Abstract
Objective
The Japan Leksell Gamma Knife (JLGK) Society has conducted a prospective multi-institute study (JLGK0901, UNIN000001812) for selected patients in order to prove the effectiveness of stereotactic radiosurgery (SRS) alone using the gamma knife (GK) for 1-10 brain lesions. Herein, we verify the validity of 5 major patient selection criteria for the JLGK0901 trial.
Materials and Methods
Between 1998 and 2010, 2246 consecutive cases with 10352 brain metastases treated with GK were analyzed to determine the validity of the following 5 major JLGK0901 criteria; 1) 1-10 brain lesions, 2) less than 10 cm3 volume of the largest tumor, 3) no more than 15 cm3 total tumor volume, 4) no cerebrospinal fluid (CSF) dissemination, 5) Karnofsky performance status (KPS) score ≥70.
Results
For cases with >10 brain metastases, salvage treatments for new lesions were needed more frequently. The tumor control rate for lesions larger than 10 cm3 was significantly lower than that of tumors <10 cm3. Overall, neurological and qualitative survivals (OS, NS, QS) of cases with >15 cm3 total tumor volume or positive magnetic resonance imaging findings of CSF were significantly poorer. Outcomes in cases with KPS <70 were significantly poorer in terms of OS.
Conclusion
Our retrospective results of 2246 GK-treated cases verified the validity of the 5 major JLGK0901 criteria. The inclusion criteria for the JLGK0901 study are appearently good indications for SRS.
Keywords: Metastatic brain tumor, stereotactic radiosurgery, gamma knife surgery, whole brain radiation therapy
INTRODUCTION
According to the Japanese Radiation Oncology Study Group (JROSG) 99-1 investigation, reported by Aoyama et al in JAMA in 2006, the efficacy of stereotactic radiosurgery (SRS) alone for 1 to 4 brain metastases has been confirmed (1). Gamma knife (GK)-SRS has been widely applied to multiple brain metastases, as reported especially by Japanese GK groups (2-4, 6-13, 16-19, 21-24). As the first step to bridge the gap between broad clinical applications and limited evidence of efficacy for treating multiple brain tumors, the Japan Leksell Gamma Knife (JLGK) Society conducted a prospective multi-institute study for selected patients in order to establish evidence of the efficacy of GK-SRS alone treatment for 5-10 brain lesions. Herein, we introduce the JLGK0901 study and verification of the validity of the inclusion criteria of this JLGK0901 study based on our retrospective review of 2246 patients treated with GK-SRS.
JLGK0901 STUDY
The JLGK0901 study plan was to select patients meeting the inclusion criteria and to follow them with both neurological examination and enhanced magnetic resonance imaging (MRI) findings. Thus, the JLGK0901 is a prospective and multi-institute, but not a randomized, study. The JLGK0901 study was designed to test the noninferiority of treating cases with 5-10 brain lesions with GK-SRS, versus 2-4, in terms of overall survival (OS). Twelve hundred cases will be registered within 3 years (final registration; February 2012) from 23 Japanese GK sites, and with a one-year observation period, the study will be finished in February 2013. As secondary endpoints of the JLGK0901 study, neurological survival (NS), qualitative survival (QS) and also neuro-cognitive function will be assessed. The JLGK0901 study committee set the 5 major selection criteria; 1) 1-10 brain lesions, 2) less than 10 cm3 volume of the largest tumor, 3) no more than 15 cm3 total tumor volume, 4) no cerebrospinal fluid (CSF) dissemination findings, 5) Karnofsky performance status (KPS) score ≥70. Cases with sarcoma, lymphoma and primary unknown cancers were excluded. The protocol stipulates follow-up, including enhanced MRI and neurological examinations, at least every 3 months. At the initial GK-SRS treatment, the standard peripheral doses must be 22 Gy if the tumor volume is <4.0 cm3, and 20 Gy if ≥4.0 but <10.0 cm3. We can change the peripheral doses plus/minus 2 Gy, depending on tumor pathology, physical status, tumor location and extra-cranial disease status. The dose can also be reduced if the tumor is located adjacent to SRS-risk organs, i.e. the brain stem, optic apparatus, cochlear and facial nerves. Intended upfront whole brain radiation therapy (WBRT) is prohibited, though there is no restriction on salvage treatment after initial GK-SRS. This trial has been registered by the Japanese ethics committee (University Hospital Medical Information Network, UMIN 0000001812, http://www.umin.ac.jp/). In early 2012, registration was completed, but interim analysis was not allowed by the protocol because of multiplicity.
PATIENTS AND METHODS
We analyzed 2246 consecutive cases with 10352 brain metastases treated with GK-SRS between 1998 and 2010. The database consisted of two cohorts studied retrospectively after institutional review board (IRB)-approval: the Chiba-series (1716 consecutive patients, January 1998 through March 2008) plus the Tokyo-series (530, April 2008 through December 2010). All aspects of patient selection, dose planning, dose selection, performing GK-SRS and collecting follow-up data were undertaken by the first author (T.S.). During the 13-year period from 1998 to 2010, all patients were treated according to the same protocol. At initial treatment, all lesions were irradiated with GK-SRS without upfront WBRT. In some cases with tumor volumes exceeding 10 cm3, staged stereotactic radiotherapy (SRT) was chosen (2) and in all those with a total tumor volume exceeding 15 cm3 and/or tumor numbers greater than 25, the radiosurgical procedures were divided into two or three sessions, to insure a total skull integral dose (TSID) of less than 10 Joules, thereby preventing acute brain swelling. New distant lesions, detected by gadolinium enhanced MRI performed every two to three months, were treated mainly with GK, sometimes with WBRT only if cerebral and/or CSF dissemination was detected. The treatment strategy was explained in detail to each patient and written informed consent was obtained from all patients before the initial GK-SRS.
A stereotactic coordinate frame (Leksell Model G stereotactic coordinate frame manufactured by Elekta Instruments AB, Stockholm, Sweden) was applied under local anesthesia supplemented with relatively deep sedation. For target coordinate determination and dose-planning, stereotactic gadolinium-enhanced T1-weighted axial MRI with a slice thickness of 2 mm, multiple slices of which covered the entire brain, were obtained. For dose planning, the Leksell GammaPlan (Elekta) was used. Before October of 2003, GK-SRS was performed using a Leksell GK Model B (1988-2003, Elekta), and a Leksell GK Model C (late 2003-2010, Elekta). The standard prescribed dose at the tumor periphery of 18-24 Gy was changed depending on tumor pathology, physical status, tumor location, tumor volume (including GK-SRT technique), extracranial disease status and so on. Cases meeting the JLGK0901 inclusion criteria were treated according to the same protocol as stipulated in the JLGK0901 protocol. All data were analyzed according to the intention-to-treat principle. The intervals from the date of GK-SRS treatment until the date of death (overall survival, OS), neurological death (neurological survival, NS) and impaired activities of daily living (ADL, qualitative survival, QS) were calculated by the Kaplan-Meier method, and compared using the log-rank test according to 5 items serving as the JLGK0901 selection criteria; 1) brain tumor number (single, 2-4, 5-10, >10), 2) maximum tumor volume (<1 cm3, 1-4 cm3, 4-10 cm3, >10 cm3), 3) total brain tumor volume (<15 cm3 vs ≤15 cm3), 4) MR findings of CSF dissemination (positive vs negative) and 5) KPS score (≥70 vs <70). Neurological death was defined as death due to any form of intracranial disease, including tumor recurrence, carcinomatous meningitis, cerebral dissemination, and other unrelated intracranial diseases. Impaired ADL was defined as an impaired neurological status as reflected by a KPS score <70 (functional preservation), as reported by Aoyama et al (1). Thus, cases without improvement in KPS scores to 70 or more, even after GKS, were excluded when evaluating QS. A p-value less than 0.01 was defined as statistically significant. All statistical analyses were performed using the JMP software program, version 9.0.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Characteristics | Covariates | Total |
|---|---|---|
| Case number | Total | 2246 |
| Age (years) | Median (min-max) | 7-94(65) |
| Gender | Male | 1350 (60.1%) |
| Female | 896 (39.9%) | |
| Extra-cranial disease | Controlled | 280 (12.5%) |
| Active | 1996 (87.5%) | |
| Pre-treatment KPS score | Median (min-max) | 50-100 (100) |
| Primary organ | Lung | 1490 (66.3%) |
| GI-tract | 277 (13.5%) | |
| Breast | 230 (10.2%) | |
| Uro-genital | 136 (6.1%) | |
| Others | 113 (5.0%) | |
| Number of brain lesions | Median (min-max) | 1-100 (3) |
| Single metastasis | 613 (27.3%) | |
| 2-4 | 766 (34.1%) | |
| 5-10 | 498 (22.2%) | |
| >10 | 369 (16.2%) | |
| Maximum lesion volume (cm3) | Median (min-max) | 0.1-47.6 (2.8) |
| Total tumor volume (cm3) | Median (min-max) | 0.1-50.0 (4.5) |
| Diagnostic lag between primary cancer and brain metastases | Synchronous | 810 (36.1%) |
| Metachronous | 1436 (63.9%) | |
| RTOG-RPA classification | Class I | 133 (5.9%) |
| Class II | 1812 (80.7%) | |
| Class III | 301 (13.4%) |
RTOG; Radiation Therapy Oncology Group
RPA; Recursive Partitioning Analysis
KPS; Karnofsky performance status
-
I)
Tumor number: 1-10
Figure 1 shows procedure times for salvage GK-SRS performed for new distant lesions in 1884 deceased cases without prophylactic WBRT. Risks of repeated GK-SRS for new lesions are obviously lower in cases with a single metastasis. The proportions of cases with 2-4 and 5-10 lesions are almost the same. However, there are significant differences between 5-10 and >10 (p=0.023).
-
II)
Less than 10 cm3 volume of the largest tumor
Figure 2 demonstrates tumor progression-free survival curves according to tumor volume treated with GK-SRS. We analyzed 10057 lesions initially irradiated with GK-SRS, excluding 295 lesions treated using a staged GK-SRT technique proposed by Higuchi and Serizawa (2). Tumor volumes were divided into 4 groups, <1 cm3 (tiny), 1-4 cm3 (small), 4-10 cm3 (medium) and >10 cm3 (large). Control of irradiated lesions with GK-SRS was defined as lack of any significant increase in tumor diameter (<20%), as in the JLGK0901 protocol. The tumor control rates at 1 year were 99.1% for 7012 tiny (mean peripheral dose; 70.9%, 24.4Gy), 93.2% for 1436 small (58.1%, 20.1Gy), 83.2% for 684 medium-sized (19.4%, 19.2Gy) and 69.1% for 225 large (50.4%, 17.3Gy) lesions. The differences were statistically significant between all pairs of adjacent tumor volume groups (all; p<0.0001).
-
III)
No more than 15 cm3 total tumor volume
Figure 3 shows OS, NS and QS curves according to total tumor volume (≤15 cm3, >15 cm3). Outcomes in terms of OS, NS and QS were significantly worse in cases with >15 cm3 total tumor volume (all p <0.0001).
-
IV)
No MR imaging evidence of CSF dissemination
Figure 4 demonstrates OS, NS and QS curves according to MR findings of CSF dissemination. OS, NS and QS of cases with MR findings of CSF dissemination were significantly poorer (p=0.0010, <0.0001, and <0.0001, respectively).
-
V)
KPS score of 70 or better
Figure 5 shows OS curves according to RTOGRPA class, as reported by Gasper. Median survival time (MST) was 24.8 months in class I, 7.8 in class II, and 3.6 in class III patients. There were significant differences (p=0.0001; I-II, p<0.0001; II-III).
Figure 1.
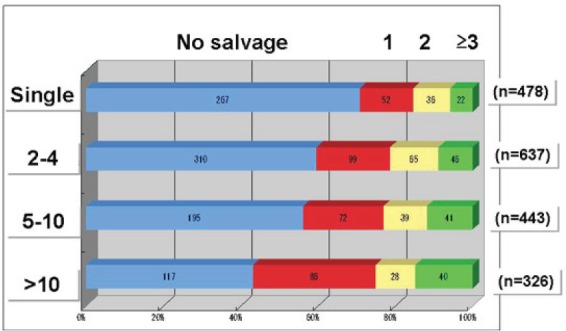
Salvage treatments for new distant lesions
The number of repeated gamma knife (GK) stereotactic radiosurgery (SRS) for new distant lesions in 1884 deceased cases treated with GK-SRS without prophylactic whole brain radiation therapy is shown in the graph. The blue indicates no salvage, red once, yellow twice and green three times or more. The upper panel shows single metastasis, the second 2-4, the third 5-10 and the lower panel more than 10. Salvage treatments were significantly less frequent in single metastasis cases. Risks of repeated GK-SRS for new lesions are obviously lower in cases with a single metastasis. The proportions of cases with 2-4 and 5-10 lesions are almost the same. However, there are some differences between 5-10 and >10 (p=0.023).
Figure 2.
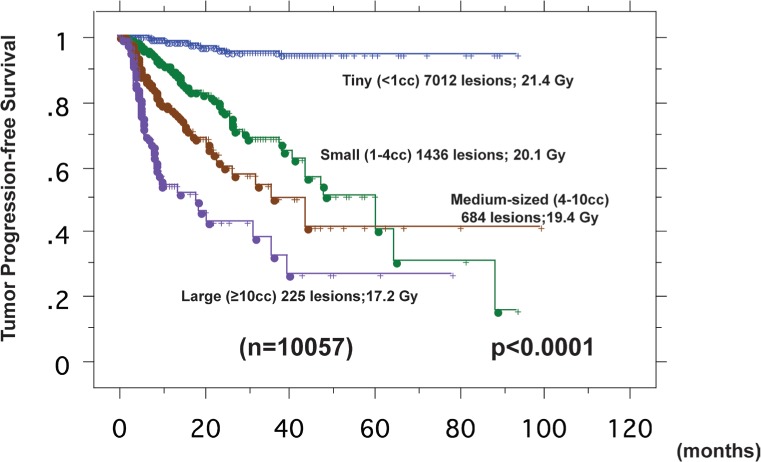
Tumor progression-free survival curves according to tumor volume
Tumor volumes were divided into 4 groups, <1 cm3 (tiny) shown in blue, 1-4 cm3 (small) in green, 4-10 cm3 (medium) in brown and >10 cm3 (large) in purple. The tumor control rates at 1 year were 99.1% for 7012 tiny (mean peripheral dose; 70.9%, 21.4Gy), 93.2% for 1436 small (58.1%, 20.1Gy), 83.2% for 684 medium-sized (19.4%, 51.2Gy) and 69.1% for 225 large (50.4%, 17.3Gy) lesions. The differences were statistically significant between each pair of adjacent tumor volume groups (all p values; <0.0001).
Figure 3.
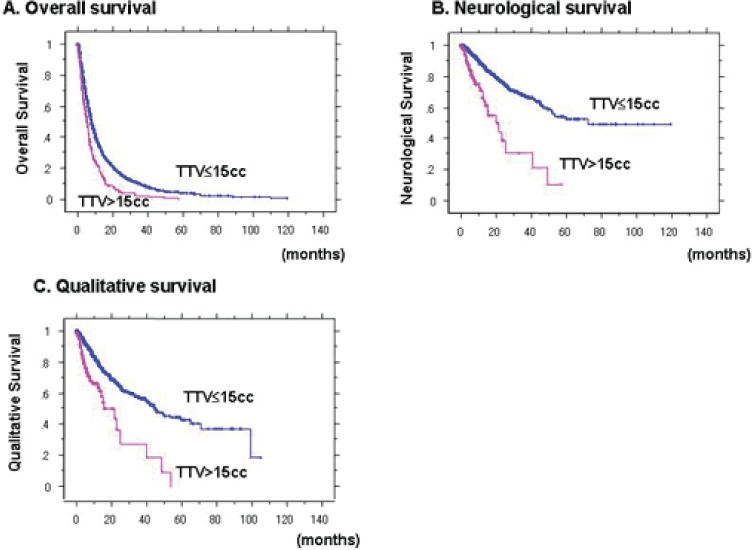
Overall survival (A), neurological survival (B) and qualitative survival (C) curves for comparison of cases with >15 cm3 versus ≤15 cm3 total tumor volume
Outcomes were significantly better for patients with ≤15 cm3 (blue) than in those with >15 cm3 (magenta) total tumor volumes, in terms of overall, neurological and qualitative survivals (all p values; <0.0001).
Figure 4.
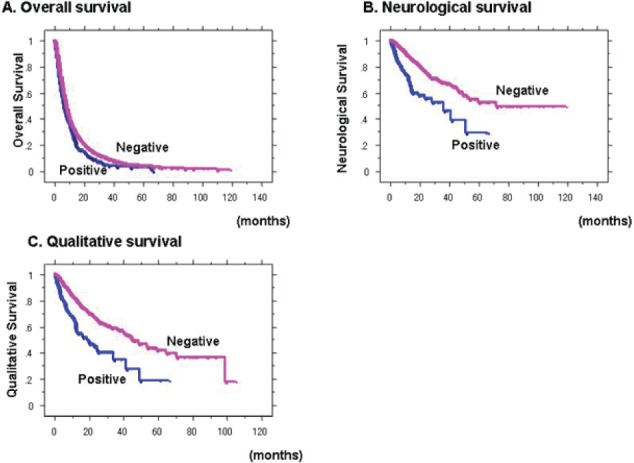
Overall survival (A), neurological survival (B) and qualitative survival (C) curves of cases with positive (blue) and negative (magenta) magnetic resonance imaging (MRI) findings of cerebrospinal fluid (CSF) dissemination OS, NS and QS of cases with MR findings of CSF dissemination were significantly poorer (p=0.0010, <0.0001, and <0.0001, respectively).
Figure 5.

Overall survival curves according to RTOG-RPA
Mean survival time (MST) was 9.4 months (24.8 months in class I indicated in blue, 7.8 in class II in magenta and 3.6 in class III in orange). There were significant differences between all pairs of classes. All p values were <0.0001.
DISCUSSION
We, the JLGK0901 study group, have determined the 5 major inclusion criteria for this protocol. The first criterion involves “tumor number”. As Aoyama has already proven the efficacy of SRS alone for 1-4 brain metastases, the frequency of salvage GK-SRS for 5-10 brain metastases was acceptable compared to that for 2-4 brain metastases (1). The concept of an upper limit of approximately 10 brain metastases for GK-SRS is now the consensus of the JLGK society. The efficacy of GK-SRS alone for treatment of 1-10 brain metastases from non-small cell lung cancer was reported by Serizawa et al. Thus, we decided to limit the tumor number to 10 in the JLGK0901 study. The second criterion is a “maximum tumor volume”. In our retrospective data, tumor control for lesions larger than 10 cm3 was not satisfactory. In the JLGK0901 study, the largest tumor volume must be less than 10 cm3 and also the maximum diameter must be less than 3 cm. The third criterion is “total tumor volume”. We have advocated the 10 Joule-TSID concept as the limitation of tumor number and size in a single GK-SRS for safety reasons, as already reported (8). This 10J-TSID is roughly equivalent to 3 Gy of mean whole brain radiation. Figure 6 demonstrates the 10J-TSID curve. Within these limits, we assume that 25 tiny, 10 small or 4 medium-sized lesions, if the tumors are approximately the same size and diffusely located in the brain, can be safely treated. This means an almost 15 cm3 total tumor volume as the upper limit of GK-SRS, with a peripheral dose of 20 Gy. Our results showed significantly poorer NS and QS, as well as OS, outcomes in cases with >15 cm3 total tumor volume. Thus, we excluded cases with >15 cm3 total tumor volumes from the JLGK0901 study. The fourth criterion is MR findings of CSF dissemination. In 2006, we reported “MR imaging findings of cerebrospinal fluid (CSF) dissemination”, such as enhancement of brain sulci, basal cisterns and/or ventricular walls, to be factors strongly predicting poor NS and QS with GK-SRS alone treatment (10). In this study, OS, NS and QS outcomes in cases with positive MR findings of CSF dissemination were significantly poorer. Therefore, we excluded cases with MR findings of CSF dissemination. The final criterion is KPS score less than 70. The OS outcomes of RPA class III patients were not acceptable. We therefore excluded cases with KPS <70, due to systemic, but not neurological causes. Thus, our retrospective results verified the validity of the 5 major JLGK0901 inclusion criteria.
Figure 6.
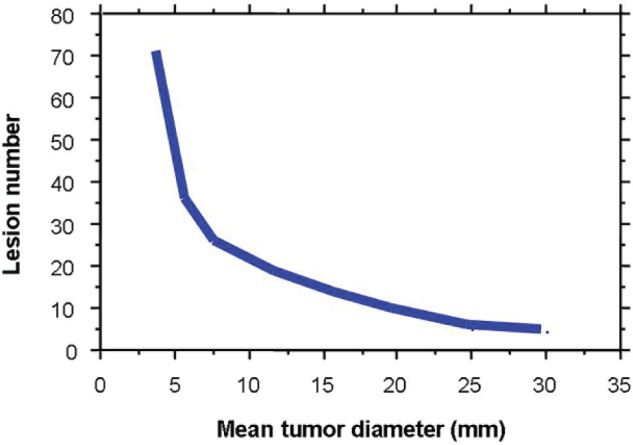
10J TSID concept
The curve indicates a total skull integral dose (TSID) of 10 Joules calculated using the GammaPlan, with 20Gy (50%) peripheral doses, for gamma knife (GK) stereotactic radiosurgery (SRS). Within these limits, we assume that 25 tiny, 10 small or 4 medium-sized lesions, if the tumors are approximately the same size and diffusely located, can be safely irradiated. This means that an almost 15 cm3 total tumor volume is the upper limit for safe GK-SRS.
Serizawa and Yamamoto have already reported the anticipated JLGK0901 study results based on two-institute retrospective data from 1508 cases (11). They proved the non-inferiority of GK-SRS for 5-10 brain metastases as compared to 2-4. The JLGK0901 protocol set a delta value of 0.3, such that their retrospective results showed the non-inferiority of treating 5-10 brain metastases as compared to 2-4 in terms of OS. If the JLGK0901 study proves the noninferiority of treating 5-10 brain metastases versus 2-4 in terms of OS, level 2-b evidence of the efficacy of GK-SRS alone treatment for 5-10 brain metastases will be established. NS, QS, and NLFS were the secondary endpoints of the JLGK0901 study. Portions of these results were published in 2009 (14). There were no significant differences in NS, QS or newlesion free survival between 2-4 and 5-10 lesions groups.
CONCLUSIONS
We retrospectively reviewed 2246 consecutive cases with brain metastases treated with GK-SRS alone and verified the validity of the five major inclusion criteria of the JLGK0901 study; 1) 1-10 brain lesions, 2) less than 10 cm3 volume of the largest tumor, 3) no more than 15 cm3 total tumor volume, 4) no cerebrospinal fluid (CSF) dissemination findings, 5) Karnofsky performance status (KPS) score ≥70. The inclusion criteria of the JLGK0901 study seemed to be good indicated for SRS.
REFERENCES
- 1. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G: Stereotactic radiosurgery plus whole brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295 (2006) 2483-2491. [DOI] [PubMed] [Google Scholar]
- 2. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, Iwadate Y, Saeki N: Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int. J. Radiation Oncology Biol. Phys. 74(5), (2009) 1543-12009 [DOI] [PubMed] [Google Scholar]
- 3. Jokura H, Takahashi K, Kayama T, Yoshimoto T: Gamma knife radiosurgery of a series of only minimally selected metastatic brain tumours. Acta Neurochir Su994; 62 (194): 77-82. [DOI] [PubMed] [Google Scholar]
- 4. Kida Y, Kobayashi T, Tanaka T: Radiosurgery of the metastatic brain tumours with gamma-knife. Acta Neurochir Su95; 63 (195): 89-94. [DOI] [PubMed] [Google Scholar]
- 5. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322, (1990) 494-1990 [DOI] [PubMed] [Google Scholar]
- 6. Serizawa T, Iuchi T, Ono J, Saeki N, Osato K, Odaki M, Ushikubo O, Hirai S, Sato M, Matsuda S: Gamma knife treatment for multiple metastatic brain tumors compared with whole-brain radiation therapy. J Neurosurg (Suppl 3) 93 (20): 3 2000 [DOI] [PubMed] [Google Scholar]
- 7. Serizawa T, Ono J, Iuchi T, Matsuda S, Sato M, Odaki M, Hirai S, Osato K, Saeki N, Yamaura A: Gamma knife radiosurgery for metastatic brain tumors from lung cancer. Comparison between small cell cancer and non-small cell cancer. J Neurosurg (Suppl 5) 97 (000): 484 2000 [DOI] [PubMed] [Google Scholar]
- 8. Serizawa T, Saeki N, Higuchi Y, Ono J, Iuchi T, Nagano O, Yamaura A: Gamma knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochir (Wien) 147 (25): 721 2005 [DOI] [PubMed] [Google Scholar]
- 9. Serizawa T, Higuchi Y, Ono J, Matsuda S, Iuchi T, Nagano O, Saeki N: Gamma knife surgery for metastatic brain tumors from lung cancer without prophylactic whole brain radiation therapy. Kondziolka (ed): Radiosurgery, Basel (Switzerland), Karger, 186- 198 2006 [Google Scholar]
- 10. Serizawa T, Higuchi Y, Ono J, Matsuda S, Nagano O, Iwadate Y, Saeki N: Gamma knife surgery for metastatic brain tumor without prophylactic whole brain radiation therapy: results in 1000 consecutive cases. J Neurosurg 105 (26): 8 2006 [DOI] [PubMed] [Google Scholar]
- 11. Serizawa T, Yamamoto M, Nagano O, Higuchi Y, Matsuda S, Ono J, Iwadate Y, Saeki N: Gamma knife surgery for metastatic brain tumors. A 2-institute study in Japan. J Neurosurg 109 (28): 118 2008 [DOI] [PubMed] [Google Scholar]
- 12. Serizawa T: Metastatic brain tumors: lung cancer. Yamamoto (ed): Japanese experience with gamma knife radiosurgery, Basel (Switzerland), Karger, 142-153, 2008 [Google Scholar]
- 13. Serizawa T: Radiosurgery for metastatic brain tumors. Int J Clin Oncol 14 (09): 28, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Serizawa T, Hirai T, Nagano O, Higuchi Y, Matsuda S, Ono J, Saeki N: Gamma knife surgery for 1-10 brain metastases without prophylactic whole-brain radiation therapy: analysis of cases meeting the Japanese prospective multi-institute study (JLGK0901) inclusion criteria. J Neurooncol 98 (10): 16, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Serizawa T, Yamamoto M, Sato Y, Higuchi Y, Nagano O, Kawabe T, Matsuda S, Ono J, Saeki N, Hatano M, Hirai T: Gamma knife surgery as sole treatment for multiple brain metastases: 2-center retrospective review of 1508 cases meeting the inclusion criteria of the JLGK0901 multi-institutional prospective study. J Neurosurg 113 (10): , 2010 [DOI] [PubMed] [Google Scholar]
- 16. Shuto T, Fujino H, Asada H, Inomori S, Nagano H: Gamma Knife Radiosurgery for Metastatic Tumours in the Brain Stem. Acta Neurochir (Wien) 145 (003): 75, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Shuto T, Fujino H, Inomori S, Nagano H: Repeated gamma knife radiosurgery for multiple metastatic brain tumours. Acta Neurochir (Wien) 146 (004): 98, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Shuto T, Inomori S, Fujino H, Nagano H: Gamma knife radiosurgery for metastatic brain tumors from renal cell carcinoma. J Neurosurg 105 (06): 55, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Shuto T, Inomori S, Matsunaga S, Fujino H: Efficacy of gamma knife surgery for control of peritumoral edema associated with metastatic brain tumors. J Neurol Neurosurg Psychiatry 79 (008): 1061, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto M, Ide M, Nishio S, Urakawa Y: Gamma knife radiosurgery for numerous brain metastases: Is this a safe treatment. Int J Radiat Oncol Biol Phys 53(5) 002): 127, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto M, Ide M, Jimbo M, Aiba M, Ito M, Hirai H, Usukura M: Gamma knife radiosurgery with numerous target points for intracranially disseminated metastases: Early experience in three patients and experimental analysis of brain irradiation doses, in Douglas Kondziolka. (ed): Radiosurgery 1997. vol 2, Basel, Karger; 1998, pp94-109 [Google Scholar]
- 22. Yamamoto M, Ide M, Jimbo M, Aiba M, Ito M, Hirai H, Usukura M: Gamma knife radiosurgery with numerous target points for intracranially disseminated metastases: Early experience in three patients and experimental analysis of brain irradiation doses, in Douglas Kondziolka. (ed): Radiosurgery 1997. vol 2, Basel, Karger; 1998, pp94-109 [Google Scholar]
- 23. Yamamoto M: Radiosurgery for metastatic brain tumors. Prog Neuro 2007; 20 2007): 106-28. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto M, Barford BE, Urakawa Y: Gamma knife radiosurgery of non-lung cancer origin: focusing on multiple brain lesions. Yamamoto (ed): Japanese experience with gamma knife radiosurgery, Basel (Switzerland), Karger, 142-153, 2008 [Google Scholar]


