Abstract
Background and Purpose
To investigate the utility of quantitative PET analysis for early prediction of local control following stereotactic body radiation therapy (SBRT).
Material and Methods
An initial test cohort of fourteen cases and a validation cohort of twenty-three cases were analyzed. All patients had metastatic or recurrent cancer and underwent PET-CTs pre- and post- SBRT to a variety of sites. Local failure was defined as biopsy proven persistent/recurrent disease or progressive disease on radiologic imaging. Using deformable registration, radiation dose was transferred to the PET-CTs. Using the prescription isodose as the volume of interest (VOI), response was assessed by generating metabolic volume histograms (MVH). MVH curves examine metabolic heterogeneity in the VOI. Exploratory analyses of the test cohort evaluated the viability of multiple iso-SUV and iso-volumetric points selected from the MVH curves to serve as novel markers of response. Standard PET response markers (maximum/mean SUV and qualitative analysis) were also assessed.
Results
In the initial cohort, ten of fourteen patients achieved local control at last follow-up, a median of 225 days following post-SBRT PET. Three out of four local failures had an increase in max SUV, while all patients who achieved local control had a reduction in max SUV (p=0.01). Exploratory analyses using multiple iso-SUV and iso-volumetric points did not yield any factors associated with local control (p>0.05). In the validation cohort, lower post- treatment max SUV (p=.03) and reduction in max SUV (p<0.05) were significantly associated with local control.
Conclusions
Reduction in max SUV following SBRT is associated with local control.
Keywords: SBRT, SBRT, PET, Response assessment, Deformable image registration
INTRODUCTION
Functional imaging using positron emission tomography (PET) and 18F-fluoro-deoxy-2-glucose (FDG) has been widely adopted in the field of oncology as a tool to assess metabolic disease activity in response to therapeutic interventions. PET has advantages over anatomical imaging in detecting early response to treatment, as changes in tumor metabolism may occur before reduction in tumor size. In one study evaluating neoadjuvant treatment for breast cancer, differences in FDG uptake between responders and non-responders were seen following a single cycle of chemotherapy.[1] Furthermore, in certain tumor types, anatomic-based imaging may not accurately reflect tumor response. This outcome has been documented in Hodgkin’s disease, where persistent mediastinal masses can be seen post-treatment despite a pathologic complete response.[2,3] Functional imaging may provide early prognostic information that can guide further therapy. A recent prospective study comparing PET versus CT to predict survival following radiotherapy with and without chemotherapy treatment for NSCLC found that a single post-treatment PET scan was a better predictor of survival than CT or pre-treatment KPS.[4]
While PET is an accepted tool for assessing outcome, there is a lack of standardization in how to measure response. Multiple approaches have been used, with two of the more common being visual assessment of tumor change and standardized uptake values (SUV). Pre-treatment SUV has been found to correlate with response to treatment[5] and reductions in SUV have been shown to correlate with histopathologic response in a variety of tumor types.[6,7,8]
Previous approaches to quantifying tumor response have used changes in average, maximum, or peak SUV within the tumor volume. These methods are useful predictors of tumor response and prognosis, but they give a numerical rather than anatomical based description of changes in FDG uptake and, therefore, do not reflect the metabolic heterogeneity within the treated target volume.[6]
We employed a PET image-guided system to quantitatively evaluate therapeutic response to SBRT by assessing metabolic heterogeneity within the treated site. Using Velocity AI software (Velocity Medical Solutions, Atlanta, GA) and deformable registration, pre- and post-treatment PET-CTs were registered to the planning CT and radiation dose information was transferred to the PET-CTs. Using the treatment prescription isodose as the volume of interest (VOI), response was assessed by generating metabolic volume histograms (MVH) for pre- and post-SBRT PETs. MVH curves examine metabolic heterogeneity in the VOI by graphically depicting the SUV by percent volume. Exploratory analyses evaluated the viability of multiple iso-SUV and iso-volumetric points selected from the MVH curve to serve as potential metabolic predictors of response. Standard PET response markers such as changes in maximum/mean SUV and qualitative analysis were also assessed.
The goal was to test feasibility of utilizing our quantitative methods for assessing treatment response with PET/CT imaging and evaluate the utility of this approach for early prediction of clinical outcomes following SBRT. After analyzing our findings from an initial test cohort, we confirmed significant results using a validation cohort.
MATERIALS AND METHODS
Patients
With IRB approval, records of the radiation oncology department were reviewed to identify adult patients with any pathologically proven solid tumor histology who had received SBRT for recurrent or metastatic disease between the years 2006 and 2008. Fourteen patients were part of the test cohort and met these inclusion criteria: (1) had undergone pre- and post SBRT PET-CT imaging, (2) had an FDG-avid tumor, (3) PET-CT was used in their radiation planning. Prior therapy including previous radiotherapy was not an exclusion criterion. The validation cohort met the same criteria and consisted of twenty-three cases treated from 2009 to 2011.
Stereotactic body radiation therapy
Each patient’s radiation treatment plan was individualized in regards to dose, fractionation, immobilization, and localization. CT-simulations were performed and the images used for three or 4-dimensional treatment planning. If deemed beneficial by the physician, respiratory gating was carried out with the Varian Realtime Position Management (RPM) system. The optimal phase(s) were then selected for planning and treatment.
All patients had PET-CT and relevant diagnostic imaging incorporated into treatment planning. Rigid and/or deformable registration of images was performed using Velocity software and metabolic target volumes were transferred to the treatment planning system.
Gross disease was outlined on the simulation CT and any additional imaging scans and an individualized margin was added to create a planning target volume (PTV). Margins varied based on the disease location, utilization of respiratory gating, and localization method, but generally ranged from 3mm to 10mm. Intensity-modulated radiation therapy (IMRT) was used for all treatments and dose was prescribed to the 100% isodose surface in all but 2 cases where the prescription volume was defined by the 80% line. Normalization was performed when necessary to ensure that the 100% isodose distribution provided adequate coverage of the PTV. Localization was achieved prior to each treatment using cone beam CT and/or on-board kV imaging. Conformality index [9] (CI) was calculated as
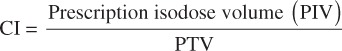
Imaging and Evaluation
All patients had whole-body FDG-PET imaging performed within the Emory Healthcare system before and after SBRT. All patients fasted for a minimum of 4-6 hours prior to intravenous injection with FDG with imaging performed 60-90 minutes post-injection. A CT scan was acquired for attenuation correction and localization purposes. Subsequently, positron emission tomography images from the skull base to the thighs were obtained. Patient weight, plasma glucose and injected dose of FDG were recorded. SUV was calculated as
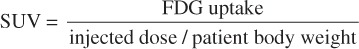
Pre- and post-treatment PET-CT images, radiation dose (RT dose) information, and planning CT images were transferred to the VelocityAI software and deformable image registration was used to register the multiple data sources. MVH curves were created and multiple iso-SUV and iso-volumetric points were selected from the MVH curve to analyze as potential metabolic markers of response for the test cohort. Minimum (SUVmin), maximum (SUVmax), and mean SUV (SUVmean) were also calculated using Velocity AI.
Maximum SUV values for each of the treated lesions were recorded from radiology reports and included in the initial analysis. These values will be referred to as SUV radiology maximum (SUVRmax) . In the cases where no maximum SUV was reported in the treated area on the post-treatment scan (i.e. no FDG uptake above background levels), the SUVmean within the VOI was used as representative of background FDG uptake.
Local failure was defined as biopsy proved persistent/recurrent disease or progressive disease on CT and/or MRI reports. Patients who did not fail locally were considered to be responders. Disease progression at other sites outside of the radiation field was defined as distant failure. In cases where the radiology reports were unclear, the treating radiation oncologist reviewed the diagnostic and simulation imaging to determine response in the radiation field. These reviewers were blinded to the results of the PET imaging.
Statistics
Paired t-test and Wilcoxon tests were performed to assess whether absolute changes in the metabolic markers were significantly different between responders and non-responders. Fisher exact test was performed to test whether a significant association existed between the direction of change in the markers and local control. Logistic regression was used to assess if the pre, post treatment or changes in the SUV markers were predictive of achieving local control. Adjustments were made for the following potential confounders in the logistic regression model: sex, age, KPS, presence of metastatic disease, previous radiation to the SBRT field, chemotherapy within 3 months of SBRT.
RESULTS
Patient and radiation treatment characteristics
Fifty-one patients received SBRT between 2006-2008 and 14 of these patients met inclusion criteria and were identified for analysis in the test cohort. The validation cohort consisted of 17 patients who received 23 treatments from 2009-2011. The median target volume, total dose, and number of fractions for the test cohort were 83cc, 17.5Gy, and a single fraction, while for the validation cohort they were 23cc, 16Gy, and a single fraction. In the test and validation cohorts respectively, three (21%) and five patients (29%) had received prior radiation to the SBRT site. Full details of the patient population and treatments can be found in Tables 1a and 1b.
Local control and PET results in the test cohort
With a median follow-up after SBRT of 9 months (range 1-22), four patients have experienced local failure and four patients have died. Two patients had both local recurrence and death. Of the four local failures, three were radiographically detected and one was detected via biopsy.
Details of the PET results and local control are listed in Table 2a. Median time from pre-treatment PET to SBRT was 41 days (range 11-128). Pre-treatment SUV parameters, had the following median values: SUVmin 0.4, SUVmean 2.7, SUVmax 10, SUVRmax 9.7. Median time from SBRT to initial post-SBRT PET was 44 days (range 14-216). Post-treatment SUV parameters had the following median values: SUVmin 0.7, SUVmean 2.3, SUVmax 7.7, SUVRmax 7.6.
Table 2.
PET response and local control
a. Test Cohort
| Case # | SUVmax | ∆SUVmax | |
|---|---|---|---|
| Pre | Post | Pre-Post | |
| Responders* | |||
| 1 | 9.8 | 5.8 | 4.0 |
| 2 | 33.8 | 15.2 | 18.6 |
| 4 | 17.2 | 8.0 | 9.2 |
| 5 | 10.1 | 7.2 | 2.9 |
| 6 | 7.2 | 3.5 | 3.6 |
| 7 | 13.2 | 9.6 | 3.6 |
| 8 | 12.5 | 11.2 | 1.3 |
| 9 | 9.3 | 7.3 | 2.0 |
| 11 | 6.3 | 4.6 | 1.7 |
| 13 | 12.4 | 7.2 | 5.1 |
| Non-Responders† | |||
| 3 | 3.9 | 4.8 | -0.9 |
| 10 | 3.2 | 9.4 | -6.3 |
| 12 | 11.8 | 10.2 | 1.6 |
| 14 | 8.3 | 8.9 | -0.7 |
| Median | 10.0 | 7.7 | 2.4 |
| Median Responders | 11.2 | 7.3 | 3.6 |
| Median Non-Responders | 6.1 | 9.2 | -0.8 |
Table 1.
Patient and radiation treatment characteristics
a. Test Cohort
| Case | Diagnosis | SBRT site | Dose/fx (Gy) | # fx | TD (Gy) | BED (Gy10) | PIV (cc) | PTV (cc) | CI | Response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | lung Adeno.* | adrenal | 18 | 1 | 18 | 50 | 29 | 23 | 1.25 | R |
| 2 | papillary RCC* | spine | 6 | 3 | 18 | 29 | 20 | 15 | 1.32 | R |
| 3 | esophageal SCC. | GE junction† | 5 | 3 | 15 | 23 | 87 | 77 | 1.13 | NR |
| 4 | Cholangiocarcinoma | abdominal LN | 20 | 1 | 20 | 60 | 537 | 467 | 1.15 | R |
| 5 | jejunal Adeno. | abdominal LN | 18 | 1 | 18 | 50 | 284 | 177 | 1.60 | R |
| 6 | lung Adeno.* | lung | 20 | 3 | 60 | 180 | 42 | 33 | 1.30 | R |
| 7 | rectal Adeno. | rectum† | 5 | 3 | 15 | 23 | 49 | 34 | 1.45 | R |
| 8 | Leiomyosarcoma* | pelvic bone | 17 | 1 | 17 | 46 | 104 | 95 | 1.10 | R |
| 9 | lung Adeno.* | spine | 12 | 1 | 12 | 26 | 13 | 10 | 1.32 | R |
| 10 | rectal Adeno. | pelvic soft tissue† | 17 | 1 | 17 | 46 | 110 | 97 | 1.13 | NR |
| 11 | breast Adeno.* | spine | 12.5 | 1 | 12.5 | 28 | 7 | 28 | 0.27 | R |
| 12 | colon Adeno.* | liver | 20 | 1 | 20 | 60 | 191 | 166 | 1.16 | NR |
| 13 | lung SCC.* | liver | 15 | 1 | 15 | 38 | 845 | 544 | 1.55 | R |
| 14 | colon Adeno.* | abdominal LN | 18 | 1 | 18 | 50 | 105 | 89 | 1.18 | NR |
| Median | 17 | 1 | 17.5 | 46 | 96 | 83 | 1.21 |
b. Validation Cohort
| Case | Diagnosis | SBRT site | Dose/fx (Gy) | # fx | TD (Gy) | BED (Gy10) | PIV (cc) | PTV (cc) | CI | Response |
| 1 | lung Adeno. | lung | 20 | 3 | 60 | 180 | 22 | 18 | 1.21 | R |
| 2 | breast Adeno.* | spine† | 7 | 3 | 21 | 36 | 237 | 214 | 1.11 | R |
| 3 | Melanoma* | IMN | 10 | 1 | 10 | 20 | 12 | 10 | 1.15 | R |
| 4 | Melanoma* | spine | 16 | 1 | 16 | 42 | 13 | 11 | 1.19 | NR |
| 5 | Melanoma* | spine | 16 | 1 | 16 | 42 | 25 | 21 | 1.15 | NR |
| 6 | pancreas Adeno. | pancreas† | 8 | 3 | 24 | 43 | 16 | 15 | 1.09 | R |
| 7 | Melanoma* | thoracic LN | 15 | 1 | 15 | 38 | 69 | 62 | 1.11 | R |
| 8 | lung Adeno* | lung | 10 | 5 | 50 | 100 | 30 | 15 | 2 | R |
| 9 | lung Adeno.* | lung | 7.5 | 6 | 45 | 79 | 84 | 96 | 0.87 | R |
| 10 | colon Adeno.* | liver | 20 | 3 | 60 | 180 | 124 | 91 | 1.36 | NR |
| 11 | h&n SSC.* | spine | 16 | 1 | 16 | 42 | 36 | 29 | 1.24 | R |
| 12 | h&n SSC.* | spine | 16 | 1 | 16 | 42 | 36 | 21 | 1.66 | NR |
| 13 | h&n SSC.* | spine | 16 | 1 | 16 | 42 | 89 | 75 | 1.18 | NR |
| 14 | h&n SSC.* | spine | 16 | 1 | 16 | 42 | 87 | 84 | 1.04 | R |
| 15 | h&n SSC.* | spine† | 16 | 1 | 16 | 42 | 32 | 29 | 1.11 | R |
| 16 | lung Adeno | lung† | 7.5 | 8 | 60 | 105 | 9 | 6 | 1.5 | R |
| 17 | h&n SSC | carotid† | 10 | 4 | 40 | 80 | 5 | 13 | 0.42 | R |
| 18 | breast Adeno.* | spine | 10 | 1 | 10 | 20 | 15 | 29 | 0.52 | R |
| 19 | colon Adeno.* | liver | 9 | 3 | 27 | 51 | 16 | 12 | 1.28 | R |
| 20 | lung SSC.* | spine | 18 | 1 | 18 | 50 | 30 | 24 | 1.25 | R |
| 21 | breast Adeno.* | spine | 16 | 1 | 16 | 42 | 58 | 47 | 1.25 | R |
| 22 | breast Adeno.* | spine | 16 | 1 | 16 | 42 | 4 | 4 | 1.15 | R |
| 23 | colon Adeno.* | spine | 18 | 1 | 18 | 50 | 49 | 23 | 2.09 | R |
| Median | 16 | 1 | 16 | 42 | 30 | 23 | 1.18 |
metastatic disease present at time of treatment
received prior radiation to this site
Abbreviations: Adeno = Adenocarcinoma; RCC= Renal cell carcinoma; SCC= Squamous cell carcinoma; SBRT = stereotactic body radiation therapy; LN = lymph node; IMN=internal mammary lymph node; fx=fraction; TD=total dose; BED (Gy10)= biologic equivalent dose; PIV = prescription isodose volume; PTV=planning target volume; CI=conformality index; R=responder; NR=non-responder
The median change between PET scans (pre- to post-SBRT) was 2.4 for SUVmax and 2.49 for SUVRmax. As seen in Table 2a three out of four patients with local failures had an increase in SUVmax, while all patients who achieved local control had a reduction in SUVmax (p=0.011). Reductions in SUVmin, SUVmean, SUVRmax were not significantly associated with achieving local control. For the non-responders, the median SUV pre- minus post-treatment (∆SUV) PET were 0.8 (∆SUVmax) and 2.5 (∆SUVRmax).
Exploratory analyses looked at multiple iso-SUV and iso-volumetric markers to determine if pre-treatment, post-treatment, or the differences between pre and post values were significantly different between the responders and non-responders. This analysis did not yield any factors associated with local control (p>0.05).
Local control and PET results in the validation cohort
With a median follow up of 97 days (range 28-278); there have been five local failures out of 23 cases in the validation cohort. All failures were detected radiographically. Results of pre- and post-SBRT for responders and non-responders are found in Table 2b. The median pre-SBRT SUVmax, post-SBRT SUVmax and ∆SUVmax were 7.46, 3.20 and 3.23 for the responders and 5.71, 7.42 and (-) 1.72 for the non-responders. Four out of five (80%) of non-responders had a negative ∆SUVmax, while all the responders have a positive ∆SUVmax.
The post-treatment SUVmax (p=0.009), the ∆SUVmax (p=0.016), and the direction of change in the ∆SUVmax (p<0.001) were significantly different between responders and non-responders. After adjusting for potential confounders in the logistic regression model, lower post-treatment SUVmax (p=0.033) and positive ∆SUVmax (p=0.045) are both significantly associated with local control.
DISCUSSION
PET-CT is a non-invasive modality that may be able to overcome the limitations of anatomic imaging [10] for measuring response to local therapy and provide earlier indication of eventual clinical outcomes. Qualitative assessment of PET-CT is often adequate and efficient for staging and overall assessment of response to systemic therapy; however, a more quantitative approach may improve the accuracy and precision when determining response to a local therapy. The most quantitative method would pair dynamic scanning with venous or arterial blood sampling, but this technique is invasive, time consuming and costly.[11] Comparisons of dynamic and semi-quantitative techniques have found a good correlation between absolute quantitative metabolic rate of FDG metabolism and SUV normalized to lean body mass, body weight or body surface area.[12]
SBRT delivers a high dose of radiation with the goal of ablating the tumor. The data supporting PET for response assessment following radiotherapy has been primarily acquired using conventional fractionation (~2 Gy per day). PET response following hypofractionated radiation has been mostly studied in non-small cell lung cancer; partially motivated by the difficulties of distinguishing residual tumor from lung fibrosis or atelectasis on CT follow high doses of radiation.[13] Limited data looking at PET one to four weeks post-SBRT suggests that early rises in SUV may be inflammatory and not indicative of treatment failure. A report of nine patients receiving SBRT for NSCLC by Ishimori et al.[14] found that 2 patients had rises in peak SUV at 1-2 weeks post-SBRT, but neither patient had evidence of local failure on follow up CT imaging at 17 and 11 months, respectively. Henderson et al. [15] reported on a prospective study of 14 patients who received SBRT for NSCLC. Of four patients who had a rise in maximum SUV at 2 weeks, none went on to develop a local failure with a median follow-up of 30 months.
b. Validation Cohort
| Case # | SUVmax | ∆SUVmax | |
|---|---|---|---|
| Pre | Post | Pre-Post | |
| Responders* | |||
| 1 | 8.91 | 2.32 | 6.59 |
| 2 | 10.46 | 7.31 | 3.15 |
| 3 | 5.84 | 2.54 | 3.30 |
| 6 | 4.47 | 2.75 | 1.72 |
| 7 | 6.16 | 5.83 | 0.33 |
| 8 | 3.26 | 2.62 | 0.64 |
| 9 | 17.42 | 8.18 | 9.24 |
| 11 | 10.64 | 2.68 | 7.96 |
| 14 | 10.75 | 4.33 | 6.42 |
| 15 | 8.57 | 3.20 | 5.37 |
| 16 | 8.16 | 3.20 | 4.96 |
| 17 | 6.76 | 4.85 | 1.91 |
| 18 | 10.65 | 2.87 | 7.78 |
| 19 | 5.24 | 3.84 | 1.40 |
| 20 | 13.20 | 4.81 | 8.39 |
| 21 | 4.85 | 2.82 | 2.03 |
| 22 | 4.38 | 1.98 | 2.40 |
| 23 | 6.43 | 3.46 | 2.97 |
| Non-Responders† | |||
| 4 | 5.33 | 6.11 | -0.78 |
| 5 | 5.71 | 7.43 | -1.72 |
| 10 | 3.65 | 7.42 | -3.77 |
| 12 | 8.00 | 12.68 | -4.68 |
| 13 | 12.96 | 4.04 | 8.92 |
| Median | 6.76 | 3.84 | 2.97 |
| Median Responders | 7.46 | 3.20 | 3.23 |
| Median Non-Responders | 5.71 | 7.42 | -1.72 |
Responders=locally controlled;
Non-Responders=local failures.
Abbreviations: Pre= prior to SBRT; Post= post SBRT; SUV; SUVmax = maximum SUV; ∆SUVmax = change in SUV maximum from pre- to post-treatment scan
Metabolic changes on delayed imaging (>1 month post-SBRT) appear to be more reliable in assessing local response to therapy. In a study of 28 patients receiving pre- and post-SBRT PET for lung cancer, Coon et al.[16] found that at a median of 5 months post-SBRT, tumor response correlated with both post-treatment SUV (P < .001) and net change in SUV (P = .02). The percent reductions in mean SUV were 94% for complete responders (CR), 48% for partial responders (PR), 28% for those with stable disease (SD), and 0.4% for those with progressive disease (PD). Feigenberg et al. [17] examined 18 NSCLC patients who had PET scans before and 3 months after SBRT. There were no local recurrences in patients who had a reduction in maximum SUV on post-SBRT PET scans, while three out of five patients who did not have a reduction in SUV post-treatment recurred locally. In their study of PET response following SBRT for lung metastasis, Fuss et al.[18] found that out of 30 patients who had post-SBRT imaging at 4-12 weeks, the two patients who did not have a reduction in SUV both failed locally.
Delayed rises in metabolic activity following ablative doses of radiation to the lung may not represent local failure. Ishmori et al. [13] found two patients who had delayed rises in SUV at >3 months after SBRT which appeared to be due to radiation pneumonitis and not local failure. In their study of 28 patients with PET following lung SBRT, Hoopes et al. [19] reported that 14% of patients had persistent FDG uptake (SUV 2.5-5.07) on delayed PET imaging (22-26 months post-treatment) without evidence of local failure. Similarly, Henderson et al. [14] found that six of 13 patients had SUVmax >3.5 at 12 months after SBRT but remained free of local failure on further follow-up.
The data for disease sites other than lung is sparser, but a few isolated reports exist. Gwak et al. [20] reported on three patients with imaging at one month and 6 months post-SBRT for spine metastasis. While changes in maximum SUV at one month did not correlate with local control, they found that at 6 months follow up, PET results correlated with clinical findings for all patients. Greco et al. [21] studied PET response following SBRT to a variety of sites in 38 patients and concluded that declines of >50% in maximum SUV at 3 months post-treatment were associated with local control. In a phase I dose escalation trial for recurrent head and neck cancer, Heron et al[22] evaluated PET and CT response at 45-60 days following SBRT in 25 patients using a ratio defined as maximum SUV post-treatment/ maximum SUV pre-treatment. PET and CT showed good agreement for CR and PD, but early CT showed PR for two cases that initially showed an increase in FDG uptake and were later deemed to be local failures. They concluded that PET was a more sensitive early biomarker of response than CT, although there is no established method to measure metabolic change.
We chose to evaluate response to SBRT in patients with metastatic and recurrent cancer, a patient population whose prior treatments can make response assessment using anatomic imaging alone difficult. Utilizing the radiation dose information and deformable image registration, we were able to directly compare an identical volume on both PET-CT scans. We selected the volume covered by the prescription isodose line as the VOI because it includes all the metabolically active tissue targeted with the treatment, received the full dose of radiation, and includes some surrounding normal tissue in which progressive disease might appear. Furthermore, there is some evidence that following radiation, increased FDG uptake in normal tissue surrounding tumor may correlate with tumor response. [23]
The main finding in our study was that reduction in maximum SUV is associated with achieving local control following SBRT with PET imaging occurring at a median of 56 days following treatment (Figures 1 & 2). This finding is similar to those seen by Coon et al.[15], Feigenberg et al.[16], and Fuss et al.[17] with PET imaging >1 month following SBRT to primary and metastatic lung tumors. Others studies, including those by Ishmori et al.[13], Henderson et al.[14], and Ishmori et al.[18], have demonstrated that moderate persistent FDG uptake in the treated area following lung SBRT may exist for months to years after treatment without any evidence of treatment failure. In our study, responders had a post-treatment median SUVmax of 4.5; a finding which supports the notion that elevated SUV is not synonymous with active tumor. Thus it appears, as has been suggested by others[24], that a more accurate approach to response assessment should involve monitoring changes in FDG uptake in a particular tumor over time rather than quantifying FDG uptake on imaging obtained at a single point in time.
Figure 1.
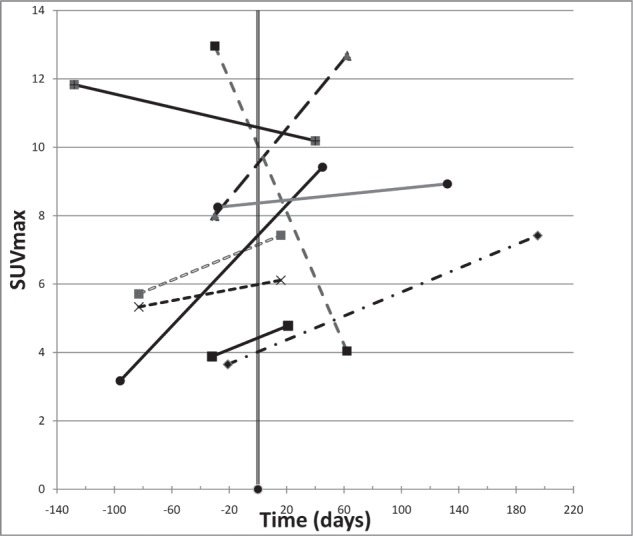
Maximum SUV (SUVmax) over time in non-responders. SBRT occurred at day zero and each of the patients had pre- and post-treatment imaging represented in time by the start and end of each line.
Figure 2.
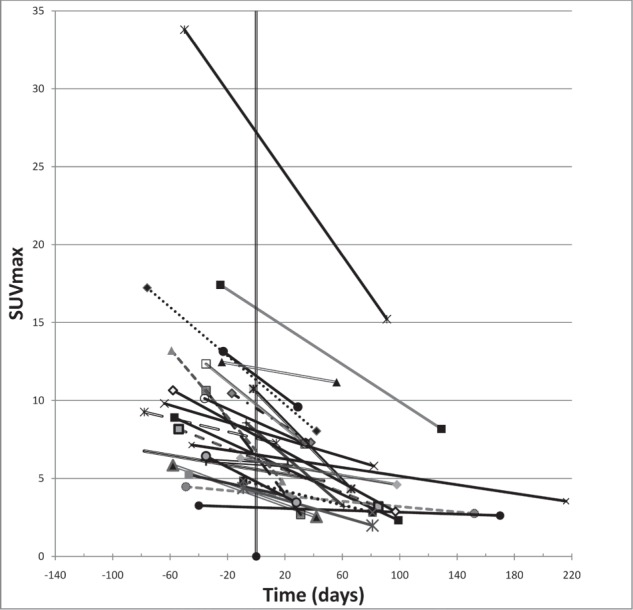
Maximum SUV (SUVmax) over time in responders. SBRT occurred at day zero and each of the patients had pre- and post-treatment imaging represented in time by the start and end of each line.
The aim of our study design was to track a specific tumor volume over serial PET-CT imaging in order to improve the accuracy and reproducibility of response assessment. While we did not identify any novel metabolic predictors of response in the MVH curve analysis, it does appear that performing a more rigorous quantitative assessment in select patient populations may be advantageous. In the test cohort, our approach correctly identified a rise in maximum SUV in three of the four patients who failed locally, none of whom were identified as local failures using PET-CT radiology reports and visual assessment. In the validation cohort, our approach identified a rise in maximum SUV in four out of five local failures, all four of whom were also correctly categorized as non-responders using qualitative assessment. Three of the four patients correctly identified using qualitative assessment had SBRT to spine targets, while all four of patients incorrectly identified by qualitative methods had organ or soft tissue radiation targets.
For the patients receiving SBRT to non-spine sites, utilizing deformable registration to directly compare SUV changes within the treated volume yielded a decided advantage, correctly identifying three of the four failures which were not detected using qualitative methods. This emphasizes the point that qualitative approaches may not be sufficient to detect small metabolic changes in pre-treated areas and tumors with low tumor to background FDG uptake. In cases like a solitary lung or vertebral body metastases , it can be straightforward for radiologists to place a VOI over the target and measure SUV on separate scans. However, in patients with metastatic disease the target may not be clearly evident, may be distorted by treatment effects, and may lie adjacent to other sites of disease. Furthermore, radiologists often to not have access to radiotherapy treatment details to guide their assessment of local response. Figure 3 shows an example of the heterogeneity of response that can be seen inside a treated volume. This patient received SBRT to a recurrent esophageal lesion and appeared to have a good response on qualitative assessment. However, the subtraction images generated using deformable registration revealed that some of the treated area has progressed.
Figure 3.
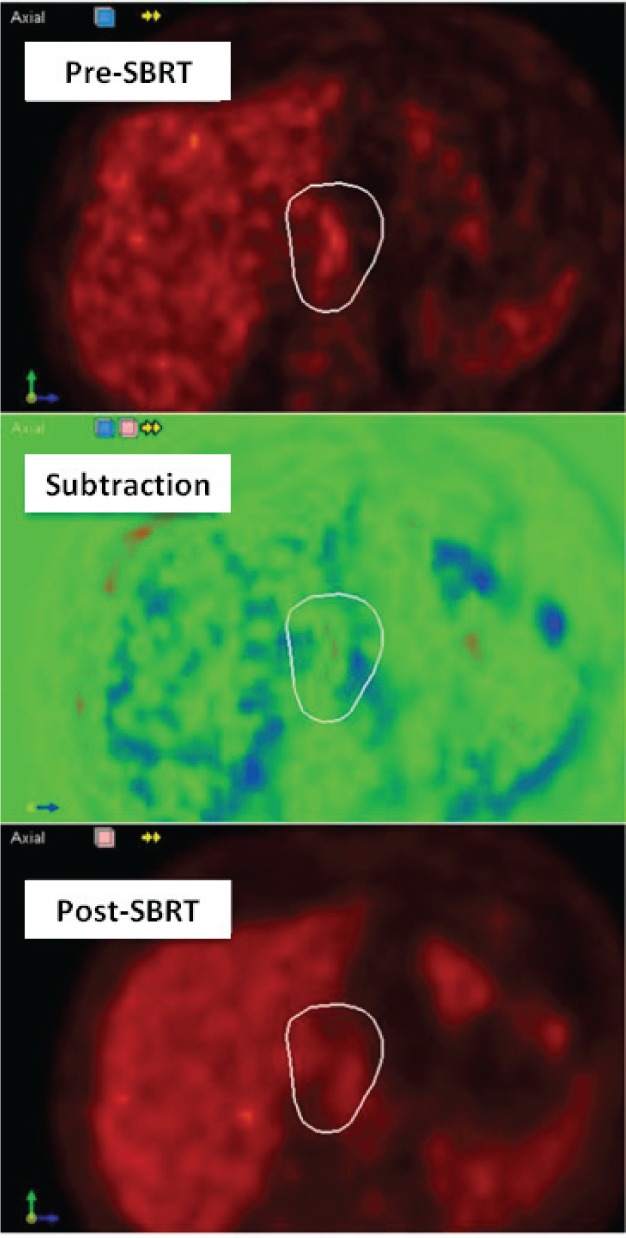
Analysis of metabolic response within the prescription isodose for Case 3 of test cohort using pre- and post-SBRT PET images and subtraction images shows a mixed response. On the subtraction image, green represents areas with no change in SUV between scans, red represents a reduction in SUV post-SBRT and blue represents a rise in SUV post-SBRT.
Although the multiple iso-SUV and iso-volumetric points selected from the MVH curve were not found to be significant predictors of local control, useful clinical information can be derived from the MVH curves and examination of metabolic heterogeneity within the target volume. Data from MVH curves can be incorporated into spatial response mapping that combines metabolic response information with anatomic imaging and dose information, thus providing a method for analyzing changes in SUV in relation to dose. This information can guide further local therapy for an individual patient and provides a basis for quantitatively measuring metabolic dose-response relationships for various tumor types and sites.
There are several limitations to the current study, foremost being the retrospective nature and small patient numbers. While we were able to detect a significant association between reduction in maximum SUV and achievement of local control, no other metabolic markers were found to be predictive of response. This may be related to the small number of patients and the heterogeneity of the population in regards to disease type, prior treatment and SBRT dose fractionation and delivery. Although a standardized PET protocol was used, several factors can interfere with the accurate measurement of SUV including blood glucose level, time from injection to scanning, calibration factors and decay correction [23] . Barriers to widespread application of this approach include that it requires additional time and requires specialized software.
CONCLUSION
Reduction in maximum SUV following SBRT appears to be associated with local control. No other surrogate endpoints from MVH analyses were found to be similarly useful. Quantitative approaches that include serial examination of the metabolic heterogeneity within tumors can provide useful information on response to treatment and may be helpful in planning further local therapy. Further studies are warranted to explore the utility of this approach in a prospective fashion.
CONFLICTS OF INTEREST STATEMENT
Dr. Fox and Dr. Crocker are entitled to royalties derived from Velocity Medical Solution’s sale of products. The terms of this agreement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
REFERENCES
- 1. Schelling M., Avril N., Nakrig J., Kuhn W., Romer W., Sattler D., Werner M., Dose J., Janicke F., Graeff H., Schwaiger M. Positron emission tomography using [18F] fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol, 2000; 18, 1689-1695. [DOI] [PubMed] [Google Scholar]
- 2. Jerusalem G., Beguin Y., Fassotte M., Najjar F., Rigo P., Fillet G. Whole-body positron emission tomography using18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphomas higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood, 1999; 94, 429-433. [PubMed] [Google Scholar]
- 3. Weihrauch M., Re D., Scheidhauer K., Ansen S., Dietlein M., Bischoff S., Bohlen H., Wolf J., Schicha H., Diehl V., Tesch H. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood, 2001; 98, 2930-2934. [DOI] [PubMed] [Google Scholar]
- 4. MacManus M., Hick R., Matthews J., McKenzie A., Rischin D., Salminen E., Ball D. Positron Emission Tomography Is Superior to Computed Tomography Scanning for Response-Assessment After Radical Radiotherapy or Chemoradiotherapy in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol, 2003; 21, 1285-1292. [DOI] [PubMed] [Google Scholar]
- 5. Kitagawa Y., Sano K., Nishizawa S., Nakamura M., Ogasawara T., Sadato N., Yonekura Y. FDG-PET for prediction of tumour aggressiveness and response to intra-arterial chemotherapy and radiotherapy in head and neck cancer. Eur J Nucl Med Mol Imag, 2003; 30, 63–71. [DOI] [PubMed] [Google Scholar]
- 6. Schelling M., Avril N., Nahrig J., Kuhn W., Romer W., Sattler D., Werner M., Dose J., Janicke F., Graeff H., Schwaiger M. Positron emission tomography using (18)F Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol, 2000; 18, 1689–1695. [DOI] [PubMed] [Google Scholar]
- 7. Benz M., Allen-Auerbach M., Eilber F., Chen H., Dry S., Phelps M., Czernin J., Weber W. Combined assessment of metabolic and volumetric changes for the assessment of tumor response in patients with soft-tissue sarcomas. J Nucl Med, 2008; 49, 1579-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wieder H., Brucher B., Zimmermann F., Becker K., Lordick F., Beer A., Schwaiger M., Fink U., Siewert J., Stein H., Weber W. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol, 2004; 22, 900–908. [DOI] [PubMed] [Google Scholar]
- 9. Shaw E., Kline R., Gillin M., Souhami L., Hirschfield A., Dinapoli R., Martin L. Radiation Therapy Oncology Group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys, 1993; 27, 1231–1239. [DOI] [PubMed] [Google Scholar]
- 10. Evilevitch V., Weber W., Tap W., Allen-Auerbach M., Chow K., Nelson SD., Eilber F., Eckardt J., Elashoff R., Phelps M., Czernin J., Eilber F. Reduction of glucose metabolic activity is more accurate than changes in size at predicting hisopathologic response to neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res, 2008; 14, 715-720. [DOI] [PubMed] [Google Scholar]
- 11. Facey K., Bradbury I., Laking G., Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess, 2007; 44, xi-267 [DOI] [PubMed] [Google Scholar]
- 12. Shankar L., Hoffman J., Bacharach S., Graham M., Karp J., Lammertsma A., Larson S., Mankoff D., Siegel B., Van den Abbeele A., Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006; 47:1059-1066. [PubMed] [Google Scholar]
- 13. Aoki T, Nagata Y, Negoro Y, et al. Evaluation of lung injury after three dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology, 2004; 230, 101–108. [DOI] [PubMed] [Google Scholar]
- 14. Ishimori T., Saga T., Nagata Y., Nakamoto Y., Higashi T., Mamede M., Mukai T., Negoro Y., Aoki T., Hiraoka M., Konishi J. 18F-FDG and 11C-methionine PET for evaluation of treatment response of lung cancer after stereotactic radiotherapy. Ann Nucl Med, 2004; 18, 669–674. [DOI] [PubMed] [Google Scholar]
- 15. Henderson M., Hoopes D., Fletcher J., Lin P., Tann M., Yiannoutsos C., Williams M., Fakiris A., McGarry R., Timmerman R. A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys, 2010; 76, 789-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coon D., Gokhale A., Burton S., Heron D., Ozhasoglu C., Neil Christie. Fractionated stereotactic body radiation therapy in the treatment of primary, recurrent, and metastatic lung tumors: The role of positron emission tomography/computed tomography-based treatment planning. Clin Lung Cancer, 2008; 9, 217–221. [DOI] [PubMed] [Google Scholar]
- 17. Feigenberg S., Yu J., Eade T., Buyyounouski M., Wang L., Langer C., Scott W., Konski A., Movsas B. FDG PET response by 3 months following stereotactic body radiotherapy for non-small cell lung cancer may be an early surrogate of local failure [abstract]. Int J Radiat Oncol Biol Phys, 2007; 69, S479-S480. [Google Scholar]
- 18. Fuss M., Salter B., Herman T., Thomas C. Metabolic PET imaging for stereotactic body radiation therapy planning and therapy response assessment of pulmonary malignancies [abstract]. Int J Radiat Oncol Biol Phys, 2005; 63, S412. [Google Scholar]
- 19. Hoopes D., Tann M., Fletcher J., Forquer J., Lin P., Lo S., Timmerman R., McGarry R. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer, 2007; 56, 229–234. [DOI] [PubMed] [Google Scholar]
- 20. Gwak H., Youn S., Chang U., Leee D., Cheon G., Rhee C., Kim K., Kim H. Usefulness of 18F−Fluorodeoxyglucose PET for radiosurgery planning and response monitoring in patients with recurrent spinal metastasis. Minim Invas Neurosurg, 2006; 49, 127-134 [DOI] [PubMed] [Google Scholar]
- 21. Greco C., Fuks Z., Kim B., Larson S., Yamada Y., Zelefsky M. Post-treatment F-18 FDG-PET standardized uptake value (SUV) predicts local control following high- dose single-fraction IGRT [abstract]. Int J Radiat Oncol Biol Phys, 2009; 75, S535-S536 [Google Scholar]
- 22. Heron D., Ferris R., Karamouzis M., Andrade R., Deeb E., Burton S., Gooding W., Branstetter B., Mountz J., Johnson J., Agiris A., Grandis J., Lai S. Stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: results of a phase I dose-escalation trial. Int J Radiat Oncol Biol Phys, 2009; 75, 1493-500. [DOI] [PubMed] [Google Scholar]
- 23. Hicks R., MacManus M., Matthews J., Hogg A., Binns D., Rischin D., Ball D., Peters L. Early FDG-PET Imaging After Radical Radiotherapy for Non-Small-Cell Lung Cancer: Inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys, 2004; 60, 412–418. [DOI] [PubMed] [Google Scholar]
- 24. Allen-Auerbach M., Weber W. Measuring response with FDG-PET: Methodological Aspects. Oncologist, 2009; 14, 369-377. [DOI] [PubMed] [Google Scholar]


