Abstract
Purpose
We investigate clinical, pathologic, and treatment paradigm-related factors affecting local control of brain metastases after stereotactic radiosurgery (SRS) with or without whole brain radiotherapy (WBRT).
Methods and materials
Patients with brain metastases treated with SRS alone, before or after WBRT were considered to determine predictors of local failure (LF), time to failure and survival.
Results
Among 137 patients, 411 brain metastases were analyzed. 23% of patients received SRS alone, 51% received WBRT prior to SRS, and 26% received SRS followed by WBRT. LF occurred in 125 metastases: 63% after SRS alone, 20% after WBRT then SRS, and 22% after SRS then WBRT. Median time to local failure was significantly less after SRS alone compared to WBRT then SRS (12.1 v. 22.7 months, p=0.003). Tumor volume was significantly associated with LF (HR:5.2, p<0.001, 95% CI:3.4-7.8).
Conclusions
WBRT+SRS results in reduced LF. Local control was not significantly different after SRS as salvage therapy versus upfront SRS.
Keywords: brain metastasis, stereotactic radiosurgery, whole brain radiotherapy, local failure, treatment paradigm, salvage therapy
1. Introduction
The combination of whole brain radiotherapy (WBRT) with stereotactic radiosurgery (SRS) has been shown in several prospective randomized studies to improve local control over either of the single modalities alone[1-4]. However, because of toxicity concerns with WBRT[3, 5] and perceived lack of added benefit for the combined approach[1], WBRT with SRS boost has been replaced by single modality treatments as the standard upfront approach for treatment of brain metastases in most patients with a limited number of brain metastases.
With the rising use of single modality therapy in the upfront treatment of brain metastases[6] as well as the increasing survival of patients with brain metastases[7], the use of salvage treatment has become increasingly important. While SRS alone generally exhibits excellent local control, the likelihood of local failure increases with increasing tumor size and survival time[8]. While most patients in this population will succumb to extracranial disease[9], local failure does increase the likelihood of neurologic death[10]. It has been unclear if treating patients in the salvage setting worsens the likelihood of local failure after SRS given the pre-selection of tumors that have already progressed through WBRT.
In this single-institution retrospective study, we examined local control of brain metastases after SRS for patients with new, recurrent or progressive brain metastasis and whether the use of SRS as salvage vs. upfront therapy has a significant impact on local control outcomes. We also aimed to identify patient, tumor, and treatment-related factors that predict local failure and survival after SRS.
2. Materials & Methods
2.1. Data Acquisition and Treatment Regimen
We retrospectively reviewed the medical records of 137 patients treated with Gamma Knife SRS for newly diagnosed, locally recurrent, or distantly progressive intracranial metastases at our institution between 2002 and 2012. Patients who underwent surgical resection of metastases were excluded. This review was conducted with Institutional Review Board approval. Prior to 2009, SRS was performed using the Leksell Gamma Knife Models B/C; from 2009 onward the Leksell Perfexion Gamma Knife system was used (Elekta AB, Stockholm, Sweden). A multi-sequence contrast-enhanced stereotactic magnetic resonance image (MRI) was obtained on the day of treatment using a 1.5 T unit prior to 2005 and a 3.0 T unit after 2005 (GE Healthcare, Waukesha, WI, USA). SRS prescription dose was determined based on previously published guidelines [11]; overall, a median dose of 19 Gy was prescribed to the 50% isodose line. WBRT was performed at various institutions according to physician and patient preference before or after SRS. Patients that received WBRT for salvage of a SRS local failure were not included in this analysis; no metastases in the SRS then WBRT group had failed prior to receiving WBRT.
Patient factors collected from the medical record included age, gender, primary histology, date of WBRT, prescribed dose of WBRT, and local recurrence at the site of SRS. A metastasis-by-metastasis database was compiled including the following factors for each individual metastasis: site of metastasis, tumor volume, treatment dose prescription, treatment volume, conformity index[12], number of shots, and beam-on-time. Brain metastases were stratified by their respective treatment paradigm: SRS alone, WBRT followed by SRS, or SRS followed by WBRT, if applicable.
2.2. Patient Follow-up and Clinical Outcomes
Patients were followed with clinical evaluation and magnetic resonance imaging of the brain initially at 4-6 weeks post-SRS and every 3 months thereafter. Local failure of a brain metastasis treated with SRS was defined as a 25% increase in tumor volume at least 90 days after SRS. Time to local failure was defined as the duration of time from the date of SRS to the date that follow-up imaging demonstrated such increase in tumor volume. Overall survival was also defined from the time of SRS. The indication for SRS was recorded from treatment planning documentation. WBRT local and distant failure were defined as progression or recurrence of a lesion treated with WBRT and new lesions not present at the time of WBRT, respectively.
2.3. Statistical Analyses
Descriptive analyses of continuous data were summarized using the mean (range) and median (interquartile range) in the case of normal and non-normal distributions, respectively. Categorical data were described as counts and frequencies. Continuous data were compared across groups with the t-test or Kruskal-Wallis test while categorical data were compared using either the Fisher’s exact or Chi-Square test. All time to event data were described with Kaplan-Meier plots and differences across strata were tested using the log-rank test. A multivariate cox proportional hazard analysis was utilized to identify covariates predictive of increased local failure. A threshold p-value of <0.2 was used for selection of covariates for consideration in the multivariate model. Selected covariates were tested for meeting the proportional hazards assumptions and for interactions across covariates. A backwards stepwise selection method was used to select the final list of covariates in the multivariate model, with a p-value <0.05 indicating statistical significance. All analyses utilized SAS v. 9.3 (Cary, NC).
3. Results
3.1. Population & Treatment Characteristics
The patient and treatment factors are described in Table 1. In total, 137 patients with 411 individual brain metastases received SRS with or without WBRT. The median patient age at time of SRS was 55 (48-63) years. Of all metastases, primary tumor histology included 193 (47%) lung, 137 (33%) breast, 59 (14%) melanoma, and 22 (5%) other (renal, colorectal, head & neck, or genitourinary) sites. The median tumor volume was 0.35 cm3 (interquartile range [IQR], 0.079-1.87 cm3) and the median number of metastases at the time of SRS was 2 (IQR, 1-4). Thirty-two patients with 51 metastases were treated with SRS alone, 70 patients with 275 metastases were treated with WBRT followed by SRS, and 35 patients with 85 metastases were treated with SRS followed by WBRT. SRS was prescribed to a median dose of 19 Gy (IQR, 16-20). WBRT was delivered to a median dose of 35 Gy (IQR, 30-37.5 Gy). The median time between WBRT and SRS in the combination treatment groups was 8.5 months (IQR, 4.8-13).
Table 1.
Patient and Treatment Characteristics by Treatment Paradigm.
| Total N (%) M (IQR) |
SRS Alone N (%) M (IQR) |
WBRT then SRS N (%) M (IQR) |
SRS then WBRT N (%) M (IQR) |
p-Value | |
| Age | 55 (48-63) | 56 (49-67) | 53 (47-61) | 59 (50-66) | 0.17 |
| Sex Male Female |
137 58 (42%) 79 (58%) |
32 16 (50%) 16 (50%) |
70 26 (37%) 44 (63%) |
35 16 (46%) 19 (54%) |
0.43 |
| Primary Tumor Lung Breast Melanoma Other |
137 69 (50%) 36 (26%) 21 (15%) 11 (8%) |
32 15 (47%) 7 (22%) 5 (16%) 5 (16%) |
70 37 (53%) 21 (30%) 11 (16%) 1 (1%) |
35 17 (49%) 8 (23%) 5 (14%) 5 (14%) |
0.19 |
| Metastases Per Patient | 2 (1-4) | 1 (1-2) | 3 (1-5) | 2 (1-3) | <0.001 |
| Total Metastases Treated | 411 | 51 (12%) | 275 (67%) | 85 (21%) | <0.001 |
| SRS Dose (Gy) | 19 (16-20) | 19 (18-21) | 18 (16-20) | 20 (18-21) | 0.006 |
| SRS Tumor Volume (cm3) | 0.35 (0.079-1.87) |
3.42 (0.28-5.62) |
0.26 (0.067-1.50) |
0.26 (0.061-1.34) |
<0.001 |
| WBRT Dose | 35 (30-37.5) |
- | 35 (30-37.5) |
31.3 (30.37.5) |
0.76 |
| Time between Treatment (mo) | 8.5 (4.8-13) |
- | 8.3 (5.5-13) |
8.7 (4.8-12.7) |
0.63 |
N: number, M: median, IQR: interquartile range, SRS: stereotactic radiosurgery, WBRT: whole brain radiotherapy, cm3: cubic centimeter, mo: months. Other: RCC, colorectal, head & neck, genitourinary.
3.2. Local Control and Survival in the Population
Median clinical and radiographic follow-up was 13.2 months (IQR, 7.5-22.6). Of the 411 metastases analyzed, 125 (30%) experienced local failure with a median time to failure of 19.6 (95% CI, 13.1-26.0) months (Table 2). Median time-to-failure from the time of SRS for metastases treated with SRS alone was 12.1 months vs. 22.7 months in those treated with WBRT followed by SRS (p=0.003). One-year local control rates for metastases treated with SRS alone, WBRT then SRS, and SRS then WBRT were 53%, 70%, and 72%, respectively (Figure 1). When stratified by indication for SRS, time to local failure did not differ significantly for metastases treated with SRS initially, for salvage of WBRT local or distant failure, or for unknown indication (Figure 2, p=0.078). Median overall survival was 13.6 months (95% CI 10.4-16.7). Median survival from the time of SRS for patients treated with SRS alone, WBRT followed by SRS, and SRS followed by WBRT was 18.7 (11.9-25.6), 11.3 (8.0-14.7), and 16.2 months (11.9-20.5), respectively (p=0.24).
Table 2.
Clinical Outcomes by Treatment Paradigm
| Total N (%) M (95% CI) |
SRS Alone N (%) M (95% CI) |
WBRT then SRS N (%) M (95% CI) |
SRS then WBRT N (%) M (95% CI) |
p-Value | |
| Total | 411 | 51 | 275 | 85 | -- |
| Local Failure | 125 (30%) | 32 (63%) | 74 (20%) | 19 (22%) | <0.001 |
| Time to Local Failure (mo) | 19.6 (13.1-26) | 12.1 (8.9-15.3) | 22.7 (8.2-37.2) | NC | 0.003 |
| 1 year Local Control | 67% | 53% | 70% | 72% | - |
| Overall Survival (mo) | 13.6 (10.4-16.7) | 18.7 (11.9-25.6) | 11.3 (8.0-14.7) | 16.2 (11.9-20.5) | 0.24 |
| 1 year Overall Survival | 55% | 68% | 46% | 60% | - |
N: number, M: median, CI: confidence interval, SRS: stereotactic radiosurgery, WBRT: whole brain radiotherapy, mo: months, NC: not calculated. Local failure: any local failure of individual metastasis at last follow-up imaging
Figure 1.
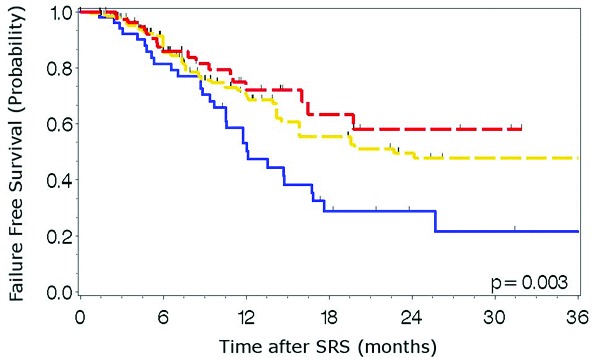
Time to local failure by treatment paradigm. Blue: SRS alone, Yellow: WBRT then SRS, Red: SRS then WBRT
Figure 2.
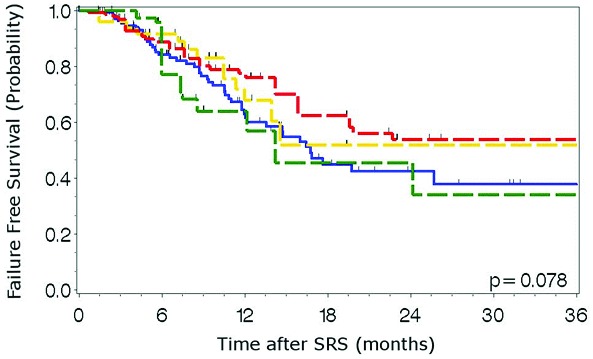
Time to local failure by indication for SRS. Blue: SRS alone, Yellow: WBRT local failure salvage, Red: WBRT distant failure salvage, Green: Other.
Univariate analysis identified other primary histology, WBRT followed by SRS and SRS followed by WBRT treatment patterns, and tumor volume as predictors of local failure after SRS (Table 3). Tumor volume (HR 5.04, 95% CI 3.5-7.3, p<0.001) persisted in the multivariate model as a significant predictor of an increased adjusted hazard (aHR) for in-field failure after SRS. Though melanoma vs. lung primary was associated with an increased likelihood of local failure (HR 4.6, 95% CI 0.92-2.67), this trend was not statistically significant (p=0.095). The use of WBRT in addition to SRS, regardless of timing, was associated with decreased local failure upon univariate analysis, though this did not persist in the multivariate model. In the univariate model for survival, breast vs. lung primary and shorter time between WBRT and SRS were associated with improved overall survival, and breast primary remained a statistically significant factor in the multivariate model (Suppl. Table 1).
Table 3.
Univariate and Multivariate Analysis of Local Failure
| Univariate Analysis | Multivariate Analysis | |||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | 0.998 | 0.981-1.015 | 0.7883 | - | - | - |
| Female v. Male | 0.706 | 0.490-0.490 | 0.0629 | - | - | - |
| Primary Tumor (v. Lung) Breast Melanoma Other |
0.837 1.567 1.441 |
0.555-1.264 0.925-2.654 0.752-2.761 |
0.3986 0.0951 0.0271 |
0.77 4.571 1.148 |
0.508-1.166 0.924-2.672 0.575-2.291 |
0.216 0.095 0.695 |
| Treatment Paradigm (v. SRS alone)<br/>WBRT then SRS<br/>SRS then WBRT | 0.531 0.438 |
0.349-0.807 0.248-0.774 |
0.003 0.0045 |
1.145 0.839 |
0.717-1.829 0.448-1.571 |
0.570 0.583 |
| Tumor Volume (cm3) | 5.039 | 3.491-7.274 | <0.001 | 5.167 | 3.409-7.832 | <0.001 |
| SRS Dose (Gy) | 0.952 | 0.906-1.001 | 0.0566 | - | - | - |
HR: Hazard Ratio, CI: Confidence Interval, SRS: stereotactic radiosurgery, WBRT: whole brain radiotherapy, cm3: cubic centimeter, Gy: Gray, Other: RCC, colorectal, head & neck, genitourinary.
4. Discussion
Local control for SRS treatment of a brain metastasis is influenced by several factors including histology[13], dose[14], and volume of the metastasis[15, 16]. In the current series, there was a strong association between large tumor volumes and SRS local failure (HR 5.04, 95% CI 3.5-7.3, p<0.001). Tumor volume has been shown to be a predictor of local control and overall survival in prior series[15, 16]. The dependence of survival on tumor volume is a complex interaction of how tumor volume affects local control, patient co-morbidities and performance status. The volume of the metastasis limits the effective dose that can safely be delivered to the tumor. For larger tumors in which local failure is of concern, use of combined WBRT with SRS boost[1], two-stage SRS[17], concurrent systemic therapy[7] or combined surgical approaches[18] have been reported as means of optimizing local control.
Supplemental Table 1.
Univariate and Multivariate Analysis for Overall Survival
| Univariate | Multivariate | |||||
| HR | 95% CI | p | HR | 95% CI | p-Value | |
| Age | 1.01 | 0.99-1.02 | 0.57 | - | - | - |
| Gender | 0.91 | 0.63-1.31 | 0.61 | - | - | - |
| Primary (v. Lung) | ||||||
| Breast | 0.58 | 0.37-0.91 | 0.02 | 0.59 | 0.38-0.92 | 0.02 |
| Melanoma | 1.32 | 0.77-2.26 | 0.31 | 1.36 | 0.79-2.36 | 0.27 |
| Other | 0.68 | 0.34-1.37 | 0.28 | 0.72 | 0.35-1.49 | 0.38 |
| Treatment Volume (cm3) | 0.94 | 0.64-1.36 | 0.73 | - | - | - |
| SRS Dose (Gy) | 0.95 | 0.89-1.01 | 0.10 | - | - | - |
| Any use of WBRT | 0.68 | 0.43-1.10 | 0.10 | - | - | - |
| Indication for SRS after WBRT (v. SRS alone) | ||||||
| WBRT LF | 1.12 | 0.67-1.89 | 0.66 | 1.18 | 0.69-1.99 | 0.55 |
| WBRT DF | 1.23 | 0.80-1.90 | 0.34 | 1.24 | 0.79-1.94 | 0.35 |
| Unknown | 0.87 | 0.44-1.70 | 0.68 | 0.97 | 0.49-1.95 | 0.94 |
| Time between WBRT and SRS (mo) | 0.96 | 0.94-0.99 | 0.005 | - | - | - |
HR: Hazard Ratio, CI: Confidence Interval, SRS: Stereotactic Radiosurgery, WBRT: Whole Brain Radiotherapy, cm3: cubic centimeter, Gy: Gray, mo: months, Other: RCC, colorectal, head & neck, genitourinary.
Several prospective studies have evaluated the role of combined SRS and WBRT versus monotherapy. In the RTOG 9508 study assessing the role of SRS boost after WBRT, the SRS boost was generally delivered within a week of completion of WBRT[1]. This was an attempt to avoid tumor repopulation prior to SRS boost. In RTOG 9508, local control was improved over WBRT alone, but there was no survival improvement. Several recent randomized trials that have also shown that there is no survival difference between patients who received combined WBRT and SRS versus SRS alone. Those studies did show that there was an improvement in local control with combined WBRT and SRS versus SRS alone[2, 3, 5]. Our report is complementary to the aforementioned prospective studies because our data show a trend toward improvement in local control even with delayed use of WBRT, suggesting that the improvement in control did not depend on delivering the consolidative radiation at a short time interval. In this study population, we found a significantly increased time to local failure with either combination of WBRT and SRS in comparison to SRS alone, but this did not persist in the multivariate analysis of local control. Given these findings and the equivalent ultimate local control of delayed WBRT, there is a stronger argument for delaying WBRT and its associated toxicities, such as subacute worsening of performance status[4] and a chronic worsening of cognition[3]. Once the chronic toxicities of WBRT develop, they are generally not reversible and tend to worsen in severity over time[19, 20]. These toxicities also must be weighed against the increased risk of distant brain failure in the SRS-alone group.
We also found no difference in local control after SRS in the treatment of WBRT failures (either local or distant) when compared with SRS for radiation-naïve metastases. We hypothesized that recurrent or new metastases after WBRT would have selected for radioresistant clonogens, leading to a propensity for local failure after SRS salvage therapy. In our population, patients who underwent SRS salvage for WBRT local or distant failure experienced an increased local failure-free survival than patients who underwent upfront SRS, though this trend was not statistically significant. This may be due to lead-time bias as patients under surveillance after prior brain radiotherapy may have earlier detection of smaller brain metastases[21].
This study is limited by its retrospective nature and by a potential selection bias in the treatment paradigm utilized. As such, the results of the current analysis are limited to hypothesis generation. In spite of the limitations, there are several useful findings from the analysis. Local control rates of three widely-utilized paradigms for the treatment of brain metastases and common indications for SRS that are frequently encountered in the management of patients with brain metastases were not statistically different. A volumetric assessment showed a strong correlation between tumor volume and local failure.
5. Conclusion
In this single-institution study, we demonstrate a relationship between local control and tumor volume. No significant difference in local control was identified when SRS salvage after WBRT was compared with SRS performed in a radiation-naïve patient.
6. Acknowledgements
The authors wish to acknowledge the support of the NCI Cancer Center Support Grant P30 CA012197, Wake Forest Baptist Comprehensive Cancer Center. The authors also wish to thank Bonny B. Morris, MSPH for her assistance with manuscript review and preparation.
Footnotes
Authors’ disclosure of potential conflicts of interest
The authors reported no conflict of interest.
Author contributions
Conception and design: Ryan T. Hughes, Paul J. Black, Brandi R. Page, John T. Lucas Jr, Shadi A. Qasem, Kounosuke Watabe, Jimmy Ruiz, Adrian W. Laxton, Stephen B. Tatter, Waldemar Debinski, Michael D. Chan.
Data collection: Ryan T. Hughes, Paul J. Black, Brandi R. Page, John T. Lucas Jr, Michael D. Chan.
Data analysis and/or interpretation: Ryan T. Hughes, Paul J. Black, Brandi R. Page, John T. Lucas Jr, Shadi A. Qasem, Kounosuke Watabe, Jimmy Ruiz, Adrian W. Laxton, Stephen B. Tatter, Waldemar Debinski, Michael D. Chan.
Manuscript writing: Ryan T. Hughes, Paul J. Black, Brandi R. Page, John T. Lucas Jr, Shadi A. Qasem, Kounosuke Watabe, Jimmy Ruiz, Adrian W. Laxton, Stephen B. Tatter, Waldemar Debinski, Michael D. Chan.
Final approval of manuscript: Ryan T. Hughes, Paul J. Black, Brandi R. Page, John T. Lucas Jr, Shadi A. Qasem, Kounosuke Watabe, Jimmy Ruiz, Adrian W. Laxton, Stephen B. Tatter, Waldemar Debinski, Michael D. Chan.
References
- 1. Andrews D.W., et al. , Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet, 2004. 363(9422): p. 1665-72. [DOI] [PubMed] [Google Scholar]
- 2. Aoyama H., et al. , Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA, 2006. 295(21): p. 2483-91. [DOI] [PubMed] [Google Scholar]
- 3. Chang E.L., et al. , Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol, 2009. 10(11): p. 1037-44. [DOI] [PubMed] [Google Scholar]
- 4. Soffietti R., et al. , A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol, 2013. 31(1): p. 65-72. [DOI] [PubMed] [Google Scholar]
- 5. Kocher M., et al. , Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol, 2011. 29(2): p. 134-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis T.L., Neal M.T., Chan M.D. The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol, 2011. 2012: p. 952345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson A.G., et al. , Impact of Systemic Targeted Agents on the Clinical Outcomes of Patients with Brain Metastases. Oncotarget, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varlotto J.M., et al. , Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys, 2003. 57(2): p. 452-64. [DOI] [PubMed] [Google Scholar]
- 9. Lucas J., et al. , Competing Risk Analysis of Neurologic vs. Non-Neurologic Death in Patients Undergoing Radiosurgical Salvage Following Whole Brain Radiotherapy Failure (WBRT): Who Actually Dies of Their Brain Metastases? International Journal of Radiation Oncology* Biology* Physics, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucas J., et al. , A Propensity Score Adjusted Analysis of Patients Receiving Up-Front SRS Versus WBRT: Does the Use of Upfront WBRT Really Affect Neurologic Death? International Journal of Radiation Oncology• Biology• Physics, 2014. 90(1): p. S315-S316. [Google Scholar]
- 11. Shaw E., et al. , Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90- 05. International Journal of Radiation Oncology Biology Physics, 2000. 47(2): p. 291-298. [DOI] [PubMed] [Google Scholar]
- 12. Shaw E., et al. , Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys, 1993. 27(5): p. 1231-9. [DOI] [PubMed] [Google Scholar]
- 13. Black P.J., et al. , Factors that Determine Local Control wiht Gamma Knife Radiosurgery: The Role of Primary Histology. Journal of Radiosurgery and SBRT, 2015. [PMC free article] [PubMed] [Google Scholar]
- 14. Shiau C.Y., et al. , Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys, 1997. 37(2): p. 375-83. [DOI] [PubMed] [Google Scholar]
- 15. Garsa A.A., et al. , Predictors of individual tumor local control after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Int J Radiat Oncol Biol Phys, 2014. 90(2): p. 407-13. [DOI] [PubMed] [Google Scholar]
- 16. Baschnagel A.M., et al. , Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery. J Neurosurg, 2013. 119(5): p. 1139-44. [DOI] [PubMed] [Google Scholar]
- 17. Devoid H., et al. , Recent Advances in Radiosurgical Management of Patients for Brain Metastases. Front Biosci, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Jensen C.A., et al. , Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg, 2011. 114(6): p. 1585-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Attia A., et al. , Treatment of radiation-induced cognitive decline. Curr Treat Options Oncol, 2014. 15(4): p. 539-50. [DOI] [PubMed] [Google Scholar]
- 20. Greene-Schloesser D., et al. , Radiation-induced brain injury: A review. Front Oncol, 2012. 2: p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lester S.C., et al. , Clinical and economic outcomes of patients with brain metastases based on symptoms: an argument for routine brain screening of those treated with upfront radiosurgery. Cancer, 2014. 120(3): p. 433-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


