Abstract
Purpose
Leksell Gamma Knife (LGK) installations replace their Co-60 sources every 5-10 years corresponding to one two Co-60 half-lives. Between source replacements the dose rate gradually declines. The purpose of this study was to assess whether the decreasing dose rates associated with radioactive decay of Co-60 may affect the radiobiological response of a given dose delivered to 9L rat gliosarcoma cells.
Method and Materials
9L rat gliosarcoma cells were irradiated using LGK U, LGK 4C, and LGK Perfexion providing three different dose rates of 0.770 Gy/ min (sources reloaded 12.0 years ago), 1.853 Gy/min (sources reloaded 5.0 years ago) and 2.937 Gy/min (sources reloaded 1.6 years ago), respectively. After irradiation of cell samples to 4.0 Gy, 8.0 Gy and 16.0 Gy using each of the LGK units, the irradiated cells were plated into petri dishes. Two weeks later cell colonies with greater than 50 cells were counted. The survival of cells was plotted as a function of dose over the range of delivered doses and fitted to a linear quadratic function of the form SD = e-αD-βD2, where α and β are terms fit using the Levenberg-Marquardt algorithm.
Conclusions
This study demonstrated no difference in tumor cell kill in the range of dose rates when using actual LGK unit with new sources or with sources decayed even for two half lives. This study focused on tumor cells. In future studies we will reassess the dose rate effect on cultured neurons to simulate response of normal healthy brain tissue to different dose rates.
Keywords: Gamma Knife, Radiosurgery, Dose Rate Effect, Relative Biological Effectiveness
INTRODUCTION
The source of radiation for Leksell Gamma Knife (LGK) stereotactic radiosurgery (SRS) is Co-60. The rate of irradiation from such a source decreases exponentially with time. Since Co-60 has half-life of 5.26 years, the rate of irradiation decreases to one-half every 5.26 years. The concept that radiobiological response of both cells and tissues decreases at lower dose rates has been well established by many radiobiological experiments on dose rate effects, and some clinical experience with fractionated external beam radiation therapy and brachytherapy1-12. Hence, there is a concern if during SRS using the LGK where gradually declining dose rates occur, the radiobiological efficacy of a given dose might also be affected.
With the increase in popularity of radiosurgery, it is essential to re-examine the fundamental radiobiological responses over the range of dose rates generally used in treatments with LGK. At the present time, the exact impact of dose rate variations of LGKs on clinical radiation sensitivity has not been established. The dose rates generally used for all models of LGKs, whether model U (LGK U)13, 14, B (LGK B), C (LGK C), 4C (LGK 4C)15-18 or the most recent model PERFEXION (LGK PFX)19-22, range typically from 3.7 Gy/min (for newly loaded sources) down to 0.9 Gy/min (for sources, which have decayed over a period of 2 half lives, i.e., about 10.5 years). Only in rare instances have dose rates below 0.9 Gy/min been used. Such low dose rates require treatment times to be very long. Most LGK installations replace their Co-60 sources every 5-7 years. The current study examined the impact of LGK dose rate on the radiation sensitivity of brain tumor cells. Three different LGK units with a 3.8-fold range of dose rates were used to irradiate 9L rat gliosarcoma cells, and the radiation sensitivity of the cells over this dose rate range was then evaluated.
METHODS AND MATERIALS
Leksell Gamma Knife Units and Phantom Design
The three LGK units used in our experiments were, LGK U, LGK 4C, and LGK PFX. There was a 3.8-fold variation in dose rates among these units (Table 1). To minimize changes in dose rate over the course of the study, irradiation of all cells was conducted over a period of 10 consecutive days. The dose rate corresponding to the middle of this 10 day period for each of the LGK units was used in all reported results. An 18 mm collimator was used for irradiation carried out using LGK U and LGK 4C; a 16 mm collimator was used with the LGK PFX. The geometry of irradiation was essentially the same on all three units, so the only difference was in dose rate.
Table 1.
Basic characteristics of Leksell Gamma Knife units used for the irradiation of cells. Because the irradiation of cells was performed over a 10 day period, range of days from the last reloading and range of dose rates are given in parenthesis. For further analysis and data reporting in this study, the average dose rate over this 10 day irradiation period was used.
| Leksell Gamma Knife Unit | Dose Rate at Reloading [Gy/min] | Time Since Reloading [days] | Experimental Dose Rate [Gy/min] |
|---|---|---|---|
| U | 3.759 | 4396 (4392-4401) | 0.770 (0.771-0.768) |
| 4C | 3.577 | 1823 (1819-1828) | 1.853 (1.856-1.850) |
| Perfexion | 3.633 | 589 (585-594) | 2.937 (2.942-2.932) |
The cells were placed at the center of an ELEKTA ABS (ELEKTA Instrument AB, Stockholm) spherical phantom and irradiated on three LGK units. The ABS phantom consists of two hemispheres with slot cut in the central area of each hemisphere. An insert made of the same material as that of the phantom was fitted into the slot from outside. At the center of this insert was a groove designed to snugly accommodate a 0.6 ml micro centrifuge tube for holding the cell suspension (Figure 1A). When placed within the insert and phantom, the micro centrifuge tube positioned the cell suspension at the center of the radiation beam – isocenter of the LGK. To assure homogenous irradiation of the cells, the bottom of the tube was first filled with 0.5 ml of 2% agar, which left a 0.15 ml, cylindrical volume (diameter 7.0 mm and height 3.8 mm) at the top of the tube to accommodate cells (Figure 1B). The irradiation dose rate at this location was carefully calibrated so that the same doses were delivered on each of the three LGK units in the same geometrical setting. Thus, the only difference between irradiation on these three LGK units was in dose rate.
Figure 1.

Irradiation of cells on the Leksell Gamma Knife (LGK) using the Elekta ABS calibration phantom. The phantom consisted of two grey hemispheres, a central black insert with groove for a micro centrifuge tube, and two clear plastic phantom holders (A). A 0.6 ml micro centrifuge tube filled with 0.5 ml of 2% agar on the bottom and the experimental cell solution was placed within the black insert for irradiation (A & B). The assembled phantom was precisely setup for irradiation on each LGK unit (example of irradiation on LGK PFX is shown on C).
Cell Preparation, Irradiation, and Plating
The 9L rat gliosarcoma cell line was used as a source of both feeder cells and cells irradiated for determination of radiation sensitivity. Feeder cells supply autocrine and paracrine factors that enhance the plating efficiency of the experimental cells.
The 9L cells were cultivated in a solution of Dulbecco’s modified Eagle’s medium (Mediatech) with 10% fetal calf serum and penicillin/streptomycin added according to the instructions of the manufacturer. They were passaged 1-2X per week at a 1:3 to 1:5 split. Three days before irradiation of experimental cells, the feeder cells were collected at 7 days after passage by addition of 0.25% trypsin with EDTA. Once the cells dislodged from the plastic substrate, the reaction was stopped by adding cultivation medium at a 5:1 ratio (cultivation solution: trypsin solution). Cells were centrifuged at 800 RPM in a swinging bucket rotor for 5 min at room temperature, and the resulting cell pellet was resuspended in cultivation media. Viable cell concentration was determined by adding 0.4% Trypan blue to a small aliquot of the suspension. The stained cells were then placed on a hemocytometer for microscopic assessment and counting. The suspension was diluted to a final concentration of 20 X 106 cells per ml and added to the top of one of the microcentrifuge tubes pre-filled with agar as described above (Figure 1B). The tube was immersed in ice to slow metabolism during transport to and from the LGK unit. They were irradiated with 50 Gy within the previously described spherical phantom (Figure 1A, B and C) and then plated in 6 well plates at a concentration of 50,000 cells per well.
Experimental cells were collected at the log phase of the growth cycle. Cells were trypsinized, centrifuged, and resuspended in the same manner as the feeder cells. Cells were diluted to 4 X 106, 2.7 X 105, 6.7 X 104, and 3.3 X 104 to create suspensions for irradiation with 16, 8, 4, and 0 (controls) Gy respectively, and the cells were stored on ice before and after irradiation prior to plating. Controls were handled in the same manner as the irradiated groups but not irradiated. Cells were plated into 6 well plates on top of the feeder layer. They were plated at 1X, 1/2X, and 1/8X the original concentration to allow for variations in survival due to irradiation and other random variables. Plates were incubated for two weeks at 37°C and 5% CO2.
Two weeks after plating, the colonies were fixed with 70% alcohol and stained with Meyer’s hematoxylin. Those cell colonies with greater than 50 cells were counted, and from the cell colonies of irradiated and un-irradiated reference cells, the survival of irradiated cells was calculated.
Data analysis
For each dose and concentration, the number of colonies formed was normalized to the number of cells plated to determine the plating efficiency. Because colonies begin to overlap and may be difficult to distinguish as plating efficiency increases, only plates in which the average colony number was less than 60 colonies were counted. Plating efficiency at the various doses PED was then normalized to the platting efficiency of non-irradiated reference samples PE0 to determine the survival, SD, after a given dose, D
(1).
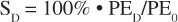
Survival was plotted as a function of dose over the range of delivered doses and fitted to a linear quadratic function of the form
(2).
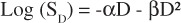
The significance of the impact of dose and LGK unit on survival were tested using general linear modeling and a commercial software package (JMP, SAS Institute, Cary, NC). The dose, square of the dose, and LGK unit were included in the model. Interactions between dose and square of the dose and LGK unit were also included to account for potential variations in the parameters due to dose rate differences among the units. Analysis of variance was used to statistically analyze differences among the α and β parameters (Equation 2) as well as the α/β ratios for the different LGK units. Values of p < 0.05 were considered significant.
RESULTS
Increasing doses of radiation substantially decreased colony formation by the 9L cells. This was evident based on both qualitative, visual inspection of the plates used for colony growth (Figure 2 A and B) and quantitative analysis of the number of colonies formed, reflected as the cell survival rates (Figure 3). The doses used in the experiment decreased survival over a wide range. The survival data for all three LGK units and thus three different dose rates was well fit by the previously described quadratic equation (Equation 2; r2 > 0.94 for all three dose rates) which results in more rapid decreases in survival with increasing dose. After the lowest dose of 4 Gy, there was an approximately 0.25 • log10 decrease in survival, whereas there was an almost 3 • log10 decrease after 16 Gy.
Figure 2.
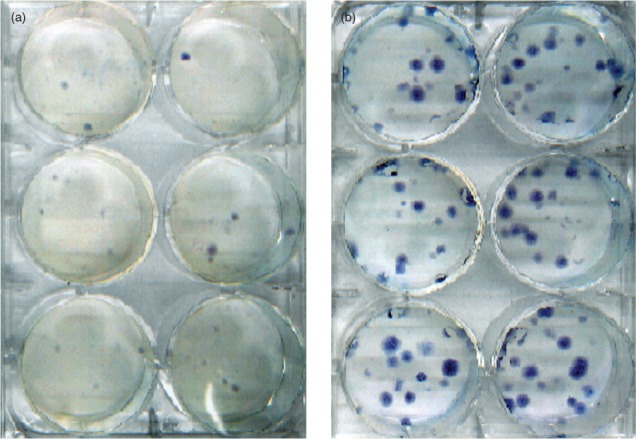
Comparison of number of cell colonies formed for irradiated (16 Gy; A) and unirradiated (B) 9L rat gliosarcoma cells. There was a substantial decrease in the number of colonies formed after 16 Gy despite being plated a concentration 120-fold higher than that used for the unirradiated cells.
Figure 3.
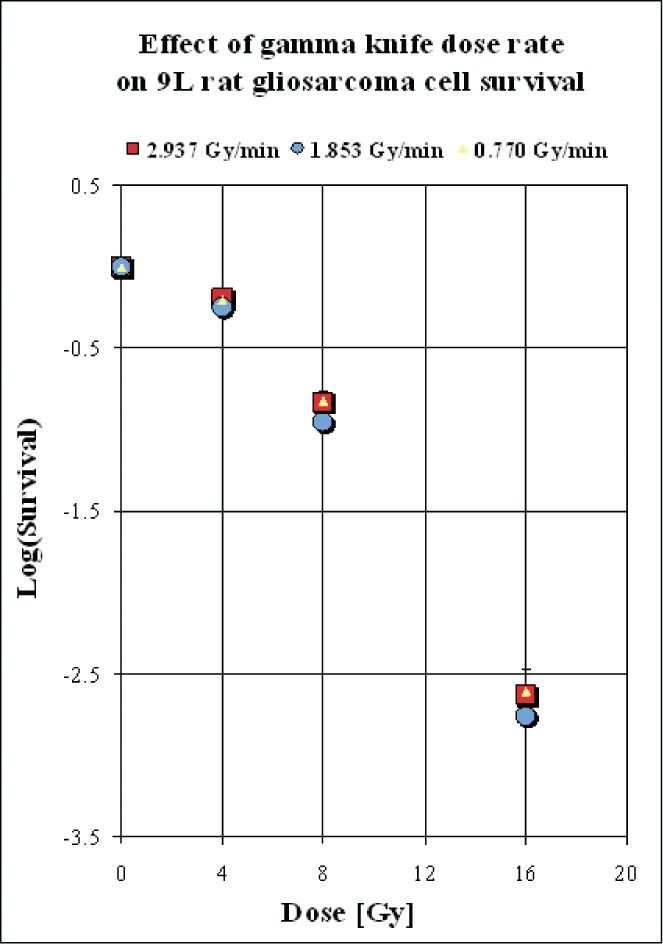
The log of survival (SD) of 9L rat gliosarcoma cells plotted as a function of radiation dose (D) for the three different dose rates. Standard error bars are only shown for the data corresponding to treatment with 16 Gy by the 4C unit. In all other cases, the bars were smaller than the associated symbol. The experiments detected no significant differences in survival due to the use of the different units and their associated dose rates.
The survival curves were very similar among all three units, and no significant differences were found by ANOVA, either in terms of the α and β parameters or in terms of the α/β ratios (Table 2). The mean α/β ratios averaged across the 4 experiments carried out for each unit ranged from 4.16 for the LGK U unit with the lowest dose rate to 7.10 for the LGK 4C unit with an intermediate dose rate to 3.90 for the LGK PFX unit with the highest dose rate.
Table 2.
The α, β, and α/β parameter estimates for the fitted survival curves when 9L cells were treated with 0-16 Gy using the LGK U, LGK 4C, and LGK PFX. Survival (S) was measured 4 times for each unit and fitted to the equation log(S) = αD – βD2 for each experiment where D is the dose in Gy. The values given for α, β, and α/β represent the mean ± SEM average over the 4 experiments for each LGK unit. There were no significant differences among the units for α, β, or the α/β ratio.
| Leksell Gamma Knife Unit | α | β | α/β |
|---|---|---|---|
| U | 0.033 ± 0.004 | 0.0082 ± 0.0007 | 4.16 ± 0.86 |
| 4C | 0.052 ± 0.002 | 0.0076 ± 0.0009 | 7.10 ± 0.98 |
| Perfexion | 0.032 ± 0.009 | 0.0083 ± 0.0007 | 3.90 ± 1.07 |
DISCUSSION
The reduction in biological effect that typically occurs with decreasing dose rate is a well known phenomenon. It is thought to arise from the fact that irradiation of a cell can lead to “sublethal damage,” which is damage that is of insufficient magnitude to induce cell death. During exposure to ionizing radiation, cellular damage accumulates. Both the length of total irradiation time and the dose rate determine whether the damage is sufficiently severe to result in lethality. At low dose rates, the cell is better able to offset the accumulating damage with repair. As the dose rate increases, the inherent repair mechanisms within a cell may no longer keep up with the accumulating damage, and, if the irradiation dose rate is sufficiently high, death may ensue.
The alterations in dose rate effect is also dependent on a variety of other factors including cell type and tissue5. For cells with a limited capability for repair of sublethal damage, there is only a moderate effect of dose rate. HeLa cells would be one example of this4. For other cells, such as Chinese Hamster ovary (CHO) cells, which have a high capacity to repair, the dose rate effect is more evident. For CHO cells, there are differences in the biological efficacy between dose rates of 0.16 Gy/min and 1.07 Gy/min1. In the present study with 9L gliosarcoma cells, we evaluated the effect of three dose rates; 0.770 Gy/min for the LGK U unit, 1.853 Gy/min for the LGK 4C unit, and 2.937 Gy/min for the LGK PFX unit. But there was no difference in cell survival. The finding of minimal or no dose rate effect may be due to the use of nervous cells in this study. In the system of spinal cord, effects of dose rate on the Relative Biological Effectiveness (RBE) of a given radiation dose have also been reported for doserates in the range of 0.03 to 1.79 Gy/min9. However, at this point, there have apparently been no reports on the impact of dose rate on the RBE of brain irradiation. Because of this, it is critically important to determine the potential impact of dose rate from various LGK units on RBE within the brain. The present study begins to address this issue by examining the impact of radiation dose-rate on brain tumor cells. The α/β ratio for the 9L brain tumor cells was in the range of 4-7, whereas the ratio for late responding normal tissues such as the brain is generally estimated as around 2. The lower α/β ratio for the normal brain could make it more susceptible to dose rate effects compared to tumor cells. Decreases in the α/β ratio are associated with a broader shoulder and greater curve to the dose-response relationship, so that the cells and associated tissues are better able to repair and recover from low dose damage but have poorer recovery from higher doses. As noted above, dose-rate effects tend to be more prominent in cells with greater repair capacities.
Theoretical studies on fractionated radiation by Fowler et al and Sterzing et al anticipated that fraction delivery times exceeding 15–30 min will show reduced treatment efficacy3, 11. Paganetti recommended that dose rates corrections should be considered as treatment times approach 20 min23. While the claims made by Paganetti were based on calculations using the lethal/potentially lethal model, Bewes et al. showed that extended treatment times in IMRT reduce the biological effectiveness of a given dose2. Similarly, Wang et al12 proposed that if delivery time is longer than 10–15 min, the prescription dose should be increased to compensate for a reduction in cell killing due to sublethal damage repair. Experimental in vitro studies by Ogino et al8 and Shibamoto et al10 showed a reduction in the cytotoxicity of a given radiation dose for irradiation times greater than 15 min and 20 min, respectively. Mu et al found a reduced cell survival at 1 min compared to the same dose being delivered in 10 min7. Similarly, Moiseenko et al demonstrated increased cell survival as delivery time was extended from 1 min to 5 min6. In contrary to the published report, we found no difference in cell survival with irradiation times in our study ranged from approximately 5 min (LGK PFX) to 20 min (LGK U) for delivered dose 16 Gy.
Bewes et al in a recent study investigated the effects of single-fraction delivery times, from acute (less than 1 min) to medium (15 min) duration2. These authors also isolated the effects of fraction delivery time from instantaneous dose rate. Bewes et al studied two critical variables, instantaneous doserate and average dose-rate. In a time-varying dose delivery, the instantaneous dose rate is the rate at which dose is accumulated at each instant of time. The instantaneous dose rate is directly proportional to the flux of incident radiation in a given medium. These investigators found that the survival fraction was independent of instantaneous dose rate. They reported a statistically significant trend to increased survival as the average dose rate was decreased, for a constant total dose.
In the case of treatment of patients on LGK, the treatment dose is generally prescribed to the 50% isodose line of a treatment plan. This means that if only a single isocenter (shot) were to be used for treatment, the treatment dose rate at 50% isodose line would be only one-half of the dose rate at the isocenter. When multiple isocenters are used to treat a lesion, and the dose prescribed is still to 50% isodose line of the treatment plan, the treatment dose rate in this case would be determined by the overall treatment time, the prescribed dose, and the calibrated dose rate at the isocenter of LGK. For example, if the total treatment time is 30 minutes to deliver the prescribed dose of 20 Gy to 50% isodose line for a specific calibrated dose rate at the isocenter, the treatment dose rate would be 0.67 Gy/min and if the treatment time were 60 minutes, the treatment dose rate in this case would be 0.33 Gy/min. Thus the treatment dose rate in the case of LGK treatments would depend on whether the treatment is done with a single isocenter or with multiple isocenters and what number of isocenters is used, which takes a longer time to treat and the calibrated dose rate at the isocenter. For a specific case of treatment of trigeminal neuralgia, Arai et al24 found that the dose rate did not affect pain control. Their dose rates at the isocenter varied from 1.21 to 3.74 Gy/min.
Our observations support the hypothesis that there is no difference in radiobiological effect on the 9L tumor cells when using LGK units whose Co-60 sources have dose rates decayed by a factor of 2.5. Future studies will focus on the effect on a neuronal cell line, which may provide additional data relative to the tolerance of nerve cells.
CONCLUSION
The reduction in biological effect that typically occurs with decreasing dose rate is a well known phenomenon. In the dose rate range commonly occurring in actual LGK radiosurgery (2.9 Gy/min to 0.8 Gy/ min) for 9L rat gliosarcoma cells, this study provides evidence that a decrease in dose rate of LGK in the range of clinical interest does not affect tumor cell survival. At this time, no adjustment in the prescribed doses based on the dose rates at the chosen isodose lines of treatment plans and the decay of Co-60 can be suggested.
ACKNOWLEDGEMENT
This project was supported by ELEKTA RESEARCH GRANT ”Biological effects of ionizing radiation on brain tumor and normal brain cells in vitro and in vivo: impact of dose rate” provided by Elekta Instrument, AB, Stockholm, Sweden.
REFERENCES
- 1. Bedford JS, Mitchell JB. Dose-rate effects in synchronous mammalian cells in culture. Radiat Res 1973; 54:316-327. [PubMed] [Google Scholar]
- 2. Bewes JM, Suchowerska N, Jackson M, et al. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT). Phys Med Biol 2008; 53:3567-3578. [DOI] [PubMed] [Google Scholar]
- 3. Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int J Radiat Oncol Biol Phys 2004; 59:242-249. [DOI] [PubMed] [Google Scholar]
- 4. Hall EJ, Bedford JS. Dose Rate: Its Effect on the Survival of Hela Cells Irradiated with Gamma Rays. Radiat Res 1964; 22:305-315. [PubMed] [Google Scholar]
- 5. Hall EJ, Brenner DJ. The dose-rate effect revisited: radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys 1991; 21:1403-1414. [DOI] [PubMed] [Google Scholar]
- 6. Moiseenko V, Duzenli C, Durand RE. In vitro study of cell survival following dynamic MLC intensity-modulated radiation therapy dose delivery. Med Phys 2007; 34:1514-1520. [DOI] [PubMed] [Google Scholar]
- 7. Mu X, Lofroth PO, Karlsson M, et al. The effect of fraction time in intensity modulated radiotherapy: theoretical and experimental evaluation of an optimisation problem. Radiother Oncol 2003; 68:181-187. [DOI] [PubMed] [Google Scholar]
- 8. Ogino H, Shibamoto Y, Sugie C, et al. Biological effects of intermittent radiation in cultured tumor cells: influence of fraction number and dose per fraction. J Radiat Res (Tokyo) 2005; 46:401-406. [DOI] [PubMed] [Google Scholar]
- 9. Scalliet P, Landuyt W, van der Schueren E. Repair kinetics as a determining factor for late tolerance of central nervous system to low dose rate irradiation. Radiother Oncol 1989; 14:345-353. [DOI] [PubMed] [Google Scholar]
- 10. Shibamoto Y, Ito M, Sugie C, et al. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int J Radiat Oncol Biol Phys 2004; 59:1484-1490. [DOI] [PubMed] [Google Scholar]
- 11. Sterzing F, Munter MW, Schafer M, et al. Radiobiological investigation of dose-rate effects in intensity-modulated radiation therapy. Strahlenther Onkol 2005; 181:42-48. [DOI] [PubMed] [Google Scholar]
- 12. Wang JZ, Li XA, D’Souza WD, et al. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity-modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys 2003; 57:543-552. [DOI] [PubMed] [Google Scholar]
- 13. Lunsford LD, Flickinger J, Lindner G, et al. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery 1989; 24:151-159. [DOI] [PubMed] [Google Scholar]
- 14. Wu A, Lindner G, Maitz AH, et al. Physics of gamma knife approach on convergent beams in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 1990; 18:941-949. [DOI] [PubMed] [Google Scholar]
- 15. Horstmann GA, Schopgens H, van Eck AT, et al. First clinical experience with the automatic positioning system and Leksell gamma knife Model C. Technical note. J Neurosurg 2000; 93 Suppl 3:193-197. [DOI] [PubMed] [Google Scholar]
- 16. Kondziolka D, Maitz AH, Niranjan A, et al. An evaluation of the Model C gamma knife with automatic patient positioning. Neurosurgery 2002; 50:429-431; discussion 431-422. [DOI] [PubMed] [Google Scholar]
- 17. Regis J, Hayashi M, Porcheron D, et al. Impact of the model C and Automatic Positionin. System on gamma knife radiosurgery: an evaluation in vestibular schwannomas. J Neurosurg 2002; 97:588-591. [DOI] [PubMed] [Google Scholar]
- 18. Tlachacova D, Schmitt M, Novotny J, Jr., et al. A comparison of the gamma knife model C and the automatic positioning system with Leksell model B. J Neurosurg 2005; 102 Suppl:25-28. [DOI] [PubMed] [Google Scholar]
- 19. Lindquist C, Paddick I. The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery 2007; 61:130-140; discussion 140-131. [DOI] [PubMed] [Google Scholar]
- 20. Niranjan A, Novotny J, Jr., Bhatnagar J, et al. Efficiency and dose planning comparisons between the Perfexion and 4C Leksell Gamma Knife units. Stereotact Funct Neurosurg 2009; 87:191-198. [DOI] [PubMed] [Google Scholar]
- 21. Novotny J, Bhatnagar JP, Niranjan A, et al. Dosimetric comparison of the Leksell Gamma Knife Perfexion and 4C. J Neurosurg 2008; 109 Suppl:8-14. [DOI] [PubMed] [Google Scholar]
- 22. Regis J, Tamura M, Guillot C, et al. Radiosurgery with the world’s first fully robotized Leksell Gamma Knife PerfeXion in clinical use: a 200-patient prospective, randomized, controlled comparison with the Gamma Knife 4C. Neurosurgery 2009; 64:346-355; discussion 355-346. [DOI] [PubMed] [Google Scholar]
- 23. Paganetti H. Changes in tumor cell response due to prolonged dose delivery times in fractionated radiation therapy. Int J Radiat Oncol Biol Phys 2005; 63:892-900. [DOI] [PubMed] [Google Scholar]
- 24. Arai Y, Kano H, Lunsford LD, et al. Does the Gamma Knife Dose Rate Affect Outcomes in Trigeminal Neuralgia Radiosurgery? Accepted for publication in Journal of Neurosurgery Suppl. [DOI] [PubMed] [Google Scholar]


