Abstract
Stereotactic radiosurgery (SRS) can be used as part of multimodality management for patients with primary central nervous system lymphoma (PCNSL). The objective of this study is to evaluate outcomes of SRS for this disease.
The International Gamma Knife Research Foundation identified 23 PCNSL patients who underwent SRS for either relapsed (intracerebral in-field or out-of-field tumor recurrences) or refractory disease from 1995-2014. All 23 patients presented with RPA Class I or II PCNSL, and were initially treated with a median of 7 cycles of methotrexate-based chemotherapy regimens (range, 3-26 cycles). Ten received prior whole brain radiation (WBRT) to a median dose of 43 Gy (range, 24-55 Gy). Sixteen presented with relapsed PCNSL, and seven presented with refractory disease. Twenty-three received 26 procedures of SRS. The median tumor volume was 4 cm3 (range, 0.1-26 cm3), and the median margin dose was 15 Gy (range, 8-20 Gy). Median follow-up from SRS was 11 months (interquartile range, 5.7-33.2 months). Twenty presented with treatment response to twenty-three tumors (12 complete, 11 partial). Fourteen patients relapsed or were refractory to salvage SRS, and local control was 95%, 91%, and 75% at 3, 6, and 12 months post SRS. Intracranial (in-field and out-of-field) and distant (systemic) PFS was 86%, 81%, and 55% at 3, 6, and 12 months post SRS. Toxicity of SRS was low, with one developing an adverse radiation effect requiring no additional intervention.
Although methotrexate-based chemotherapy regimens with or without WBRT is the first-line management option for PCNSL, SRS may be used as an alternative option in properly selected patients with smaller relapsed or refractory PCNSL tumors.
Keywords: radiosurgery, primary central nervous system lymphoma, salvage, International Gamma Knife Research Foundation
Introduction
Although primary central nervous system lymphomas (PCNSL) are uncommon, accounting for 4 percent of CNS malignancies, the incidence of these tumors has slightly risen especially in the elderly population and in those with infection with human immunodeficiency virus (HIV) leading to acquired immunodeficiency syndrome1,2. Unlike other CNS malignancies, surgery does not commonly play a role except in establishing a diagnosis. Treatment is based on a high-dose methotrexate-based (MTX) chemotherapy regimen alone or with consolidative whole brain radiotherapy (WBRT). Patients with good performance status and renal function are initially treated with induction MTX-based chemotherapy regimens followed by WBRT, resulting in a predicted median survival as high as 8.5 years3. Poor-performance patients can be managed with WBRT alone, resulting in inferior rates of tumor control and survival compared to combined modality treatment4.
In the elderly, WBRT is often deferred following complete response to MTX-based chemotherapy regimens, due to concerns of unacceptable neurotoxicity5. On the other hand, younger patients, generally <60 years of age, with good performance status and renal function, can be treated with high-dose MTX-based chemotherapy regimens followed by WBRT for improved outcomes2,6. Although survival has improved following the introduction of MTX-based chemotherapy regimens, 35%-60% of patients may relapse or become refractory to first-line treatment, requiring salvage treatment with regimens including temozolomide, topotecan, rituximab, high-dose cytarabine, or high-dose chemotherapy with autologous stem cell transplantation6-9. However, none of these regimens have been established as standard of care, resulting in a heterogeneity of treatment options, and addressing the need for standardized regimens established through prospective trials or derived from large multi-institutional experiences especially in patients presenting with relapsed or refractory PCNSL.
SRS has been used primarily in the salvage setting for patients presenting with relapse of small tumors10,13. Since a favorable therapeutic ratio may be achieved using this treatment modality, especially with a potentially low risk of treatment-induced neurotoxicity, we evaluated the outcomes of SRS in patients with PCNSL. Due to the rarity of this condition, we collected retrospective data provided by 6 medical centers to evaluate the outcomes of PCNSL patients treated with SRS.
Materials and Methods
Study population
Individual institutional review board approval was obtained for each medical center participating in this study. All participating centers are members of the International Gamma Knife Research Foundation (IGKRF). The following centers contributed to this study: New York University (N=1), Universite de Sherbrooke (N=1), Tapei Veterans General Hospital (N=2), University of Manitoba (N=1), University of Virginia (N=4), and University of Pittsburgh (N=14). All patients were diagnosed with PCNSL, were treated with Gamma Knife SRS from 1995-2014, and were assessed by each center for inclusion into this study. A database with variables including age, gender, Karnofsky Performance Status (KPS), date of diagnosis, histopathologic diagnosis, imaging information, chemotherapy details, WBRT treatment details, tumor response to initial treatment, date of relapse or progression following initial treatment, location of tumor relapse (intracerebral in-field, intracerebral out-of-field, or systemic tumor recurrences) or progression of refractory disease, date of salvage with SRS, tumor volume, SRS dose (margin and maximum), SRS treatment response, date and location of relapse or progression of refractory disease following SRS, date of death or last follow-up, and details of adverse treatment effect were sent to all participating centers. Due to the multifocal nature of PCNSL, patients presenting with relapsed or refractory disease were included in this analysis if there was imaging confirmation of localized disease within the brain (i.e. 1-3 tumors). Data was collected by participating investigators, removing all patient identifiers, and were sent to principal investigators at NYU for final data analysis. For each patient, we used recursive partitioning analysis (RPA), which includes age and KPS[3].
Parameters associated with overall survival (OS) and intracranial progression-free survival (PFS) were assessed including presence of multifocal disease at time of diagnosis, tumor volume (≥4 cm3 or <4 cm3; dichotomized by a median tumor volume of 4 cm3 for the evaluated cohort), gender (male or female)12, age (≥50 years or <50 years)3, , location in deep structure of brain (yes or no)[13], prior treatment with WBRT, SRS dose (≥15 Gy or <15 Gy; dichotomized by a median SRS dose of 15 Gy for the evaluated cohort), and tumor response to SRS (partial versus complete). SRS treatment response (complete=CR or partial response=PR) was evaluated on the first post-treatment follow-up imaging studies with either MRI or CT. CR was defined as complete disappearance of enhancing or nonenhancing tumor, and PR was defined as shrinkage of tumor volume with residual enhancing or nonenhancing tumor at the site of treatment. Concurrent treatment with systemic therapy and SRS was defined as treatment with systemic therapy either on the same day or within 2 months of treatment with SRS. Concurrent treatment with systemic therapy and SRS was also evaluated with respect to OS and PFS. Following SRS treatment, radiographic imaging was reviewed by the treating physicians and their respective institution’s neuroradiologists to interpret whether the patient’s treated tumors resulted in adverse radiation effects (ARE), tumor progression or pseudo-progression14.
Radiosurgery technique
At each participating center, patients in this study were treated with SRS using Gamma Knife technique. Radiosurgery has been described in detail in previous reports15. In brief, SRS was performed under local anesthesia using a Leksell Gamma Knife (Models U, B, C, 4C, or Perfexion®; Stockholm, Sweden), depending on the technology available in all participating centers. After application of a stereotactic head frame, high-resolution MRI studies were obtained unless the patient had a contraindication to MRI (ferrous aneurysm clip, etc.) and therefore was imaged using cranial computed tomography (CT). SRS target volume, delineated on Gamma Plan, maximal and margin dose to the tumor were determined at the time of dose planning. SRS treatment dose was determined based on the size of the contoured tumor, location of the tumor, and whether the patients were treated with prior radiation treatment. No margin was added to the segmented tumor volume. Following SRS, patients were typically discharged the same day and follow-up MRI or CT was performed every 2-3 months thereafter or according to the participating institution’s follow-up protocol to assess tumor treatment response.
Statistical analysis
OS, PFS (including intracranial in-field, out-of-field, and/or systemic tumor recurrences), and local control (LC) from time of SRS were estimated and plotted using the Kaplan-Meier Method. Univariate analyses of significance were also performed using the Kaplan-Meier Method. Significance was assessed using the log-rank statistic with a two-sided p < 0.05 set as the level of significance. All calculations were performed using IBM SPSS, version 21, (SPSS; Chicago, IL, USA).
Results
Patient characteristics
Twenty-three patients were included in this multi-institutional analysis with detailed patient characteristics shown in Table 1. Median age was 62 (range, 21-84 years). The majority of patients were male (57%), and were RPA Class II (78%). All patients had KPS≥70. One patient had history of HIV infection. Diagnostic evaluation with biopsy confirmed diffuse large B-cell lymphoma in all patients evaluated in this study, and the majority of patients were treated with MTX-based chemotherapy regimens (84%). Six patients initially presented with multifocal disease. Following treatment with MTX, 10 patients received WBRT. Ten patients were treated with second-line treatment regimens with temozolomide and high-dose cytarabine (N=1), autologous stem cell transplantation (N=1), high-dose cytarabine followed by rituximab (N=6), or high dose-cytarabine (N=2).
Table 1.
Patient and radiosurgery characteristics
| Age | |
| • Median (range) | 62 (21-84) |
| Gender (N=23 patients) | |
| • Male | 13 (57 %) |
| • Female | 10 (43 %) |
| KPS (N=23 patients) | |
| 70-100 | 23 (100%) |
| RPA (N=23 patients) | |
| • I | 5 (22%) |
| • II | 18 (78%) |
| Histopathology (N=23 patients) | |
| • Diffuse large B-cell lymphoma | 23 (100%) |
| Cycles of methotrexate-based chemotherapy regimens | |
| • Median (range) | 7 (1-26 cycles) |
| Prior external beam radiation dose | |
| • Median dose, Gy (range) | 43 Gy (24-55 Gy) |
| Number of months between methotrexate-based chemotherapy and SRS | |
| • Median (range) | 2 months (1-159 months) |
| Tumor location (N=26 tumors) | |
| • Paraventricular | 3 (12%) |
| • Cerebellum | 5 (18%) |
| • Frontal lobe | 4 (15%) |
| • Parietal lobe | 4 (15%) |
| • Temporal lobe | 3 (12%) |
| • Occipital lobe | 2 (8%) |
| • Thalamus | 1 (4%) |
| • Basal ganglia | 2 (8%) |
| • Medulla oblongata | 1 (4%) |
| • Retina | 1 (4%) |
| Tumor presentation prior to SRS (N=23 patients) | |
| • Relapsed | 16 (70%) |
| • Refractory with progression | 7 (30%) |
| Total tumor volume (cm3) | |
| • Median (range) | 4 cm3 (0.1-26 cm3) |
| Maximum tumor diameter (cm) | |
| • Median (range) | 2 cm (0.2-3.0 cm) |
| Margin dose (Gy) | |
| • Median (range) | 15 Gy (8-20 Gy) |
| Maximum dose (Gy) | |
| • Median (range) | 30 Gy (16-40 Gy) |
| Concurrent treatment with SRS and chemotherapy (N=23 patients) | |
| • Yes | 6 (26%) |
| • No | 17 (74%) |
KPS Karnofsky Performance Status, RPA Recursive Partitioning Analysis, SRS Stereotactic Radiosurgery
Tumor response and local control
Following SRS in twenty-three patients, twenty patients presented with treatment response to twenty-three tumors (12 CR, 11 PR) at the time of their first follow-up MRI or CT 2-3 months following SRS (Figure 1). One patient had a PR and CR to two intracerebral out-of-field recurrences treated with SRS in the same treatment session. Another patient had CR following SRS for his first intracerebral in-field recurrence of PCNSL, and PR following a second procedure of SRS for a second intracerebral out-of-field recurrence. A third patient had CR following SRS for her first intracerebral out-of-field recurrence of PCNSL, and CR for a second intracerebral out-of-field recurrence treated with SRS. Five patients were disease-free at most recent follow-up.
Figure 1.
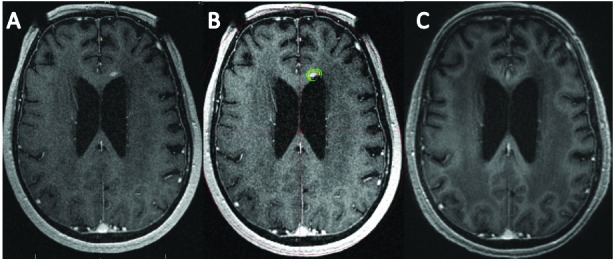
A) Axial multiplanar reconstruction (MPR) MR image obtained at time of SRS for treatment of recurrent PCNSL tumor in the left aspect of the genu of the corpus callosum. B) The tumor volume was 0.1 cm3 and was treated to a margin dose of 20 Gy (Yellow=20 Gy, Green=12 Gy). C) Post-SRS MPR MR image 8 months later demonstrates complete regression of tumor.
Fourteen patients had relapsed or progression of refractory disease following SRS. Twelve patients had 13 relapsed tumors (n=6 intracerebral out-of-field, n=6 intracerebral in-field, n=1 systemic tumor recurrences), and 2 had disease refractory to salvage SRS, One patient presented with intracerebral in-field recurrences to 2 discrete tumors treated with SRS. Median time to tumor progression of refractory disease or relapse following SRS was 8 months. Local control at 3, 6, and 12 months from time of SRS was 95%, 910%, and 75%, respectively.
Overall survival and intracranial progression-free survival
One-year OS from time of SRS was 47% (Figure 2). PFS at 3, 6, and 12 months from time of SRS was 86%, 81%, and 55%, respectively (Figure 3). None of the clinical variables analyzed were associated with decreased OS from time of SRS including, presence of multifocal disease at diagnosis, male gender, age ≥50 years, prior treatment with WBRT, SRS dose, concurrent use of systemic therapy with SRS, location in deep-seated structure of brain, or partial tumor response to SRS. However, tumor volume ≥4 cm3 trended towards significance with respect to worse OS (p=0.08). The cause of death was reported to be due to progression of intracerebral lymphoma in two patients. None of the studied clinical variables were associated with decreased PFS from time of SRS., except for tumor volume ≥4 cm3 (p=0.049).
Figure 2.
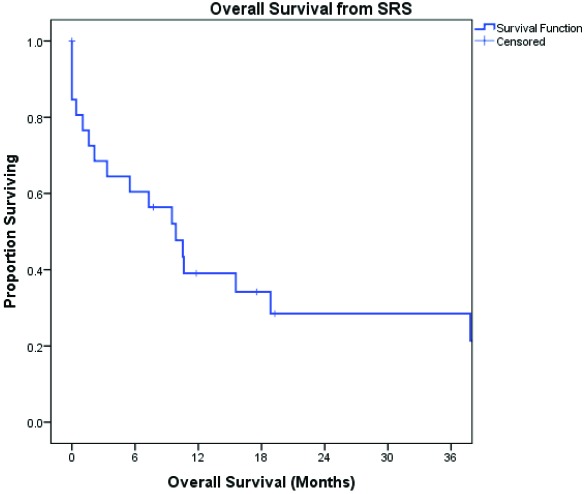
OS from time of SRS
Figure 3.
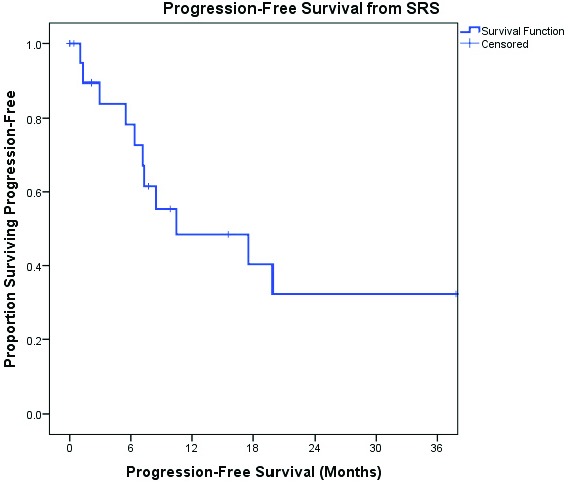
PFS from time of SRS
Following SRS, one patient developed a grade 1 ARE, which was seen on follow-up imaging. The patient did not develop symptomatic change requiring additional intervention. The treated tumor completely regressed on subsequent imaging studies.
Discussion
Approximately one third of PCNSL patients will present with disease refractory to first-line MTX-based chemotherapy regimens, requiring utilization of salvage therapy16. Additionally, up to 60% of patients will present with relapsed disease following initial tumor response to first-line treatment6. Although there is no current standard approach to the second-line management of relapsed or refractory PCNSL, the outcomes of this study demonstrate that patients can be safely and effectively be treated with SRS in the second-line setting, with one patient developing a grade 1 ARE.
SRS has primarily been used in the palliative setting for patients with relapsed or refractory PCNSL17-19,10 (Table 2). In this multi-institutional study, all patients in this study were treated with SRS for relapsed or refractory tumors, with 20 patients demonstrating clinical response (CR and PR). These findings are consistent with other case series, including a single institution retrospective series of 9 patients with 17 relapsed tumors, demonstrating treatment response in 76% of treated tumors10. Another single institution retrospective series of 22 patients also showed that in 18 patients who had received prior first-line therapy, there was 100% treatment response in tumors treated with SRS17. Despite treatment response, prognosis is still poor for patients treated with SRS, with a median time to tumor progression or relapse of 8 months, and a 1-year OS and PFS of 47% and 55%. These findings are consistent with the results of other retrospective and small phase II studies evaluating the use of other salvage therapies including combination therapy with rituximab and temozolomide or topotecan, but they are inferior to the results of a study evaluating the use of intensive chemotherapy with hematopoietic stem-cell rescue7,9,8.
Table 2.
Selected series outcomes of relapsed or refractory PCNSL patients treated with SRS
| Author | n | Single or Multi-institutional | Data Collection | Median Margin Dose | Median Tumor Volume | 1 Year PFS/OS | 1 year LC |
| Sakamoto et al. 10 | 9 | Single | Retrospective | 12 Gy | 3.5 cm3 | 22%/58% | 89% |
| Kenai et al. 17 | 22 | Single | Retrospective | 16.5 Gy | 4 cm3 | Not Reported | 100% |
| Matsumoto et al. 18 | 6 | Single | Retrospective | Not Reported | Not Reported | Median PFS/OS: 11 months/17 months | 67% |
| Kumar et al. 19 | 14 | Single | Retrospective | 15.5 Gy | 6.7 cm3 | Median PFS/OS: 4 months/9.5 months | 78% |
| Current Study | 23 | Multi | Retrospective | 15 Gy | 4 cm3 | 55%/47% | 75% |
PCNSL Primary Central Nervous System Lymphoma, SRS Stereotactic radiosurgery, PFS Progression-free survival, OS Overall survival, LC Local Control
Older age, a variable commonly associated with poorer outcomes, demonstrated no significant association with poorer OS or PFS in this current study3. Tumor volume ≥4 cm3 was associated with inferior PFS on univariate analysis, which suggests that SRS may not be as adequate of a salvage option in patients presenting with larger refractory or relapsed tumors.
Important limitations of this study include sample size, which may limit the interpretation of clinical factors that may be associated with better prognosis in patients presenting with this rare tumor. Although SRS can potentially reduce neurotoxicity compared to WBRT in elderly patients treated in the salvage setting, another limitation of this study is the absence of neurocognitive testing in this cohort. In addition, the retrospective nature of this study allows for inevitable selection bias with introduction of confounding variables. Lastly, as noted in other similar studies, follow-up is short, with a median follow-up of 11 months following initial SRS treatment.
Despite these limitations, this international research consortium effort further supports the findings demonstrated in prior institutional series of PCNSL patients initially managed with first-line MTX-based chemotherapy regimens and salvaged with SRS17-19,10. Although tolerability of alternative salvage treatments, including high dose chemotherapy and stem-cell transplantation, may be difficult in this population, patients in this study were safely and quickly treated with acceptable rates of tumor control and survival. Due to the favorable therapeutic ratio possible through the use of SRS, prospective evaluation with SRS combined with therapeutic agents including immunotherapy should be considered in patients with relapsed or refractory PCNSL20.
Conclusion
Although MTX-based chemotherapy regimens with or without WBRT is the first-line management option for patients with PCNSL, SRS may be safely used as an alternative local therapy option in properly selected patients with smaller relapsed or refractory PCNSL tumors. Further prospective studies may benefit from the insight provided by these results, to further improve the clinical outcomes of this rare disease.
Acknowledgements
Authors’ disclosure of potential conflicts of interest
Dr. Shin reports grants from Feinberg Lymphoma, during the conduct of the study; Dr. Lunsford is a consultant and stockholder for AB Elekta. All other authors reported no conflict of interest.
Author contributions
Conception and design: Samuel M. Shin, Joshua S. Silverman, and Douglas Kondziolka
Data collection: : Samuel M. Shin, Joshua S. Silverman, Greg Bowden, David Mathieu, Huai-Che Yang, Cheng-chia Lee, Moses Tam, Paul Szelemej, Anthony M. Kaufmann, Or Cohen-Inbar, Jason Sheehan, Ajay Niranjan, L. Dade Lunsford, and Douglas Kondziolka
Manuscript writing: Samuel M. Shin
Final approval of manuscript: Samuel M. Shin, Joshua S. Silverman, Greg Bowden, David Mathieu, Huai-Che Yang, Cheng-chia Lee, Moses Tam, Paul Szelemej, Anthony M. Kaufmann, Or Cohen-Inbar, Jason Sheehan, Ajay Niranjan, L. Dade Lunsford, and Douglas Kondziolka
References
- Villano JL, Koshy M, Shaikh H, et al. : Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. British Journal of Cancer. 2011;105 (9):1414–1418. doi:10.1038/bjc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor T, Loeffler JS: Primary CNS lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(8):1281–1288. doi:10.1200/jco.2005.04.881. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Ben-Porat L, Panageas KS, et al. : Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(36):5711–5715. doi:10.1200/jco.2006.08.294. [DOI] [PubMed] [Google Scholar]
- Nelson DF, Martz KL, Bonner H, et al. : Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. International Journal of Radiation Oncology, Biology, Physics. 1992:23(1):9–17 [DOI] [PubMed] [Google Scholar]
- DeAngelis LM, Seiferheld W, Schold SC, et al. : Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2002;20(24):4643–4648 [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Reni M, Foppoli M, Martelli M, et al. : High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet (London, England). 2009;374(9700):1512–1520. doi:10.1016/s0140-6736(09)61416-. [DOI] [PubMed] [Google Scholar]
- Enting RH, Demopoulos A, DeAngelis LM, et al. : Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004:63 (5):901–903 [DOI] [PubMed] [Google Scholar]
- Voloschin AD, Betensky R, Wen PY, et al. : Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. Journal of Neuro-oncology. 2008;86(2):211–215. doi:10.1007/s11060-007-9464-. [DOI] [PubMed] [Google Scholar]
- Soussain C, Suzan F, Hoang-Xuan K, et al. : Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2001;19(3):742–749 [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Oya N, Mizowaki T, et al. : Initial experiences of palliative stereotactic radiosurgery for recurrent brain lymphomas. Journal of Neuro-oncology. 2006;77(1):53–58. doi:10.1007/s11060-005-7698-. [DOI] [PubMed] [Google Scholar]
- Nicolato A, Gerosa MA, Foroni R, et al. : Gamma Knife radiosurgery in AIDS-related primary central nervous system lymphoma. Stereotactic and Functional Neurosurgery. 1995;64 Suppl 1:42–55 [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY, et al. : Survival among patients with primary central nervous system lymphoma, 1973-2004. Journal of Neuro-oncology. 2011;101(3):487–493. doi:10.1007/s11060-010-0269-. [DOI] [PubMed] [Google Scholar]
- Iwadate Y, Suganami A, Ikegami S, et al. : Non-deep-seated primary CNS lymphoma: therapeutic responses and a molecular signature. Journal of Neuro-oncology. 2014;117(2):261–268. doi:10.1007/s11060-014-1379-. [DOI] [PubMed] [Google Scholar]
- Cox JD, Stetz J, Pajak TF. (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). International Journal of Radiation Oncology, Biology, Physics. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Kano H, Harrison GL, et al. : Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. Journal of Neurosurgery. 2011;114(3):792–800. doi:10.3171/2010.8.jns1046. [DOI] [PubMed] [Google Scholar]
- Langner-Lemercier S, Houillier C, Soussain C, et al. : Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro-oncology, 2016. doi:10.1093/neuonc/now03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenai H, Yamashita M, Nakamura T, Asano T, et al. : Gamma Knife surgery for primary central nervous system lymphoma: usefulness as palliative local tumor control. Journal of Neurosurgery. 2006;105 Suppl:133–138. doi:10.3171/sup.2006.105.7.13. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Horiike S, Fujimoto Y, et al. : Effectiveness and limitation of gamma knife radiosurgery for relapsed central nervous system lymphoma: a retrospective analysis in one institution. International Journal of Hematology. 2007;85(4):333–7. doi:10.1532/IJH97.0620. [DOI] [PubMed] [Google Scholar]
- Kumar R, Laack N, Pollock BE, et al. : Stereotactic radiosurgery in the treatment of Recurrent CNS Lymphoma. World Neurosurgery. 2015;84(2):390–7. doi: 10.1016/j.wneu.2015.03.062. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Shin SM, Brunswick A, et al. : The biology of radiosurgery and its clinical applications for brain tumors. Neuro-oncology. 2015;17(1):29–44. doi:10.1093/neuonc/nou28. [DOI] [PMC free article] [PubMed] [Google Scholar]


