen presentation, such as MHC-II, CD80, and CD86, and also increases the expression of various M2-specific immunomodulatory mediators including IL-10, vascular endothelial growth factor (VEGF), and various matrix metalloproteinases (MMPs) (46, 47), promoting growth and invasion of the tumor. Moreover, STAT3 targets pro-proliferation genes (48) that may contribute to microglia proliferation in brain tumor. Several surface markers such as CD163, CD204 (16, 34, 49), and arginas-1 (33) are present in M2 cells. These M2 cells promote the function of Th2 cells and frequently express IL-10, which is a strong anti-inflammatory mediator (9, 50). M2 microglia-derived IL-10 helps to create an immunosuppressive microenvironment to promote tumor survival (6, 8, 9). A recent study showed that co-culture of M2 macrophages with glioma cells significantly increased tumor proliferation when compared with co-culture with an M1 subtype, and this effect was suppressed by blocking the expression of STAT3 (51). These results suggest that the polarization of microglia/macrophage profoundly affects tumor growth in the brain (Fig. 2).
Figure 2. Differential roles of activated microglia/macrophages in the brain tumor.
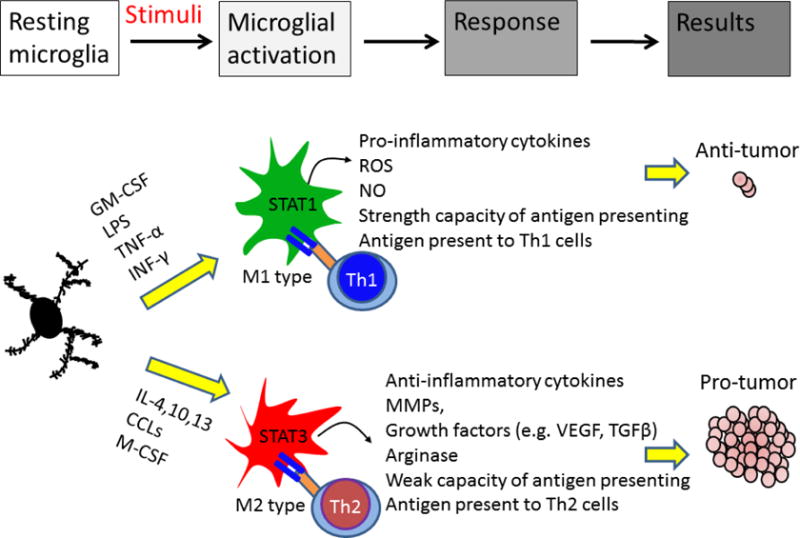
Microglia/macrophages have both pro- and anti-tumor potentials. In response to granulocyte-macrophage colony stimulating factor (GM-CSF), lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ) stimuli, microglia/macrophage can be polarized to M1 phenotype. M1 cells exhibit anti-tumor immunity by producing cytotoxic factors and presenting tumor antigen to T helper type 1 cells (Th1) cells. STAT1 activation in M1 cells induces pro-inflammatory cytokines production and increases T-cell-mediated cytolytic activity, leading to tumor cell damage. In response to interleukin-4 (IL-4), chemokine (C-C motif) ligands (CCLs) and macrophage colony-stimulating factor (M-CSF), microglia/macrophage polarize into M2 phenotype. M2 cells express STAT3 that induces anti-inflammatory factors. M2 cells also modulate Th2 cells, which promotes tumor progression. In addition, M2 cells can promote tissue repair and angiogenesis, resulting in tumor progression.
Accumulating evidence suggests that microglia/macrophages in the brain tumor are skewed to the M2 phenotype and that the cytokines IL-4, IL-6, IL-10, and TGF-β (44, 52, 53), secreted from the tumor, induce M2 microglia/macrophage activation. Accordingly, high numbers of CD163+ and CD204+ microglia/macrophages are detected in glioma patients, (52) and their levels positively correlate with poor clinical prognosis of human glioma (51). By contrast, a higher ratio of CD74+ M1 cells is positively associated with better survival of human glioblastoma patients (54). Activated M2 microglial cells have been shown to promote colonization of breast cancer cells in the brain (13). In contrast, activated M1 microglial cells induced apoptosis of metastatic lung cancer cells in vitro (55). Both M1 and M2 microglia are detected within brain tumor mass. Even M1 microglia is able to suppress tumor growth and cause tumor cell death, the immunological functions of M1 microglia in the brain tumor including cytotoxicity, phagocytosis, and antigen presentation are impaired (56). Thus, metastasis tumor cell can escape the immune attack of M1 microglia and then colonize in the brain microenvironment. The balance of upregulating M2 pro-tumor response and attenuated M1 anti-tumor immune response determine the promotion of tumor growth and invasion.
5. CROSS-TALK BETWEEN MICROGLIA/MACROPHAGES AND TUMOR CELLS BY MULTIPLE FACTORS THAT STIMULATE BRAIN TUMOR PROGRESSION
Reactive gliosis including microgliosis is a hallmark of neurodegeneration, neuron injury and brain tumors (6, 57). In neuron, reactive microglial is known to lead local neuronal DNA damage and neuron death through secretion of proinflammatory mediators or ROS. By contrast, chronic reactive microgliosis increase production of cytokines and chemokines that accelerate growth and invasion of tumors. Reactive microgliosis creates favorable and more permissive brain microenvironment for promoting tumor growth/invasion. On the other hand, tumor-derived factors not only promote their own growth but also induce another wave of microglial activation resulting in an autocrine self-propelling inflammatory response in the brain. Here, we discuss several important molecules from microglia/macrophages and tumor cells that are involved in modulating reactive microgliosis in the progression of brain tumors (Fig. 3 and Table 1).
Figure 3. Reactive microgliosis promotes brain tumor progression.
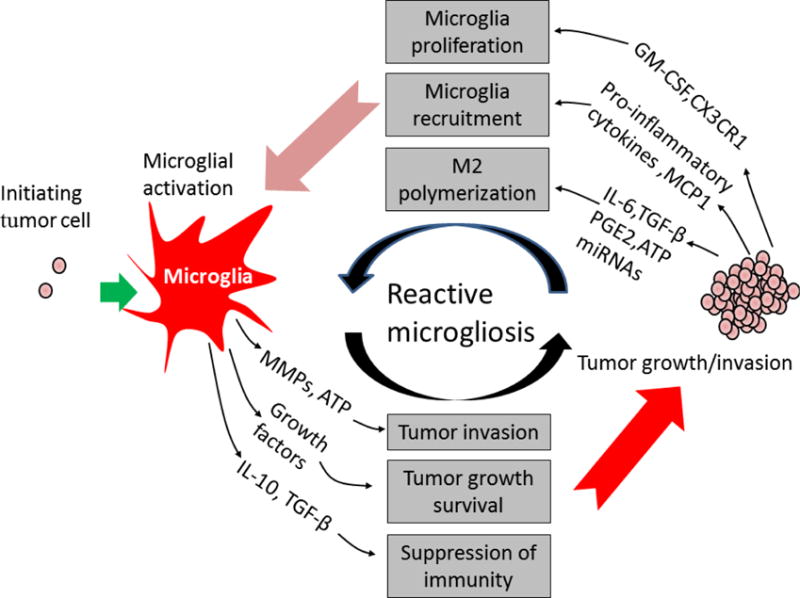
Microglial cells become hyper-activated through two mechanisms in brain tumor microenvironment. First, microglial cells become active, produce cytokines, growth factors and matrix-metalloproteases (MMPs) in response to initial tumor cell stimuli. Microglia-secreted factors then promote tumor growth and invasion. Second, tumor cells release growth, chemoattractant, and chemokine factors that recruit and induce another wave of microglial activation, resulting in a perpetuating cycle of microglia activation in the brain tumor. IL-6: Interleukin IL-6, IL-10: Interleukin 10, TGF-β: Transforming growth factor, PGE2: Prostaglandin E2, GM-CSF: granulocyte-macrophage colony stimulating factor, MCP-1: Monocyte chemoattractant protein-1, ATP: Adenosine triphosphate, miRNAs: microRNAs.
Table 1.
Tumor- and microglia-derived factors affect brain tumor progression and microglial polarization
| Tumor-derived factor | Microglia-derived factor | Receptor | Outcome | Ref. |
|---|---|---|---|---|
| MCP-1 | CCR2 in microglia | Recruitment of microglia | 7, 59–67 | |
| GM-CSF | GM-CSFR in microglia | Microglial proliferation | 68 | |
| G-CSF | GM-CSFR in microglia | Promoting brain tumor proliferation | 68, 69 | |
| CX3CL1 | CX3CR1 in microglia | Recruitment of microglia | 70–75 | |
| IL-10 | IL-10 (Major) | Il-10R in both | Immunosuppression, M2 activation, Promoting cancer stem cell growth | 8, 9 |
| IL-6 | IL-6 | IL-6R in both | Tumor migration, M2 activation | 78–81 |
| IL1-β, TNF-α | IL1-β, TNF-α | IL-1R,TGFR in both | Tumor growth, increasing BBB permeability | 25, 82–84 |
| Prostaglandin | Prostaglandin | PGER in both | Immunosuppression | 88, 89, 91 |
| TGF-β | TGFR in tumor | Stem cell proliferation, immunosuppression | 90–94 | |
| TGF-β | TGFR in microglia | Recruitment of microglia | 90 | |
| GDNF | GDNFR in microglia | Recruitment of microglia | 95 | |
| HGF/SF | c-Met in microglia | Microglia migration | 96 | |
| HGF/SF | c-Met in tumor | Angiogenesis | 96–99 | |
| MMPs | MMPs | Tumor migration, invasion, infiltration of microglia, degradation of extracellular matrix, angiogenesis | 100–105 | |
| MT1-MMP | Pro-MMP2 cleavage | |||
| ATP | P2X7R in microglia | M2 activation, enhancing motility of microglial cells | 106–116 | |
| microRNAs | Promotion or inhibition of tumor | 117–129 | ||
| miR-124 | Microglia quiescence | 127 | ||
| Soluble factors | TLRs in microglia | Degradation of extracellular matrix, | 131 | |
| S100B | RAGE in microglia | M2 activation, angiogenesis | 132, 133 | |
| Wnt signaling | Wnt signaling | WntR in Both | Brain metastasis, cytokines production | 13,134–136 |
| VEGF | VEGFR+ in endothelial cells | Building functional vascular tubes | 140–142 |
5.1 Cytokines/chemokines
Cytokines/chemokines and their receptors are thought to be important for trafficking immune cells in the peripheral nervous system (58). Although the identity of tumor-derived cytokines/chemokines that modulate the recruitment of microglia remains unclear, several common chemokines and receptors have been found to be up-regulated in brain tumors, including monocyte chemoattractant protein-1 (MCP-1), Granulocyte/macrophage-colony stimulating factor (GM-CSF), CX3CL1, and CCL (59). MCP-1/CCL2 is believed to be a major contributor in microglia/macrophage recruitment to gliomas and breast cancer brain metastases in vivo and in vitro (7, 59–64). MCP-1 is also responsible for increasing microglial proliferation in glioma (60). Brain tumor cell-derived MCP-1 binds to its specific receptor, CCR2, on the microglia, facilitating the recruitment of microglia into the tumor site (65). Expression of MCP-1 positively correlates with the higher grade of malignant glioma (66), suggesting that MCP-1 in the glioma not only induces the recruitment of microglial cells into glioma but also increases tumor growth. MCP-1 is also implicated in breast cancer progression. A high level of MCP-1 in breast cancer cells was shown to promote migration and infiltration of macrophage into the brain through CCR2. Blocking CCL2 with neutralizing antibodies decreased macrophage infiltration and tumor growth in a mouse model of breast cancer (64, 67). GM-CSF has a similar effect as MCP-1 in enhancing microglial proliferation (68). In addition to GM-CSF, brain tumor tissues express high levels of the receptor of granulocyte-colony stimulating factor (G-CSF). Unlike GM-CSF, G-CSF does not promote microglial proliferation (68); however, it was shown to promote brain tumor proliferation by autocrine mechanisms (69). Glioma and breast tumor brain metastases exhibit a high level of mRNA and protein expressions of CX3CL1 (70, 71). By contrast, CX3CR1 expression was shown to be absent in tumor cells, but it was found to be expressed in microglia themselves that lacked expression of CX3CL1 (71). CX3CL1 functions as a chemoattractant for macrophages and microglial cells (72–74). Treating microglia with exogenous CX3CL1 increased the ability for migration and adhesion of microglia to glioma cell in vitro, whereas blocking of CX3CR1 by CX3CR1 antibodies reduced CX3CL1-induced adhesion ability of microglia when compared to a control group (71). The expression of CX3CR1 is associated with tumor metastasis to the brain (75). Indeed, CX3CR1 overexpression in the brain metastasis of breast cancer patients (75), suggesting that CX3CR1 is associated with tumor metastasis to the brain. Increasing expression of CX3CR1 in the tumor mass is due to tumor-recruited microglia/macrophages because abundant microglia and macrophages are recruited within brain tumor mass (6, 7, 13, 76). Tumor-derived CX3CL1 attracts microglia/macrophage infiltration. These microglia/macrophages release cytokines and chemokines that promote the migratory ability of tumor cells to the brain. In view of the above facts, it is understood that these chemokines significantly increase the ability of microglia in proliferation and inflitration in brain tumor/metastasis, and therefore, these factors could serve as treatment targets.
The results of RNase protection assays showed that the expression of 53 genes encoding cytokines or cytokine receptors were altered in human glioma cell lines and brain tissue (77). In situ hybridization analyses showed that both—microglial cells and astrocytes— contribute to anti-inflammatory IL-10 gene expression in glioblastoma tissue (8). Furthermore, primary cells from human glioma specimens showed that microglia/macrophages are the major sources of IL-10 expression in gliomas (9), suggesting that IL-10 secreted by microglia create an immunosuppressive microenvironment for growth of glioma. In addition, IL-6 secreted by glioma and microglia was shown to stimulate the production of MMP-2 that induces glioma-cell migration and invasiveness (78–81). Although tumor-derived soluble factors mainly induce polymerization of M2-like microglia/macrophages, some factors also induce a partial shift of microglia/macrophages toward the M1 phenotype in the brain tumor. Interestingly, several previous studies have shown that M1 specific markers or associated pathways are not only detected but also positively correlated with glioma growth, and that IL1-β and TNF-α directly promote glioma growth (25). In other studies, IL1-β and TNF-α were shown to stimulate brain microvessel endothelial cells, leading to increasing permeability of the blood-brain barrier (82–84) and immune cell infiltration from the peripheral system. However, several studies showed that M1 cytokines (e.g., IFN-γ, TNF-α, IL-1β, IL-2, and IL-12) and their receptors were virtually absent in glioma, brain metastatic cell lines, and in human tissues whereas M2 immunosuppressive cytokine (i.e. IL-6, TGF-β) were greatly predominant in these cells (77, 85–87). Therefore, in brain tumor, the balance of upregulating M2 pro-tumor response and attenuated M1 anti-tumor immune response determine the promotion of tumor growth and invasion.
5.2 Prostaglandin and transforming growth factor-β
Prostaglandin E2 and TGF-β are important immunosuppressants in brain tumor. Previous studies have shown that prostaglandin E2 inhibits the innate and adaptive immune responses of immune cells via the activation of adenosine monophosphate (88, 89). Both, glioma and microglial cells, are known to produce prostaglandin. Microglial cells are the major source of cyclooxygenase-2, which is the key enzyme responsible for arachidonic acid conversion to active prostaglandins (90, 91). Microglial activation increases the expression of cyclooxygenase-2, which may contribute to the increase in prostaglandin production in the brain tumor. Tumor-released TGF-β has been shown to recruit microglia/macrophage to glioma (90). Furthermore, microglia-derived TGF-β stimulates the migration of glioma cell (92) and increases the proliferlation of CD133+ glioma cells (glioma stem-like cells) (90), whereas the blocking of TGF-β abolishes the effects of microglia on glioma invasiveness (90, 91). Moreover, several reports have shown that TGF-β1 is required for the maintenance of self-renewal property of glioma stem-like cells (93, 94), suggesting that TGF-β secreted from microglia/macrophage plays a role in the maintenance of cancer stem-like cells associated with growth, migration, and invasion of brain tumors.
5.3 Growth factors
Glial cell-derived neurotrophic factor (GDNF) secreted by tumor cells was identified as a strong chemoattractant for microglia. The downregulation of GDNF by siRNA in mouse glioma cells was shown to diminish the attraction of microglia, whereas the overexpression of GDNF promoted microglia-attraction of glioma cells (95). In addition to GDNF, other growth factors [e.g., hepatocyte growth factor/scatter factor (HGF/SF)] from tumor cells are able to chemotactically attract microglial cells in vitro (96). The glioma-released HGF/SF targets the transmembrane tyrosine kinase receptor, c-Met, on microglia, thereby increasing the migratory ability of microglia in a co-culture model with glioma cells (96). This effect can be readily abolished with HGF antibody treatment (96), suggesting that the tumor-derived growth factors play a role in microglia chemotaxis. Interestingly, activation of HGF/SF and c-Met have been shown to promote angiogenesis by stimulating endothelial cell migration and proliferation (96, 97). Microglial cells also produce HGF/SF and express c-Met (96, 98, 99), which may contribute to angiogenesis in the brain tumor. These results provide a new insight into the role of growth factors not only in promoting tumor growth but also in enhancing tumor invasion and angiogenesis by modulating the activation of microglia.
5.4 Matrix metalloproteinases
MMPs are known to degrade extracellular matrix, which promotes tumor migration and invasion. The expression analysis of MMPs in the glioma model revealed that MMP-1, 2, 3, 8, 9, 13, and 14 genes are upregulated in the tumor and microglial cells. Among them, MMP2 is also one of the major proteases found in mouse and human gliomas (100, 101). Both, microglial and tumor cells produced MMP2. Secreted MMP2 is in a pro-form that needs to be cleaved to an active form by membrane type 1-matrix metalloproteinase 1 (MT1-MMP) to facilitate glioma cell motility. In the normal brain, MT1-MMP expression in microglia is relatively low and is detectable only in the white matter (102). However, the expression of MT1-MMP is elevated in the event of occurrence of brain tumors (103). Consistent with these results, 80% of brain metastasis from lung adenocarcinoma and 50% of that from breast cancer were positive in MT1-MMP immunostaining (104). Markovic and colleagues found that MT1-MMP was especially expressed in microglia that was in close contact with glioma cells, whereas glioma cells expressed MT1-MMP in brain tumor only at a lower level (103). Knockdown of MT1-MMP in microglia by shRNA effectively reduced the growth of glioma in vivo, suggesting that MT1-MMP expression in microglial cells plays an essential role in glioma progression. Furthermore, upregulating MMP-2 and MMP-9 induced degeneration and retraction of astrocytic end-feet (105), which increases the blood-brain barrier permeability, resulting in the infiltration of macrophages, T cells, and cancer cells into the brain parenchyma (19, 20). Thus, microglia can serve as activators for the degradation of extracellular matrix for primary brain tumor invasion and brain metastasis. Moreover, upregulating MMPs on microglia contributes to peripheral cancer and immune cell infiltration.
5.5 Cell-cell communication through extracellular vesicles
Tumor and microglia/macrophages are able to release extracellular microvesicles known as exosomes into the microenvironment and circulation. The released exosomes may serve as carriers for cell-cell communication, which affects brain tumor progression/metastasis and controls microglia activation in an autocrine and paracrine fashion. Exosomes are ~40 to 100 nm in size and can encapsulate various molecules, including metabolites, proteins, and nucleic acids (106, 107). For example, the exosomal adenosine triphosphate (ATP), abundantly released from tumor cells, has been shown to bind to the purinergic receptor P2X7 (P2X7R) on the surface of microglia/macrophages, resulting in microglia/macrophage activation and the production of macrophage inflammatory protein-1α (MIP-1α) and MCP-1 (108). Treatments with P2X₇R antagonists or oxidized ATP reduced the expression of MIP-1α and MCP-1 in microglia/macrophages, while it suppressed brain tumor progression (108). Exosomal ATP was also found to induce microglia ramification (109), enhance the motility of microglial cells (110, 111), and promote M2 phenotype (112), but at the same time inhibit M1 phenotype activation (113). In addition to tumor cells, microglial cells produce and release ATP (114) which may directly induce microglial activation through an autocrine mechanism. The microglial cell-derived exosomal ATP stimulates the production of MCP-1 in tumor cells (115), which in turn promotes microglia cell infiltration into the tumor mass. ATP is a well-known mitotic factor for glioma cells that promotes tumor growth. Although high levels of ATP (5 mM) induce cell death in normal cells, glioma cells present resistance to death, induced by ATP stimulation (116). These results suggest that tumor cell- and microglial cell-derived exosomal ATP induces cell death of the normal tissue surrounding the tumors, which may potentially set the stage for tumor cells for rapid growth and invasion.
RNA molecules in exosomes include mRNAs, microRNAs (miRNAs), and long non-coding RNAs. MicroRNAs function as novel classes of oncogenes or tumor suppressors and are frequently located at the chromosomal fragile sites in cancer genomes (117, 118). For example, the exosomal miR-223 plays an oncogenic role by promoting the invasive potential of breast cancer cells (119). In contrast, other exosomal miRNAs including miR15b, miR124, miR-137, miR-146b, and miR-451 inhibit brain tumor invasion and regulate the tumor cell-cycle progression (120–124). Among these, miR-124 is the brain-enriched miRNA that modulates neuronal development, tumor progression, and microglial activation (125–127). Compared with that in the normal brain tissues, the expression of miR-124 is significantly down-regulated in brain tumor tissues and cell lines (122, 123, 128). Hongping et al. showed that the overexpression of miR-124 reduced tumor sphere formation, inhibited stemness, and suppressed tumor cell invasion (122). Interestingly, high expression of miR-124 is detected in resting microglia of the normal CNS, whereas it appears to be downregulated in activated microglial cells (127). Transfection of miR-124 in microglia/macrophage and animals directly inhibited the immunogen–induced microglia/macrophage activation and suppressed cytokine production (127). Furthermore, knockdown of miR-124 in microglia/macrophages resulted in microglia/macrophage activation in vitro and in vivo (127), suggesting that miR-124 could be a key regulator of microglia quiescence. In addition to tumor-derived miRNAs, astrocyte-secreted miRNAs also affected microglia activation. Loss of PTEN, an important tumor suppressor, in the tumor cells significantly increased IBA1+ expression on microglia, promoted tumor growth and elevated risk of breast cancer brain metastasis through miR-19a secretion from astrocytes (129). These results suggest that miRNAs in the brain microenvironment mediate microglial activation and tumor progression.
Proteins in exosomes have also been shown to play critical roles in controlling microglia/macrophage activation. For example, heat shock proteins (HSPs) such as HSP90, HSP70, and HSP32, induce the production of IL-6 and TNF-α, and increases the rate of phagocytosis in microglial cells (130). Taken together, exosomal miRNAs and proteins in brain tumor are important not only for promoting tumor progression/metastasis but also for modulating microglia/macrophage activation.
6. SIGNALING PATHWAYS OF MICROGLIA/MACROPHAGES FOR BRAIN TUMOR GROWTH AND INVASION
Reciprocal communications between microglia and tumor cells activate multiple key signaling pathways as summarized in Fig. 4. Several important pathways that play pivotal roles in brain tumor growth and invasion are discussed below.
Figure 4. Cross-talk between tumor cells and resident cells in the brain.
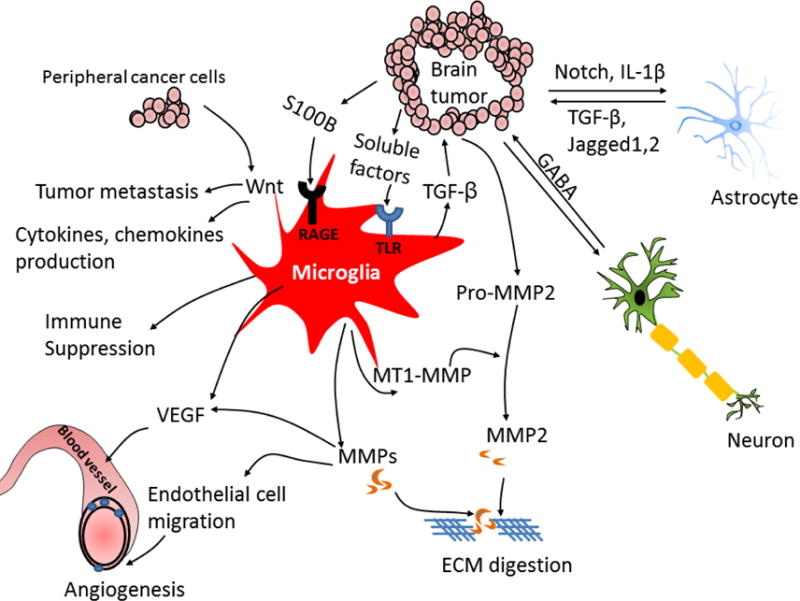
Various secreted soluble factors from tumor cell stimulate microglia and astrocyte activation. The tumor-derived soluble factors bind toll-like receptors (TLRs) on microglia that induces p-38 MAPK activation, resulting in up-regulation of matrix-metalloproteases (MMPs) and membrane type 1-matrix metalloproteinase (MT1-MMP). Microglia-released MMPs then cause extracellular matrix (ECM) digestion that promotes tumor invasion and macrophages/T cells infiltration. In addition, secreted transforming growth factor (TGF-β) from microglial cells triggers the release of pro-MMP2 from tumor cells which is cleaved into active form MMP2 by microglia-released MT1-MMP. Microglia-secreted vascular endothelial growth factor (VEGF) enhances angiogenesis. S100 calcium-binding protein B (S100B) induces receptor for advanced glycation end products (RAGE) activation on microglial cells that induces production of anti-inflammatory cytokines, leading to immune suppression. The metastatic tumor cell induces production of cytokines and chemokines in activated microglia via activation of Wnt signal. Upregulating Wnt signaling in microglia promotes tumor colonization and metastasis. Tumor cells induce astrocyte activation by production of interleukin-1β (IL-1β). Activated astrocytes release TGF-β, Jagged and other factors, which promotes tumor growth and mediates cancer stem cells self-renewal. Neuron-released neurotransmitter gamma-aminobutyric acid (GABA) promotes tumor progression. The interaction of tumor and resident cells induces multiple pathway activation that creates favorable microenvironment for tumor growth. BBB: blood–brain barrier, EGFR: epidermal growth factor receptor, HER: human epidermal growth factor receptor, IL: interleukin, JAG: jagged, MMP: matrix metalloproteinase, TGF: transforming growth factor, VEGF: vascular endothelial growth factor.
6.1 Toll-like receptor 2 signal
The soluble factors released from a glioma can be recognized by various Toll-like receptors (TLRs), in particular TLR 2, on microglia/macrophage. Treating the primary microglial cells with a TLR 2 agonist was shown to up-regulate the downstream molecules, MyD88 and p38 MAPK, that promoted the productions of MT1-MMP and MMP-9, leading to glioma cell invasion due to the degradation of extracellular matrix (103). In addition, blocking TLR 2 on microglial cells or deletion of microglial MT1-MMP reduced MT1-MMP expression on microglia and impaired the growth of glioma (103), suggesting that the activation of the TLR 2 signal on microglia enhances brain tumor invasion. A more recent study has identified glioma-produced versican as an endogenous TLR 2 ligand that can trigger p38 MAPK signaling activation followed by increasing MT1-MMP production in microglial cells (131). Interestingly, microglia-released TGF-β was shown to induce the production of pro-MMP2 in glioma cells, which was subsequently cleaved into active MMP2 by microglia-secreted MT1-MMP. This positive circuit between microglial cells and tumor cells promotes brain tumor growth and invasion.
6.2 S100B-RAGE-STAT3 signal
M2 microglia polarization is important for maintaining local immunosuppression. Thus, upon attracting microglia, tumors establish an immunosuppressive microenvironment to promote their growth. Several lines of evidence indicate that the activation of S100B-RAGE-STAT3 signaling stimulates polarization of M2. Tumor-secreted S100 calcium binding protein B (S100B) activates receptor for advanced glycation end products (RAGE) on microglia, which induces STAT3 activation, resulting in the suppression of microglial M1 function, in turn reflected by the inhibition of TNF-α and IL-1β production (132). Zhang et al. showed that blocking RAGE expression inhibited glioma-induced STAT3 activation and suppressed the production of M2-type cytokines such as IL-10 in microglial cells (132). Importantly, activated RAGE signal in microglia/macrophage not only maintains M2-like phenotype but also affects angiogenesis. Indeed, genetic depletion of RAGE in glioma cells and mice was shown to abrogate angiogenesis by downregulating the expression of VEGF (133). Reconstitution of RAGE knockout mice with wild-type microglia or macrophages normalized glioma vascularity, suggesting that RAGE signaling in microglia/macrophages was sufficient to promote angiogenesis in glioma (133).
6.3 Wnt signal
Tobias et al. demonstrated that activated microglia cells significantly promote colonization of breast tumor tissues in cancer metastasis (13). Moreover, the presence of microglia also enhanced the invasion rate of human and murine breast cancer cells in the brain, when compared to a control group (13). They have also shown that the direction of microglia movement can serve as a guiding rail for malignant cells to move toward the neighboring tissue of tumor plaques (13). Interestingly, inhibiting microglia function by a Wnt inhibitor significantly diminished total invasion of the tumor cells (13). WnT signal is essential for communication between the microglia/macrophages and brain-metastasis tumor cells (134–136). Microglial activation-promoting brain metastasis often depends on activation of Wnt signaling (13, 136, 137), and treatment of microglial cells with a Wnt antagonist completely abolishes microglia-induced tumor invasion (13, 136). On the other hand, treating microglia/macrophages with Wnt increases the production of IL-6, IL-12, TNF-α, and MMP via the activation of AP-1/c-Jun (136, 138). Consistent with this ex vivo study, the Wnt signaling is elevated in breast cancer patients with brain metastasis (139). Therefore, activation of Wnt signaling in microglia promotes brain metastasis in part by upregulating microglial cytokine production.
6.4 Angiogenic factor
For the metastatic spread of cancer cells, the growth of the vascular network is important in order to supply nutrients and oxygen to tumor cells to promote their growth and invasion. The effect of microglia/macrophages on angiogenesis has been well documented. Microglia/macrophages are found to be located at vascular branching points and release VEGF that stimulates and guides the VEGFR+ endothelial cells to build functional vascular tubes. Deletion of macrophages by a genetic approach significantly decreases the migration ability of endothelial cells and reduces branching in the vascular plexus (140). Microglia-derived MMPs have been shown to induce production of angiogenic factors and stimulate angiogenesis in glioma (141). Moreover, microglia-derived MMPs degrade the extracellular matrix, which allows endothelial cells to invade into tumor tissues during angiogenesis in glioma (141, 142). Therefore, activated microglial cells play a pivotal role in constructing abundant vascular networks that promote tumor growth.
6.5 Signaling in other brain residential cells
In addition to microglia, tumor cells communicate with other residential brain cells that contribute to tumor growth. Beside microglia, astrocytes are the most abundant glial cells in the brain. Cancer cells stimulate the production of pro‐inflammatory cytokines in astrocytes, which also promotes tumor growth and metastasis. We have recently shown that tumor-produced prostaglandin could activate astrocytes to release CCL7, which in turn promoted self-renewal of the tumor-initiating cells (143). Furthermore, we showed that breast tumor cells in the brain express high levels of IL-1β which activates astrocytes to upregulate Jagged-1 which in turn interacts with the Notch signal in cancer stem cells (CSCs) and promotes self‐renewal of CSCs (144). Similarly, the tumor-promoting effect of Notch and the jagged-2 pathway has also been explored in other brain-metastasis models (145). The effect of the neuron-secreted neurotransmitter gamma-amino butyric acid (GABA) on brain tumor has also been explored. High levels of GABA and its receptor are detected in both, primary brain tumor and brain metastatic tissue (146). GABA is converted to a succinate form by GABA transaminase, resulting in the subsequent production of NADH to satisfy the energy and growth requirements of neuron cells (147). GABA enhances proliferation of brain-metastatic cells, whereas GABA transaminase inhibitor abolishes the proliferative effect of GABA on breast tumor (146). Together, these findings suggest that, in addition to microglia, neurons and astrocytes communicate with tumor cells to create appropriate tumor microenvironment that would promote brain tumor growth and metastasis (Fig. 4).
7. INTERACTION BETWEEN MICROGLIA CELLS AND CANCER STEM CELLS IN THE BRAIN
It is becoming increasingly clear that CSCs can drive tumor growth, invasion, and immune evasion. Brain tumors, especially glioblastomas, are believed to arise from glioblastoma stem cells (GSCs). High-density microglia/macrophages are detected in and around the GSC niche, suggesting that the inflammatory cells and inflammatory mediators may be indispensable components for GSCs growth. GSCs-secreted chemo-attractant factors recruit microglial cells into the tumor mass, while the recruited microglial cells release soluble factors that create a favorable microenvironment to help the growth and enhance the invasiveness of GSCs [28]. Liang Yi et al. found that the GSCs had a stronger ability to recruit microglia/macrophages than other glioma cells (148). Compared to the non-GSC glioma cells, GSCs expressed 2- to 3-fold higher level of CCL2, CCL5, and CCL7, 7-fold higher level of VEGF, and nearly 50-fold higher level of neurotensin. Among these, VEGF can induce proliferation of microglia (149) and inhibit myeloid progenitor maturation to develop tumor-associated macrophages, which promotes malignant progression (150). Neurotensin increases the migratory ability of microglia (151). Furthermore, GSCs were found to affect the polarization of microglia/macrophage. Treatment of resting microglia/macrophages with conditioned medium from GSCs promoted polarization of microglia/macrophages to an M2 phenotype and inhibited the capability of phagocytosis (42, 131). Recently, GSC-secreted periostin was found to function as a new potent chemo-attractant to recruit macrophages through the activation of integrin αvβ3 signal (25). Importantly, inhibiting the αvβ3 signal by blocking peptides impairs macrophage recruitment and suppresses GSC invasion (25). Furthermore, periostin-integrin αvβ3 signal has been found to maintain the microglia/macrophages phenotype at M2 subtype, which contributes to GSC growth in brain tumors (25).
Activated microglia/macrophage also affects the growth of GSCs. Microglia/macrophage-secreted IL-10 was shown to promote the growth of GSC (131). In addition, activated microglia/macrophages produce high levels of TGF-β1, which induces MMP-9 production and increases GSC invasiveness, whereas knockdown of the TGF receptor reduces the invasiveness of GSCs in vivo (90). Moreover, IL-6 has also been identified as a growth factor for glioma stem cells (152), which suggests that microglia-derived IL-6 may promote GSCs growth.
A recent study showed that naïve microglial cells curb GSC invasion. Isolated microglial cells from non-glioma patients released MCP-1 and IL-8 that reduced the sphere-forming ability of GSCs and inhibited brain tumor growth, whereas isolated microglial cells from glioma patients were unable to do so (42). Supplementing GSCs with naïve microglial-conditioned medium caused cell cycle arrest and reduced proliferation of GSCs (42). Moreover, growth- and differentiation-related genes were significantly down-regulated in GSCs when they were treated with naïve microglial-conditioned medium (42). These results suggest that the crosstalk between GSCs and microglia/macrophages promotes GSC growth and invasion.
8. INHIBITING MICROGLIA ACTIVATION AS THERAPEUTIC STRATEGY FOR BRAIN TUMOR
As described above, brain tumor growth is dependent on various signal stimulations from the microenvironment and on the various secreted factors from microglia/macrophages that promote brain tumor growth, invasion, and colonization. Thus, inhibition of the microglia/macrophages-derived signals is considered a potential anti-neoplastic-targeted therapy to block the growth of brain tumor. Here, we discuss several approaches that target and modulate the functions and activation of microglia/macrophage.
8.1 Immunotherapies
Several immunotherapies are under development for the treatment of patients with brain tumor. A recent study showed that immunotherapy using activated natural killer (NK) cells in combination with the antibody mAb9.2.27 diminished tumor growth by inhibiting tumor proliferation and promoting apoptosis (153). Moreover, this approach effectively increased the expression of ED1+ and MHC class II+ on microglia, which increased the function of microglia for tumor antigen presentation and cytotoxicity (153). A recombinant immunotoxin supplement was noted to block the folate receptor β on microglia/macrophages, causing depletion of microglia/macrophages and reduce glioma growth in nude mice (154). Likewise, ablation of CD11b+ cells in ganciclovir-treated CD11b-HSVTK mice decreased the brain tumor size and improved animal survival (155). Up-regulating M1 microglia/macrophage function can be an immunotherapeutic approach to enhance anti-tumor immunity in the brain. Indeed, IL-12, LPS, and INF-γ effectively increased microglial cytotoxicity and phagocytic activity that eliminated cancer cell growth in vivo (156–158). Likewise, stimulation of M1 microglia/macrophage by the TLR3 agonist poly (I: C) increased tumor cell death and inhibited growth and invasion of the tumor (159). In addition, inhibiting the CSF-1 receptor has been shown to reduce M2 macrophage polarization and inhibit tumor growth (160). These findings indicate that immunotherapies or the elevation of microglial cytotoxicity function may be an amenable therapeutic strategy to treat brain tumors.
8.2 Antibiotic interference
Antibiotic drugs can also be used as anti-glioma therapies by modulating microglia/macrophage function. Minocycline hydrochloride, a small, highly lipophilic antibiotic, has been shown to suppress tumor invasion by inhibiting the expression of MT1-MMP and p38 MAP kinase in microglial cells (161, 162). Furthermore, treating mice with cyclosporine significantly down-regulated the levels of IL-10 and GM-CSF, which in turn inhibited infiltration of microglia/macrophages and decreased glioma growth (34). Moreover, amphotericin B has been shown to enhance microglia/macrophage activation, which leads to the arrest of brain tumor-initiating cell cycle and inhibition of cell differentiation (42).
8.3 Drug delivery by microglia/macrophages
Taking advantage of microglial phagocytic properties, recent studies used nanoparticles to modulate the function of microglial cells in the tumor (163, 164). Cyclodextrin-based polymer nanoparticle (CDP-NP) is taken up by microglial cells with no toxicity. The CDP-NP-labeled microglial cells are found to surround the tumor, suggesting that these labeled microglia/macrophages could potentially be used as nanoparticle drug carriers into malignant brain tumors (163).
8.4 Radiation and antiangiogenic therapy
The radiation therapy (RT) is the front-line treatment for brain tumors and metastasis; however, it eventually fails owing to the recurrence of the tumor. Whole brain irradiation is accompanied with the production of hypoxia-inducible factor-1 and stromal cell–derived factor-1 (SDF-1), which enhances recruitment of macrophage and increases angiogenesis at the tumor-invasion front (165, 166). However, using a combination of RT with a small molecule inhibitor of SDF-1, AMD3100, abrogated tumor regrowth in nude mice by preventing RT-induced macrophage recruitment (166).
Antiangiogenic therapy is able to delay brain tumor progression, but the benefit of this approach is still limited. This is because antiangiogenic therapy induces recruitment of microglia/macrophages in the tumor bulk and infiltrative regions. (167, 168). Moreover, a clinical study showed that a higher population of microglia/macrophages correlated with poor survival after anti-VEGF therapy in glioma (167). This suggests that microglia/macrophages may participate in escaping from RT and antiangiogenic therapy, which results in tumor recurrence. Thus, microglia/macrophages may be potential biomarkers for predicting resistance to RT or as a treatment target for recurrent brain tumor.
9. CONCLUSIONS AND PROSPECTIVES
Tumor-secreted soluble factors induce microglial activation and recruit microglial cells into the tumor site in the brain, while these secreted factors also modulate the immune function of microglia. Activated microglial cells release multiple pro-tumor factors, which in turn promote tumor growth and invasion. It is now evident that the paracrine loops between cancer and microglial cells profoundly affect microglial pro- and anti-tumor functions, resulting in brain tumor promotion or inhibition. However, there are still many unsolved questions. For example, the crucial factors and mechanisms that mediate the interaction of microglia/macrophages with cancer cells in the brain tumor/metastasis remain largely unknown. Likewise, the interaction of microglia/macrophages and other microenvironmental cells in the brain (e.g., astrocyte) might contribute to tumor growth, but the detailed mechanisms involved in their communications remain unclear. Although microglial cells and macrophages are recruited into the tumor mass and promote tumor growth, whether they execute distinctively different functions in brain tumor progression is still unknown. In addition, brain metastasis is a multistep process; we do not know how metastatic cells escape the immune attack of microglia/macrophages to colonize the brain microenvironment. These unanswered questions need further investigation, and understanding the role of microglia/macrophages in brain tumor could contribute to the development of new therapy for brain tumor. The functional impairment of microglia/macrophages occurs in the early stages of brain tumor/metastasis; therefore, early intervention by microglia/macrophage activation may also provide a potential therapeutic direction in brain tumor/metastasis.
Acknowledgments
This study was supported by grants R01CA173499, R01CA185650, R01CA205067 from NIH.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32:4655–62. [PubMed] [Google Scholar]
- 3.Polivka J, Jr, Polivka J, Holubec L, Kubikova T, Priban V, Hes O, Pivovarcikova K, Treskova I. Advances in Experimental Targeted Therapy and Immunotherapy for Patients with Glioblastoma Multiforme. Anticancer Res. 2017;37:21–33. doi: 10.21873/anticanres.11285. [DOI] [PubMed] [Google Scholar]
- 4.Chinot OL, de La Motte Rouge T, Moore N, Zeaiter A, Das A, Phillips H, Modrusan Z, Cloughesy T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334–40. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 5.Staberg M, Michaelsen SR, Rasmussen RD, Villingshoj M, Poulsen HS, Hamerlik P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol (Dordr) 2017;40:21–32. doi: 10.1007/s13402-016-0301-9. [DOI] [PubMed] [Google Scholar]
- 6.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40:252–9. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 7.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–55. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 8.Huettner C, Czub S, Kerkau S, Roggendorf W, Tonn JC. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17:3217–24. [PubMed] [Google Scholar]
- 9.Wagner S, Czub S, Greif M, Vince GH, Suss N, Kerkau S, Rieckmann P, Roggendorf W, Roosen K, Tonn JC. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–6. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padalecki SS, Guise TA. Actions of bisphosphonates in animal models of breast cancer. Breast Cancer Res. 2002;4:35–41. doi: 10.1186/bcr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, Siam L, Hoffmann A, Trumper L, Stadelmann C, Bechmann I, Hanisch UK, Binder C. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58:1477–89. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- 14.Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–16. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 15.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol. 2013;2013:285246. doi: 10.1155/2013/285246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima K, Tohyama Y, Maeda S, Kohsaka S, Kurihara T. Neuronal regulation by which microglia enhance the production of neurotrophic factors for GABAergic, catecholaminergic, and cholinergic neurons. Neurochem Int. 2007;50:807–20. doi: 10.1016/j.neuint.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74:269–77. doi: 10.1007/BF00688191. [DOI] [PubMed] [Google Scholar]
- 20.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92:288–93. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 21.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 22.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 23.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–51. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, Szulzewsky F, Yerevanian A, Chen Z, Heinzmann D, Rasmussen RD, Alvarez-Garcia V, Kim Y, Wang B, Tamagno I, Zhou H, Li X, Kettenmann H, Ransohoff RM, Hambardzumyan D. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–94. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller A, Brandenburg S, Turkowski K, Muller S, Vajkoczy P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer. 2015;137:278–88. doi: 10.1002/ijc.29379. [DOI] [PubMed] [Google Scholar]
- 27.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61. doi: 10.1097/00006123-200004000-00035. discussion 961–2. [DOI] [PubMed] [Google Scholar]
- 28.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–71. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 30.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50:298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- 31.Murata J, Ricciardi-Castagnoli P, Dessous L’Eglise Mange P, Martin F, Juillerat-Jeanneret L. Microglial cells induce cytotoxic effects toward colon carcinoma cells: measurement of tumor cytotoxicity with a gamma-glutamyl transpeptidase assay. Int J Cancer. 1997;70:169–74. doi: 10.1002/(sici)1097-0215(19970117)70:2<169::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1120–6. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellert-Miklaszewska A, Dabrowski M, Lipko M, Sliwa M, Maleszewska M, Kaminska B. Molecular definition of the pro-tumorigenic phenotype of glioma-activated microglia. Glia. 2013;61:1178–90. doi: 10.1002/glia.22510. [DOI] [PubMed] [Google Scholar]
- 34.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 37.Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol. 2001;166:5168–75. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 38.Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56:732–40. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- 39.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 40.Pace JL, Russell SW. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981;126:1863–7. [PubMed] [Google Scholar]
- 41.Pace JL, Taffet SM, Russell SW. The effect of endotoxin in eliciting agents on the activation of mouse macrophages for tumor cell killing. J Reticuloendothel Soc. 1981;30:15–21. [PubMed] [Google Scholar]
- 42.Sarkar S, Doring A, Zemp FJ, Silva C, Lun X, Wang X, Kelly J, Hader W, Hamilton M, Mercier P, Dunn JF, Kinniburgh D, van Rooijen N, Robbins S, Forsyth P, Cairncross G, Weiss S, Yong VW. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Jones LM, Broz ML, Ranger JJ, Ozcelik J, Ahn R, Zuo D, Ursini-Siegel J, Hallett MT, Krummel M, Muller WJ. STAT3 Establishes an Immunosuppressive Microenvironment during the Early Stages of Breast Carcinogenesis to Promote Tumor Growth and Metastasis. Cancer Res. 2016;76:1416–28. doi: 10.1158/0008-5472.CAN-15-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–84. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Devgan G, Darnell JE, Jr, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A. 2001;98:1543–8. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Handel MV, Schartner JM, Hagar A, Allen G, Curet M, Badie B. Regulation of IL-10 expression by upstream stimulating factor (USF-1) in glioma-associated microglia. J Neuroimmunol. 2007;184:188–97. doi: 10.1016/j.jneuroim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T, Suzu S, Nakamura H, Kuratsu J, Takeya M. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci. 2012;103:2165–72. doi: 10.1111/cas.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 53.Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, Xu K, Wang Y, Wu A. IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011;38:3585–91. doi: 10.1007/s11033-010-0469-4. [DOI] [PubMed] [Google Scholar]
- 54.Zeiner PS, Preusse C, Blank AE, Zachskorn C, Baumgarten P, Caspary L, Braczynski AK, Weissenberger J, Bratzke H, Reiss S, Pennartz S, Winkelmann R, Senft C, Plate KH, Wischhusen J, Stenzel W, Harter PN, Mittelbronn M. MIF Receptor CD74 is Restricted to Microglia/Macrophages, Associated with a M1-Polarized Immune Milieu and Prolonged Patient Survival in Gliomas. Brain Pathol. 2015;25:491–504. doi: 10.1111/bpa.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay BH, Chang AY. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006;12:161–70. doi: 10.2119/2006-00033.He. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51:279–85. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Swarnkar S, Goswami P, Nath C. Astrocytes and microglia: responses to neuropathological conditions. Int J Neurosci. 2011;121:589–97. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- 58.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 59.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–27. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 60.Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S, Weller M. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54:388–92. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 61.Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, Iwama T, Takami T. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–7. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 62.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 65.Galasso JM, Stegman LD, Blaivas M, Harrison JK, Ross BD, Silverstein FS. Experimental gliosarcoma induces chemokine receptor expression in rat brain. Exp Neurol. 2000;161:85–95. doi: 10.1006/exnr.1999.7249. [DOI] [PubMed] [Google Scholar]
- 66.Kuratsu J, Yoshizato K, Yoshimura T, Leonard EJ, Takeshima H, Ushio Y. Quantitative study of monocyte chemoattractant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993;85:1836–9. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- 67.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–84. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 68.Giulian D, Ingeman JE. Colony-stimulating factors as promoters of ameboid microglia. J Neurosci. 1988;8:4707–17. doi: 10.1523/JNEUROSCI.08-12-04707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999;155:1557–67. doi: 10.1016/S0002-9440(10)65472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park MH, Lee JS, Yoon JH. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol. 2012;106:386–92. doi: 10.1002/jso.23095. [DOI] [PubMed] [Google Scholar]
- 71.Held-Feindt J, Hattermann K, Muerkoster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, Mehdorn HM, Mentlein R. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Exp Cell Res. 2010;316:1553–66. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Ferretti E, Pistoia V, Corcione A. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm. 2014;2014:480941. doi: 10.1155/2014/480941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–95. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, Soria JC, Cristofanilli M. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–51. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 76.Sarmiento M. Use of confocal microscopy in the study of microglia in a brain metastasis model. Methods Mol Biol. 2013;1041:337–46. doi: 10.1007/978-1-62703-520-0_29. [DOI] [PubMed] [Google Scholar]
- 77.Hao C, Parney IF, Roa WH, Turner J, Petruk KC, Ramsay DA. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 2002;103:171–8. doi: 10.1007/s004010100448. [DOI] [PubMed] [Google Scholar]
- 78.Ghosh A, Chaudhuri S. Microglial action in glioma: a boon turns bane. Immunol Lett. 2010;131:3–9. doi: 10.1016/j.imlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–78. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33:312–9. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]
- 81.Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23:1553–9. doi: 10.3892/or_00000795. [DOI] [PubMed] [Google Scholar]
- 82.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 1999;64:1941–53. doi: 10.1016/s0024-3205(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 83.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 84.Abraham CS, Deli MA, Joo F, Megyeri P, Torpier G. Intracarotid tumor necrosis factor-alpha administration increases the blood-brain barrier permeability in cerebral cortex of the newborn pig: quantitative aspects of double-labelling studies and confocal laser scanning analysis. Neurosci Lett. 1996;208:85–8. doi: 10.1016/0304-3940(96)12546-5. [DOI] [PubMed] [Google Scholar]
- 85.Murphy GM, Jr, Bitting L, Majewska A, Schmidt K, Song Y, Wood CR. Expression of interleukin-11 and its encoding mRNA by glioblastoma cells. Neurosci Lett. 1995;196:153–6. doi: 10.1016/0304-3940(95)11862-q. [DOI] [PubMed] [Google Scholar]
- 86.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 Tumour-Associated Macrophages (TAMs) and Th1/Th2 Cytokine Profiles in Patients with NSCLC. Cancer Microenviron. 2016;9:1–11. doi: 10.1007/s12307-015-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rincon M, Tugores A, Lopez-Rivas A, Silva A, Alonso M, De Landazuri MO, Lopez-Botet M. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988;18:1791–6. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- 89.Rivkin I, Rosenblatt J, Becker EL. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975;115:1126–34. [PubMed] [Google Scholar]
- 90.Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, Jiang XF, Yang L, Zhang P, Qian C, Cui YH, Zhang X, Bian XW. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189:444–53. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 91.Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S, Kaminska B. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion–an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–30. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 92.Merzak A, McCrea S, Koocheckpour S, Pilkington GJ. Control of human glioma cell growth, migration and invasion in vitro by transforming growth factor beta 1. Br J Cancer. 1994;70:199–203. doi: 10.1038/bjc.1994.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, Cuartas I, Garcia-Dorado D, Poca MA, Sahuquillo J, Baselga J, Seoane J. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–27. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 94.Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–14. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 95.Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Synowitz M, Glass R, Kettenmann H. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol. 2013;125:609–20. doi: 10.1007/s00401-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 96.Badie B, Schartner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery. 1999;44:1077–82. doi: 10.1097/00006123-199905000-00075. discussion 1082–3. [DOI] [PubMed] [Google Scholar]
- 97.Rosen EM, Laterra J, Joseph A, Jin L, Fuchs A, Way D, Witte M, Weinand M, Goldberg ID. Scatter factor expression and regulation in human glial tumors. Int J Cancer. 1996;67:248–55. doi: 10.1002/(SICI)1097-0215(19960717)67:2<248::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 98.Di Renzo MF, Bertolotto A, Olivero M, Putzolu P, Crepaldi T, Schiffer D, Pagni CA, Comoglio PM. Selective expression of the Met/HGF receptor in human central nervous system microglia. Oncogene. 1993;8:219–22. [PubMed] [Google Scholar]
- 99.Yamagata T, Muroya K, Mukasa T, Igarashi H, Momoi M, Tsukahara T, Arahata K, Kumagai H, Momoi T. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem Biophys Res Commun. 1995;210:231–7. doi: 10.1006/bbrc.1995.1651. [DOI] [PubMed] [Google Scholar]
- 100.Mayes DA, Hu Y, Teng Y, Siegel E, Wu X, Panda K, Tan F, Yung WK, Zhou YH. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res. 2006;66:9809–17. doi: 10.1158/0008-5472.CAN-05-3877. [DOI] [PubMed] [Google Scholar]
- 101.Guo P, Imanishi Y, Cackowski FC, Jarzynka MJ, Tao HQ, Nishikawa R, Hirose T, Hu B, Cheng SY. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–90. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada T, Yoshiyama Y, Sato H, Seiki M, Shinagawa A, Takahashi M. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta Neuropathol. 1995;90:421–4. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- 103.Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kalin R, van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106:12530–5. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshida S, Takahashi H. Expression of extracellular matrix molecules in brain metastasis. J Surg Oncol. 2009;100:65–8. doi: 10.1002/jso.21296. [DOI] [PubMed] [Google Scholar]
- 105.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 107.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65:331–5. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Fang KM, Wang YL, Huang MC, Sun SH, Cheng H, Tzeng SF. Expression of macrophage inflammatory protein-1alpha and monocyte chemoattractant protein-1 in glioma-infiltrating microglia: involvement of ATP and P2X(7) receptor. J Neurosci Res. 2011;89:199–211. doi: 10.1002/jnr.22538. [DOI] [PubMed] [Google Scholar]
- 109.Wollmer MA, Lucius R, Wilms H, Held-Feindt J, Sievers J, Mentlein R. ATP and adenosine induce ramification of microglia in vitro. J Neuroimmunol. 2001;115:19–27. doi: 10.1016/s0165-5728(01)00257-0. [DOI] [PubMed] [Google Scholar]
- 110.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–82. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lambert C, Ase AR, Seguela P, Antel JP. Distinct migratory and cytokine responses of human microglia and macrophages to ATP. Brain Behav Immun. 2010;24:1241–8. doi: 10.1016/j.bbi.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–86. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Imura Y, Morizawa Y, Komatsu R, Shibata K, Shinozaki Y, Kasai H, Moriishi K, Moriyama Y, Koizumi S. Microglia release ATP by exocytosis. Glia. 2013;61:1320–30. doi: 10.1002/glia.22517. [DOI] [PubMed] [Google Scholar]
- 115.Jantaratnotai N, Choi HB, McLarnon JG. ATP stimulates chemokine production via a store-operated calcium entry pathway in C6 glioma cells. BMC Cancer. 2009;9:442. doi: 10.1186/1471-2407-9-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrone FB, Horn AP, Stella J, Spiller F, Sarkis JJ, Salbego CG, Lenz G, Battastini AM. Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J Neurooncol. 2005;71:135–40. doi: 10.1007/s11060-004-1383-1. [DOI] [PubMed] [Google Scholar]
- 117.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–71. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 119.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang M, Li D, Zhao Y, Ge R, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380:205–10. doi: 10.1016/j.bbrc.2008.12.169. [DOI] [PubMed] [Google Scholar]
- 121.Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–65. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 122.Xia H, Cheung WK, Ng SS, Jiang X, Jiang S, Sze J, Leung GK, Lu G, Chan DT, Bian XW, Kung HF, Poon WS, Lin MC. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem. 2012;287:9962–71. doi: 10.1074/jbc.M111.332627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620–32. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–31. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, Zhou L, Chen Z, Ng HK. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009;40:1234–43. doi: 10.1016/j.humpath.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16:601–3. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- 131.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, Badie SA, Badie B. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–98. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, Wu S, da Fonseca AC, Weng L, Yamamoto Y, Yamamoto H, Natarajan R, Badie B. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74:7285–97. doi: 10.1158/0008-5472.CAN-14-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klemm F, Bleckmann A, Siam L, Chuang HN, Rietkotter E, Behme D, Schulz M, Schaffrinski M, Schindler S, Trumper L, Kramer F, Beissbarth T, Stadelmann C, Binder C, Pukrop T. beta-catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434–42. doi: 10.1093/carcin/bgq269. [DOI] [PubMed] [Google Scholar]
- 135.Bleckmann A, Siam L, Klemm F, Rietkotter E, Wegner C, Kramer F, Beissbarth T, Binder C, Stadelmann C, Pukrop T. Nuclear LEF1/TCF4 correlate with poor prognosis but not with nuclear beta-catenin in cerebral metastasis of lung adenocarcinomas. Clin Exp Metastasis. 2013;30:471–82. doi: 10.1007/s10585-012-9552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chuang HN, van Rossum D, Sieger D, Siam L, Klemm F, Bleckmann A, Bayerlova M, Farhat K, Scheffel J, Schulz M, Dehghani F, Stadelmann C, Hanisch UK, Binder C, Pukrop T. Carcinoma cells misuse the host tissue damage response to invade the brain. Glia. 2013;61:1331–46. doi: 10.1002/glia.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, Kumar Puli L, Farber K, Harkany T, Schulte G. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–31. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 140.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, Miura N, Yla-Herttuala S, Fruttiger M, Makinen T, Eichmann A, Pollard JW, Gerhardt H, Alitalo K. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–13. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–41. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665–76. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 143.Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, Chan MD, Zhou X, Qasem SA, Pochampally R, Mo YY, Watabe K. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290:9842–54. doi: 10.1074/jbc.M114.602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5:384–96. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, Kim H, Joo KM, Lee DS, Price JE, Bang SI, Park WY. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 2008;14:4059–66. doi: 10.1158/1078-0432.CCR-07-4039. [DOI] [PubMed] [Google Scholar]
- 146.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E, Jandial R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A. 2014;111:984–9. doi: 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Larsson OM, Schousboe A. Kinetic characterization of GABA-transaminase from cultured neurons and astrocytes. Neurochem Res. 1990;15:1073–7. doi: 10.1007/BF01101706. [DOI] [PubMed] [Google Scholar]
- 148.Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 149.Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol. 2002;132:93–8. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- 150.Johnson B, Osada T, Clay T, Lyerly H, Morse M. Physiology and therapeutics of vascular endothelial growth factor in tumor immunosuppression. Curr Mol Med. 2009;9:702–7. doi: 10.2174/156652409788970634. [DOI] [PubMed] [Google Scholar]
- 151.Martin S, Dicou E, Vincent JP, Mazella J. Neurotensin and the neurotensin receptor-3 in microglial cells. J Neurosci Res. 2005;81:322–6. doi: 10.1002/jnr.20477. [DOI] [PubMed] [Google Scholar]
- 152.Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB, Rich JN. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poli A, Wang J, Domingues O, Planaguma J, Yan T, Rygh CB, Skaftnesmo KO, Thorsen F, McCormack E, Hentges F, Pedersen PH, Zimmer J, Enger PO, Chekenya M. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–46. doi: 10.18632/oncotarget.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, Hirano H, Arita K, Matsuyama T. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol Immunother. 2009;58:1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–85. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hwang SY, Yoo BC, Jung JW, Oh ES, Hwang JS, Shin JA, Kim SY, Cha SH, Han IO. Induction of glioma apoptosis by microglia-secreted molecules: The role of nitric oxide and cathepsin B. Biochim Biophys Acta. 2009;1793:1656–68. doi: 10.1016/j.bbamcr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 157.Mora R, Abschuetz A, Kees T, Dokic I, Joschko N, Kleber S, Geibig R, Mosconi E, Zentgraf H, Martin-Villalba A, Regnier-Vigouroux A. TNF-alpha- and TRAIL-resistant glioma cells undergo autophagy-dependent cell death induced by activated microglia. Glia. 2009;57:561–81. doi: 10.1002/glia.20785. [DOI] [PubMed] [Google Scholar]
- 158.Chiu TL, Peng CW, Wang MJ. Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncol Rep. 2011;25:1373–80. doi: 10.3892/or.2011.1213. [DOI] [PubMed] [Google Scholar]
- 159.Kees T, Lohr J, Noack J, Mora R, Gdynia G, Todt G, Ernst A, Radlwimmer B, Falk CS, Herold-Mende C, Regnier-Vigouroux A. Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neuro Oncol. 2012;14:64–78. doi: 10.1093/neuonc/nor182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, Kettenmann H. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25:624–8. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 162.Weingart JD, Sipos EP, Brem H. The role of minocycline in the treatment of intracranial 9L glioma. J Neurosurg. 1995;82:635–40. doi: 10.3171/jns.1995.82.4.0635. [DOI] [PubMed] [Google Scholar]
- 163.Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B. Tumor-associated macrophages are predominant carriers of cyclodextrin-based nanoparticles into gliomas. Nanomedicine. 2010;6:382–90. doi: 10.1016/j.nano.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.VanHandel M, Alizadeh D, Zhang L, Kateb B, Bronikowski M, Manohara H, Badie B. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. J Neuroimmunol. 2009;208:3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 165.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda DG, Ancukiewicz M, Plotkin SR, Chi AS, Gerstner ER, Eichler AF, Dietrich J, Stemmer-Rachamimov AO, Batchelor TT, Jain RK. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15:1079–87. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Piao Y, Liang J, Holmes L, Zurita AJ, Henry V, Heymach JV, de Groot JF. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol. 2012;14:1379–92. doi: 10.1093/neuonc/nos158. [DOI] [PMC free article] [PubMed] [Google Scholar]


