Abstract
Summary
Indications and treatment goals for SRS have changed since the publication of RTOG 90-05. We present initial retrospective outcomes from a new dose selection algorithm in use at our institution felt to be more contemporary with doses being used in the radiosurgery community today and report our local control and toxicity outcomes. This dose selection algorithm will be subject to a forthcoming prospective phase 2 trial.
Introduction
To evaluate safety and efficacy of an institutional dose selection algorithm in the treatment of brain metastases (BM) with single fraction radio-surgery (SRS).
Methods and Materials
The medical records of 65 patients with ≤10 BM treated with GK at our institution between April 2012 and October 2012 were reviewed retrospectively. The prescription doses used in this study ranged from 16-22Gy and were based upon RTOG 90-05 guideline doses subsequently modified at our institution depending on lesion number, lesion volume, institutional experience and prior history of whole brain radiation therapy (WBRT). Primary endpoint was local recurrence (LR) with additional outcomes measured including distant intracranial recurrence (DIR), death without local recurrence (DWLR) and alive and disease free (ADF). Fine Gray competing risk analysis was used to examine factors affecting local recurrence.
Results
Median follow up was 8.9 months (range 1.0-29.6months) and 12 month overall survival was 37% (95% CI 24.9-49.1%). Overall local recurrence rate was 7.7%. On competing risks regression analysis, no variable was significantly associated with local recurrence, including previous whole brain radiotherapy (WBRT), (SHR 1.21 [95%CI 0.13-11.5], p=0.87 and radioresistant versus radiosensitive histology (SHR 0.51 [95% CI 0.06-7.73], p=0.55). No patient developed grade 3 or higher neurotoxicity at 12 months following GK.
Conclusions
Initial local control and toxicity results from our institutional dose selection algorithm are reported here. Comparison of our results with RTOG 90-05 is difficult due to significant differences in the patient population and their treatments. The applicability of this algorithm merits further investigation across multiple centers for the purpose of treatment and clinical trial standardization in single fraction SRS and will be the subject of a forthcoming phase 2 prospective study within our own institution.
Keywords: brain metastases, dose selection, stereotactic radiosurgery, gamma knife
1. INTRODUCTION
The incidence of brain metastases (BM) in patients with a metastatic cancer diagnosis has been estimated to be as high as 30-40% [1-4]. With advances in systemic therapy resulting in longer survival, the management of brain metastases has become increasingly important. Whole brain radiation therapy (WBRT) remains a standard of care in the management of BM however it can be associated with an increased incidence of neurocognitive adverse effects.[5] Stereotactic radiosurgery (SRS) is therefore becoming an increasingly popular management option in these patients both as sole first line treatment as well as salvage treatment after initial SRS or WBRT. [6]
RTOG 90-05 determined maximum safe and tolerated doses for single fraction radiosurgery as salvage treatment for both recurrent previously irradiated primary brain tumors and brain metastases. [7] Recommended tolerated doses were defined by tumor diameter (marginal doses of 24, 18 and 15Gy for <20, 21-30 and 31-40 mm respectively) and these dosing guidelines are still commonly used today. Since the publication of the results of this trial over 15 years ago however, the indications and goals for SRS in treatment of BM have changed significantly and it is possible that an updated standardization of these dosing guidelines may be more applicable in clinical practice today. We present initial safety and efficacy results of use of a dose selection algorithm developed within our institution from the original 90-05 guidelines for single fraction radiosurgery in patients with BM treated with GK and scanned using a 1.5 Tesla MRI.
2. METHODS AND MATERIALS
The Medical Records for all patients undergoing SRS for BM at our institution treated with Gamma Knife radiosurgery (GK) between April 2012 and October 2012 were reviewed. Patients with ≤ 10 brain metastases on GK-planning MRI were included for analysis. All GK patients at our institution were enrolled prospectively onto an Institutional Review Board approved Data Repository prior to treatment and informed consent or waiver of consent was obtained in all cases. Patients with extremely radiosensitive tumor histologies (lymphoma, leukemia), evidence of leptomeningeal dissemination and resection of cranial metastases presenting for SRS cavity boost were excluded.
Baseline demographics were recorded for all patients treated including age, gender, previous history of WBRT, number of lesions treated and performance status, previous SRS, tumor histology, tumor location and volume and previous intracranial surgery.
2.1. Treatment
All patients were treated using the Leksell Perfexion Gamma Knife (Elekta Medical Systems Inc, Atlanta, GA). Planning MRI scan was obtained on the morning of GK treatment following application of the stereotactic head frame. Patients were scanned on a Siemens Magnetic Resonance Imaging Scanner. Double contrast gadolinium was administered in all cases unless the patient had significant renal dysfunction or contrast allergy and 1 mm slices were obtained for planning purposes. The images were transferred to Gamma Plan Software version 10.1 (Elekta Instruments, Atlanta GA) for the purposes of treatment planning. The planning target volume (PTV) was defined as the contrast enhancing tumor volume on the T1 weighted post contrast MRI images and the PTV was the same volume as the gross tumor volume (GTV) i.e. no margin was added to GTV to form PTV.
2.2. Dose selection algorithm
The dose selection algorithm was created using an initial sub-stratification of the guidelines in RTOG 90-05 (24 Gy for <4.5cc. 18Gy for 4.6-14.0 cc. and 15 Gy for >14.0 cc.) then modified based on institutional experience is shown in Table 2. Critical criteria involved in prescription dose selection included tumor histology, use of prior WBRT, individual tumor volume and number of metastases to be treated (1-4 or 5-10).
Table 2.
Dose selection algorithm accounting for treatment volume, number of metastases, tumor histology and prior WBRT.
| Planning Treatment Volume | RTOG 90-05 | Number of Metastases | Radioresistant / Prior WBRT | Radioresistant / No Prior WBRT | Radiosensitive / Prior WBRT | Radiosensitive / No Prior WBRT |
|---|---|---|---|---|---|---|
| 0-4.5 cc | 24 Gy | 1-4 | 22 Gy | 22 Gy | 22 Gy | 22 Gy |
| 5-10 | 20 Gy | 20 Gy | 20 Gy | 20 Gy | ||
| 4.6-7.0 cc | 18 Gy | 1-4 | 22 Gy | 22 Gy | 20 Gy | 22 Gy |
| 5-10 | 20 Gy | 20 Gy | 18 Gy | 20 Gy | ||
| 7.1-8.5 cc | 18 Gy | 1-4 | 20 Gy | 20 Gy | 20 Gy | 20 Gy |
| 5-10 | 18 Gy | 20 Gy | 18 Gy | 18 Gy | ||
| 8.6-11.0 cc | 18 Gy | 1-4 | 20 Gy | 20 Gy | 18 Gy | 20 Gy |
| 5-10 | 18Gy | 18 Gy | 18 Gy | 18 Gy | ||
| 11.1-14.0 cc | 18 Gy | 1-4 | 20 Gy | 20 Gy | 18 Gy | 20 Gy |
| 5-10 | 16 Gy | 18 Gy | 16 Gy | 18 Gy | ||
| 14.1-22.0 cc | 15 Gy | 1-4 | 18 Gy | 18 Gy | 16 Gy | 18 Gy |
| 5-10 | 16 Gy | 16 Gy | 16 Gy | 16 Gy | ||
| 22.1-34.0 cc | 15 Gy | 1-4 | 16 Gy | 16 Gy | 15 Gy | 16 Gy |
| 5-10 | 16 Gy | 16 Gy | 15 Gy | 16 Gy |
Overall, doses were raised for smaller lesion volume, radioresistant pathology (including melanoma, sarcoma and renal cell carcinoma) and in those patients without prior WBRT in order to try to obtain maximal treatment benefit. Doses were lowered in larger volume lesions, patients with 5-10 metastases and previous WBRT in order to reduce toxicity.
For lesions <4.5cc we lowered the dose from 24Gy to 22 Gy as in our experience these doses are equivalent. Lesions between 4.5 cc and 14.0cc would normally be treated with 18 Gy as per RTOG guidelines. For the purposes of our algorithm, we raised the dose and stratified by diameter to between 20 and 22 Gy. If a patient had previous WBRT then we reduced the dose by 2Gy. For lesions >14.0cc (normally treated to 15Gy in RTOG 90-05), our starting point was 18Gy for dose selection with a 2 Gy reduction in dose for previous WBRT. A 1Gy reduction in dose was applied for volume greater than 22.0cc.
Only lesions located within the brainstem or adjacent the optic apparatus were not treated based on the algorithm as marginal prescription dose for these lesions does not exceed 18Gy and 8Gy respectively in our institution.
2.3. Outcome measures
Primary endpoint was local recurrence (LR) as evaluated by RECIST criteria i.e an increase in the size of the lesion of ≥ 20%. Outcomes were recorded as local recurrence (LR), death without local recurrence (DWLR), distant intra-cranial recurrence (DIR) and alive and disease free (ADF). Patients were censored at DWLR or when WBRT was used at the time of DIR. Neurological toxicity was measured using the RTOG radiation morbidity criteria (Table 3). Patients were evaluated both clinically and radiographically at 6 weeks post Gamma Knife treatment and at 3 monthly intervals thereafter with an MRI scan at each of these time points. Treatment-related imaging changes (possible radiation necrosis)– were included within LR in order to avoid the need to histopathologically validate imaging findings.[8]
Table 3.
RTOG scale for radiation related morbidity criteria
| RTOG score | Definition |
|---|---|
| 0 | No change |
| 1 | Fully functional status (i.e., able to work) with minor neurologic findings, no medication needed |
| 2 | Neurologic findings present sufficient to require home case/ nursing assistance may be required/ medications including steroids/anti-seizure agents may be required |
| 3 | Neurologic findings requiring hospitalization for initial management |
| 4 | Serious neurologic impairment which includes paralysis, coma or seizures>3 per week despite medication/hospitalization required |
2.4. Physics
Information on planning matrices was recorded including lesion location, tumor volume, treatment volume and prescription dose along with isodose line. Where possible, we aimed for the lowest achievable conformity index (defined as prescription isodose volume divided by planning target volume) between 1.0 and 2.0. The entire PTV volume was covered by the prescription isodose and the most common prescription isodose line was the 50% line.
2.5. Statistical analysis
The goal for an acceptable lesion local control rate was 80% based on a local recurrence rate of 20% at 1 year in the RTOG 90-05 trial. Fine-Gray competing-risks analysis accounting for age, gender, number of metastases treated (1-4 vs. 5-10), tumor histology (radioresistant versus radio-sensitive), and prior WBRT was performed to examine factors affecting local control taking into account the competing risk of death. Time to endpoint was calculated from the day of Gamma Knife treatment.
3. RESULTS
3.1. Patient characteristics
A total of 65 consecutive patients were treated for 1-10 BM at our institution between April 2012 and October 2012 were eligible for analysis. 53% of the patients were male and 47% were female (Table 1). Median age was 64 years (range 38 86 years). Primary pathology included NSCLC 40.0% (n=26), melanoma 23.1% (n=15) and breast 13.8% (n=9). At the time of analysis, 41% of patients were alive. 12 (18.5%) patients had WBRT prior to SRS and the median interval between WBRT and SRS in these patients was 11.3 months (range 2.2 to 67.5 months).
Table 1.
Patient Demographics
| n | % | ||
|---|---|---|---|
| Gender | Male | 33 | 50.8 |
| Female | 32 | 49.2 | |
| Karnofsky Performance Status | KPS >70 | 54 | 83.1 |
| <70 | 9 | 13.8 | |
| unknown | 2 | 3.1 | |
| Radiosensitive histology | Breast | 9 | 13.8 |
| NSCLC | 26 | 40.0 | |
| Colorectal | 1 | 1.5 | |
| Other | 6 | 9.2 | |
| Radioresistant histology | Renal | 6 | 9.2 |
| Melanoma | 15 | 23.1 | |
| Sarcoma | 2 | 3.1 | |
| Number of metastases at treatment | 1-4 | 47 | 72.3 |
| 5-10 | 18 | 27.7 | |
| WBRT prior to SRS | Yes | 12 | 18.5 |
| No | 53 | 81.5 |
The single largest indication for GK (62.5%) was for prophylactic treatment of patients with new asymptomatic BM found on routine screening or surveillance imaging. Presenting symptoms in the remaining 40% of patients included a mix of headache, seizure, gait instability, weakness / clumsiness, mental status changes, and cranial nerve palsy – often in combination. (36% (n=23) of patients had single metastases at time of GK, 30% (n=19) had 2-4 metastases and 34% (n=22) had 5-10 metastases at time of radiosurgery.
3.2. Lesion characteristics
A total of 236 lesions were treated. Lesion characteristics are shown in Table 4. The most common location of metastases was in the frontal lobe (35.8%) 93.2% of lesions were less than 20mm in diameter, 5.9% were 20-30mm in diameter and 0.9% 30-40mm. 89.1% of lesions were ≤4.5cc in volume (equivalent to spherical diameter of 2.04 cm). 5.0% of lesions were treated to the 40-49% isodose surface, 56.2% to the 50-59% isodose, 29.3% to the 60-65% isodose and 9.5% to the 70% or greater isodose surface.
Table 4.
Lesion characteristics
| n | % | ||
|---|---|---|---|
| Lesion location | Frontal | 83 | 35.2 |
| Temporal | 33 | 14.0 | |
| Parietal | 30 | 12.7 | |
| Occipital | 35 | 14.8 | |
| Cerebellar | 39 | 16.5 | |
| Other | 16 | 6.8 | |
| Lesion diameter | <20mm | 220 | 93.2% |
| 20-30mm | 14 | 5.9% | |
| 30-40mm | 2 | 0.9% | |
| Lesion volume | 0-4.5 cc | 223 | 94.5 |
| 4.6-7.0 cc | 8 | 3.4 | |
| 7.1-8.5 cc | 1 | 0.4 | |
| 8.6-11.0 cc | 1 | 0.4 | |
| 11.1-14.0 cc | 1 | 0.4 | |
| 14.1-22.0 cc | 1 | 0.4 | |
| 22.1-34.0 cc | 1 | 0.4 |
3.3. Outcome
Median follow up was 8.9 months (range 1.0-29.6 months). 6 month overall survival was 64% (95% CI 51-75%) and 12 month overall survival was 37% (95% CI 25-49%). 35.9% of patients (n=23) died without local recurrence (DWLR) and 12.5% (n=8) were alive and disease free (ADF) at last follow up. Freedom from LR+DIR analyzed by patient at 12 months was 31.0% (95% CI 17.1-46.4%). Of the 236 lesions treated, there were no local failures at 6 months and only 4 (1.7%) local lesional failures at 12 months. Fine Gray competing risk regression analysis was used to estimate of a patient’s 12 month local recurrence-free survival (taking into account the competing risk of death) was 84.7% (95%CI 64.0-94.0%).
The results of a Fine Gray regression analysis with regards to local control are shown in Figures 1a and 1b and Table 5. No variable was significantly associated with local control, including treatment volume and 5-10 metastases (vs. 1-4) (SHR 6.11 [95% CI 0.64-58.8], p=0.12). There was no significant difference in competing risk analysis with regards to radioresistant versus radiosensitive histology (figure 1a), (SHR 0.51 [95% CI 0.06-7.73], p=0.55).
Figure 1.
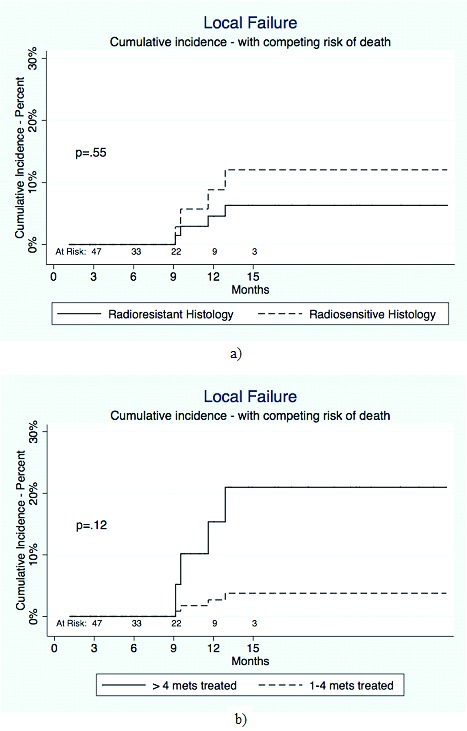
Table 5.
results of competing risk analysis for local control
| Hazard ratio | 95% CI | p value | |
|---|---|---|---|
| Radioresistant (vs. radiosensitive) | 0.51 | 0.06-7.73 | 0.55 |
| 1-4 ( vs. 5-9) | 6.11 | 0.64-58.8 | 0.12 |
| WBRT (vs. no WBRT) | 1.21 | 0.13-11.5 | 0.87 |
| Male Gender (vs. female) | 1.0 | 0.15-7.0 | 0.97 |
| Age (continuous variable) | 0.98 | 0.84-1.15 | 0.83 |
3.4. Local recurrence
The actuarial incidence of local recurrence and/or radionecrosis (lesions which could have met the criteria for local recurrence due to an increase in size post treatment but which may have been due to radionecrosis rather than local treatment failure were included in the LR category) at 12 months was 6.2% and overall at time of analysis was 7.7%. All of the lesions which recurred were less than 2.0cm in diameter. Of the 5 patients who developed local recurrence, LR occurred at 8 months post GK in 2 patients. Both opted for palliative care rather than further active management. The third patient developed LR at 13 months post GK and was treated with WBRT. She was therefore censored at this time. This was a patient with a history of breast cancer with multiple treatments with GK for recurrent CNS disease. She is currently alive 29 months post WBRT. The fourth patient with a history of breast cancer underwent surgical resection of a progressive lesion 10 months post GK. Histology was predominantly consistent primarily with radiation necrosis although a small focus of adenocarcinoma was found in one specimen. The patient subsequently progressed extra-cranially and died 3 months post-surgery/ 13 months post GK. The fifth patient developed slowly progressive LR at 10 months post GK which subsequently stabilized. At current follow up 24 months post GK, no salvage treatment has been required in this case and the patient remains alive and well.
No patient developed grade 3 or higher neurotoxicity at 6 or 12 months following GK nor did any patient develop seizures or require surgery post GK for toxicity.
4. DISCUSSION
The landmark RTOG 90-05 study published in 2000, established safe and tolerable doses for single fraction radiosurgery (SRS) of both recurrent primary brain tumors and brain metastases (BM) following standard fractionated external beam radiation. [7] This study was conducted as a dose escalation trial with the primary aim of keeping CNS toxicity following SRS at less than 20%.
Since the publication of RTOG 90-05, however, SRS has gained increasing popularity as a treatment for BM. Advances in systemic therapy, including the use of many new targeted and immune-based therapies in cancers such as melanoma and NSCLC [9,10,11], have resulted not only in an increasing number of patients diagnosed with brain metastases, but also more patients who are living longer after treatment of their brain metastases. This has resulted directly in an increased adoption of the use of SRS for post-WBRT salvage therapy [7] and emphasized the need to use an SRS treatment dose that is likely to ensure long-term control of treated lesions with a minimal risk of toxicity.
In addition, the indications for SRS in treatment of brain metastases since the publication of RTOG 90-05 have changed significantly. The recognition of the need for regular screening and surveillance of the brain using higher resolution MRI [12, 13,14] in patients with systemic metastases has resulted in an increasing number of small, asymptomatic brain lesions being detected. Most of these patients are neurologically asymptomatic and given the superiority of SRS in local lesional control as well as the possibility of developing delayed neurocognitive side effects following WBRT, there has been a shift away from first-line WBRT for treatment of BM [6]. Indeed many radiosurgeons today would advocate first-line treatment with SRS alone for high functioning patients with 5-10 brain metastases [15,16]. Dosing guidelines for the use of SRS without prior WBRT have never been published. Accounting for all these factors raises the question as to whether a new dose selection criteria refining the parameters laid down in RTOG 90-05 is needed in the modern era of SRS.
Based on our own institutional practice, it was observed that despite the standard RTOG 90-05 guideline doses of 15, 18 and 24Gy, radiosurgeons at our institution would often prescribe modified doses in between these numbers i.e. 16, 20 and 22Gy taking into account lesion size and number to be treated (as an estimate of survival), histopathology and history of previous WBRT. In order to standardize our practice and so as to allow us to compare outcomes and toxicities, we developed a dose selection algorithm based predominantly on a combination of the experience of our most senior radiosurgeons and past SRS cases given our previously published reports of comparable outcomes to national standards. The application of the algorithm was initiated in April 2012 and remains in effect at our institution today. The goal of this retrospective study was then primarily to demonstrate safety and efficacy of this standardized dose algorithm for SRS and to establish that the algorithm could be implemented without significant reduction in local or distant control.
Kaplan Meier estimate of a patient’s 12 month lesional recurrence free survival was 81.4% which compares very favorably to the 1 year actuarial incidence of local progression in RTOG 90-05 of 48%. The two patient populations however are far from equivalent. RTOG 90-05’s schema permitted only single lesions to be targeted (either isolated lesions or dominant lesions in patients with multiple lesions) whereas only a third (32%) of the patients in our study had single metastases. The RTOG 90-05 study also allowed for CT based planning which would likely increase the irradiated volume and may impact the local control and radionecrosis rate when compared to the high resolution MRI used in our study. Patients with both primary glial tumors and brain metastases were included in RTOG 90-05 which would significantly impact the reported local control rate and all patients received re-irradiation of a previously treated lesion – some to doses as high as 60Gy in the original treatment region which would increase the likelihood of radiation necrosis. This contrasts sharply with the indications for radiosurgical treatment in this study where all patients undergoing SRS had brain metastases and 81.0% were radiation treatment naïve. Lastly, a comparison of the volumes treated between the 2 studies also highlights differences between the treatment populations. In the RTOG 90-05 study 25.6% (40 out of 156) of the lesions had diameters of <20mm compared with this study where 93.2% of the lesions were <20mm diameter. The significant differences in patient population between RTOG 90-05 and our study only highlights again the need to revisit dosing guidelines specific for brain metastases.
In RTOG 90-05, surgical resection was encouraged for suspicious radiographic changes of RN and the rate of RN was found to be 11% at 2 years. Radiation necrosis is notoriously difficult to diagnose and to differentiate from tumor progression radiographically. Estimates of the incidence of RN vary from anywhere between 7 to 24% [17,18] and this is increasing as patients survive longer following SRS for BM and undergo more intensive post treatment surveillance imaging. Several studies have shown a dose related correlation with RN including Kjellberg and Abe [19] who created a dose versus volume model that was thought to predict risk of radiation necrosis. The possibility of radiation necrosis was not specifically addressed in this study given its short follow-up period as these cases were included in our definition of local failure. Even if all cases of local recurrence in this study were in fact radiation necrosis, an overall rate of 7.7% would be acceptable in comparison with rates reported in RTOG 90-05. The impact of our dose selection algorithm on rates of radiation necrosis will be examined further in our prospective phase 2 study which will have 2 year follow-up data.
Limited data exists following the publication of RTOG 90-05 on the optimal SRS dose for brain metastases. To our knowledge, in the only large study looking specifically at this topic, Shehata et al. [20] examined the optimal SRS dose for 468 patients with brain metastases < 2cm in diameter and the influence of WBRT on local control. The authors concluded that WBRT was the single most influential factor in local lesional control and that WBRT + SRS doses of 20 Gy resulted in 99% lesional control versus 91% if doses less than 20Gy were used. No improvement in local control but rather an increase in treatment related toxicity was seen in doses higher than 20Gy administered after WBRT. Of note in this study, only 17.5% of patients received WBRT prior to GK and the use of WBRT was not found to contribute to improved local control or increased toxicity.
This study was completed in anticipation of our prospective phase 2 trial using the same algorithm as we switch to 3 Tesla MR imaging for SRS planning. Given the initial promising results, we hope ultimately, following completion of this phase 2 study, to establish our dose selection algorithm for possible wider multi-institutional adoption as a framework for standardizing SRS dosing in the modern era of brain metastases management.
A major limitation of our retrospective analysis was the short follow up period and smaller patient numbers; we will present 2 year follow up data in our prospective trial. Also, since the analysis was retrospective, not all cases of radiosurgery performed at our institution used the rationalized dosing and it is possible that this could introduce significant bias in outcome data. Lastly, the created algorithm has been formulated based upon experience of local radiosurgeons at our institution and as such therefore subject to institutional practice biases. While we hope to address these limitations and gather further toxicity data in our forthcoming prospective phase 2 protocol, we also recognize that multi-institutional validation of our results will also be required.
5. CONCLUSIONS
Initial good local control and toxicity results from our institutional dose selection algorithm for radiosurgical treatment of up to 10 brain metastases are reported. The results are difficult to compare to those reported in RTOG 90-05 given the significant differences in treatment population emphasizing the need for brain metastasis specific radiosurgery dose guidelines. The applicability of this algorithm merits further investigation in a multi-institutional setting for the purpose of treatment and clinical trial standardization in single fraction SRS and will be the subject of a forthcoming phase 2 prospective study within our own institution.
Glossary
Abbreviations
- SRS
stereotactic radiosurgery
- BM
brain metastases
- GK
Gamma Knife
- WBRT
whole brain radiation therapy
- LR
local recurrence
- DIR
distant intra-cranial recurrence
- DWLR
death without local recurrence
- ADF
alive and disease free
Footnotes
Authors’ disclosure of potential conflicts of interest
Drs. Colaco, Bindra, Contessa, Bond, Knisely and Chiang have no conflicts of interest to disclose.
Dr. Yu receives research funding from 21st Century Oncology and the PhRMA Foundation. These funding sources had no involvement in the design, analysis, or preparation of the manuscript. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. No portion of this work has been presented or published prior to manuscript submission.
Author contributions
Conception and design: Rovel J. Colaco, James B. Yu, Veronica L. Chiang, Jonathan P.S. Knisely, Ranjit S. Bindra, Joseph N. Contessa.
Data collection: James S. Bond, Rovel J. Colaco, Veronica L. Chiang.
Data analysis and interpretation: James B. Yu, Rovel J. Colaco.
Manuscript writing: Rovel J. Colaco, Veronica L. Chiang, Jonathan P.S. Knisely.
Final approval of manuscript: Veronica L. Chiang, James B. Yu, Ranjit S. Bindra, Joseph N. Contessa.
REFERENCES
- 1.Norden AD, Wen PW, Kesari S. “Brain metastases,” Current Opinion in Neurology, vol. 18, no. 6, pp. 654–661, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Lohr F, Pirzkall A, Hof H, Fleckenstein K. Debus J. Adjuvant treatment of brain metastases. Semin Surg Oncol 2001; 20: 50–56. [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012; 14: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:37-46. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, Allen PK. Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044. [DOI] [PubMed] [Google Scholar]
- 6.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with and without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363:1665-1672. [DOI] [PubMed] [Google Scholar]
- 7.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000; 47: 291–98. [DOI] [PubMed] [Google Scholar]
- 8.Shah R, Vattoth S, Jacob R, Manzil FF, O’Malley JP, Borghei P, Patel BN, Curé JK. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012. Sep-Oct;32(5):1343-59 [DOI] [PubMed] [Google Scholar]
- 9.Knisely JP, Yu JB, Flanigan J, Snzol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence DP, Hamid O, McDermott DF, Puzanov, Sznol M., Clark J., Logan, Hodi F. S., Heller K. N., K. A. Phase 3 trial of ipilimumab monotherapy in melanoma patients with brain metastases. J Clin Oncol; 2010:28 [Abstract 8523] [Google Scholar]
- 12.Barth M, Nöbauer-Huhmann IM, Reichenbach JR, Mlynárik V, Schöggl A, Matula C, Trattnig S. High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla. Invest Radiol. 2003; 38(7):409-14. [DOI] [PubMed] [Google Scholar]
- 13.Trattnig S, Ba-Ssalamah A, Noebauer-Huhmann IM, Barth M, Wolfsberger S, Pinker K, Knosp E. MR contrast agent at high-field MRI (3 Tesla). Top Magn Reson Imaging. 2003; 14(5):365-75. [DOI] [PubMed] [Google Scholar]
- 14.Ba-Ssalamah A, Mlynarik V, Barth M, Barth M, Wolfsberger S, Pinker K, Knosp E. Magnetic resonance imaging contrast enhancement of brain tumors at 3 tesla versus 1.5 tesla. Investigative Radiology 2002; 37(3):114-9. [DOI] [PubMed] [Google Scholar]
- 15.Knisely JP, Yamamoto M, Gross CP, Castrucci WA, Jokura H, Chiang VL. Radiosurgery alone for 5 or more brain metastases: expert opinion survey. J Neurosurg. 2010. December; 113 Suppl:84-9. [DOI] [PubMed] [Google Scholar]
- 16.Raldow AC, Chiang VL, Knisely JP, Yu JB. Survival and intracranial control of patients with 5 or more brain metastases treated with gamma knife stereotactic radiosurgery. Am J Clin Oncol. 2013; 36(5):486-90 [DOI] [PubMed] [Google Scholar]
- 17.Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: A case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg 2001; 94:899-904. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Clarke E, Lanzetta G, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011; 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjellberg RN, Abe M: Stereotactic Bragg peak proton radiosurgery results, in Lunsford LD (ed): Modern Stereotactic Neurosurgery. Boston: Martinus Nijhoff, 1988, pp. 463–470 [Google Scholar]
- 20.Shehata MK, Young B, Reid B, Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004; 59(1):87-93. [DOI] [PubMed] [Google Scholar]


