Abstract
Objectives
To review the literature and report our experience with the use of stereotactic body radiation therapy (SBRT) to treat multiple primary lung cancers (MPLCs).
Methods
A retrospective review of 18 patients with 36 separate MPLC lesions (6 synchronous pairs and 12 metachronous pairs) was performed. Of these 18 patients, 16 were not surgical candidates and 2 declined to have surgery. Of the 36 lesions treated, 27 received SBRT, 6 had received prior fractionated RT, and 3 had prior surgical resection. Radiotherapy doses for SBRT ranged from 48 to 56 Gy (Median = 50 Gy) in 4 to 13 fractions (Median = 5 fractions) and treatment plans used 4D-CT simulation scans in all patients.
Results
The median follow-up was 20 months after initial SBRT. We observed local control in 22 of 27 (81.5%) of the lesions treated with SBRT. The actuarial overall survival at 2 years from completion of initial SBRT course was 62%. Metastatic disease occurred in 3 of the 6 deceased patients. Clinically evident pneumonitis was observed in 3 of the 18 pts (17%), which resolved completely with steroid therapy.
Conclusions
SBRT appears to be a safe and effective treatment for MPLC both solely or after prior fractionated RT or surgical resection. SBRT for MPLC is a reasonable treatment option for patients who are not optimal candidates for surgery or who decline surgery.
Keywords: Stereotactic Body Radiation Therapy (SBRT), Multiple Primary Lung Cancer, Radiotherapy, Lung Cancer
1. INTRODUCTION
Lung cancer is the leading cause of cancer mortality in the world [1] and the second cause of overall mortality in the United States. Given that smoking is the greatest risk factor for this disease, it is not uncommon for patients to be diagnosed with multiple synchronous or metachronous lung cancers. The prognosis for patients with non-small cell lung cancer (NSCLC) has been shown to be particularly poor for multiple primary lung cancers (MPLCs) with synchronous or metachronous tumors, and for unilateral or bilateral tumors [2-5]. An estimated 1 to 4% of NSCLC occur as multiple primary lung cancers (MPLCs) [2, 6].
Surgical resection remains the primary modality of treatment for these patients and there is a substantial body of literature supporting the efficacy of this approach [7-11]. Unfortunately, not all patients are deemed surgical candidates secondary to underlying comorbidities or limited performance status. In addition, surgical resection can be particularly challenging for multiple lung cancers due to concerns for limited pulmonary reserve. Also, there are some patients who decline surgery despite being operative candidates.
The successful treatment of these patients presents significant challenges for their treating physicians. One possible intervention is stereotactic body radiotherapy (SBRT). Stereotactic body radiotherapy is an emerging and rapidly-evolving new technology for treatment of lung tumors. SBRT is regarded as an appropriate method of definitive treatment of inoperable early stage lung cancer with an excellent toxicity profile and local control rate [12, 13]. SBRT can also be utilized to treat patients with previous lung resection when further surgical treatment is not an option due to limited pulmonary reserve. Also, SBRT has shown favorable results in MPLC for patients with prior thoracic radiotherapy[14]. Previously, we have reported our own institutional experience using modern techniques[15, 16] including stereotactic body radiotherapy [17] in early stage lung cancer demonstrating good local control and favorable toxicity profile.
Although there is limited published data regarding this approach, SBRT appears to be a viable modality of therapy for inoperable multiple primary lung cancers. Sinha & McGarry [18] reported no disease progression in 8 of 10 cases with MPLCs treated with SBRT with minimal pulmonary toxicity (≤ RTOG grade 2).
We report a series of 18 patients from our institution treated with our most current SBRT techniques in this study. We also review the literature supporting multidisciplinary approaches to treatment of multiple primary lung cancers.
2. MATERIALS & METHODS
This research was conducted with approval by the Institutional Review Board (IRB) of the University of California San Diego. From February of 2007 to August of 2011 we reviewed the records of patients treated with SBRT for early stage NSCLC. We identified 18 patients who were treated for multiple primary lung cancers [Table I]. Of the 18 patients, 2 declined to have surgery and the remaining 16 were deemed poor surgical candidates due to comorbid medical conditions and/or poor pulmonary reserve. The lesions were synchronous for 6 of the patients and metachronous for the remaining 12 patients.
Table 1.
Patient Characteristics
| Patient Data | |
|---|---|
| Age, mean | 74.6 years |
| Gender (n =18) | Male 56% |
| Female 44% | |
| Smoking history (n =18) | 89% smokers |
| Reason for not having surgery (n =18) | Not a candidate 89% |
| Refused surgery 11% | |
| Timing of diagnosis of MPLC (n =18) | Metachronous 67% |
| Synchronous 33% | |
| Modality of treatment, by lesion (n = 36) | SBRT 75% |
| Prior fractionated RT 17% | |
| Prior surgical resection 8% | |
| Size of lesions treated with SBRT, mean (n = 27) | 2.7 cm |
| Stage, lesions treated with SBRT (n = 27) | T1aN0M0, IA 48% |
| T1bN0M0, IA 15% | |
| T2aN0M0, IB 26% | |
| T2bN0M0, IIA 7% | |
| T3aN0M0, IIB 4% | |
| Histology, lesions treated with SBRT (n=27) | Squamous carcinoma 19% |
| Adenocarcinoma 15% | |
| NSCLC NOS 11% | |
| Biopsy non-diagnostic 7% | |
| Biopsy not performed 48% | |
| Tumor location, lesions treated with SBRT (n=27) | Peripheral 74% |
| Central 26% |
Of the 27 lesions treated with SBRT, 12 of them were biopsy-proven NSCLC. All lesions treated were found to be hypermetabolic on PET CT scan. All of the patients had at least one lesion found to be lung cancer on pathologic examination, except two patients in whom biopsy was non-diagnostic and one patient who could not tolerate a biopsy. Tumors without pathologic confirmation of NSCLC were clinically diagnosed as primary lung cancer based on radiographic and clinical evidence when the risks of additional biopsy were felt to be greater than the clinical benefit of obtaining additional biopsy specimens. Martini & Melamed [2] established a criteria for diagnosing multiple primary lung cancers, however this criteria was derived from a surgical series where full pathologic specimen analysis was possible. These criteria could not be applied to many of the patients in our population where repeated biopsy was precluded by poor pulmonary function and deemed clinically to have more risk than benefit. For this scenario in the present series the diagnosis of MPLC was based on clinical information, imaging patterns and timing, as well as multidisciplinary tumor board consensus.
A description of the methods for SBRT at our institution has been published previously [17]. Briefly, CT simulations were obtained and a 4D-CT was created for treatment planning purposes. Varian (Palo Alto, CA) treatment planning software and linear accelerators were used in treatment. The ITV was contoured on the image set. Lesions in the upper one-third of the lungs were expanded 5mm uniformly to generate a PTV. Lesions in the lower two-thirds of the lungs were expanded 5–10 mm axially and 10mm in the superior-inferior direction to generate a PTV. Plans were created with IMRT in 4-7 coplanar beams, and also using RapidArc (Varian, Palo Alto, CA) treatment delivery. The range of doses prescribed was 48 – 56 Gy (Median = 50 Gy) in 4-13 fractions (Median = 5 fractions) [Figure 1]. The majority of patients were treated with 4 or 5 fractions, although in some patients the treatment was delivered by the same methodology in larger fraction numbers than a strict definition of SBRT. In general plans were normalized such that 100% of the PTV received at least 100% of the dose. Maximum point doses were below 110% and within the PTV. Setup was performed by skin marking alignment with lasers, orthogonal films, and daily cone-beam CT images.
Figure 1.
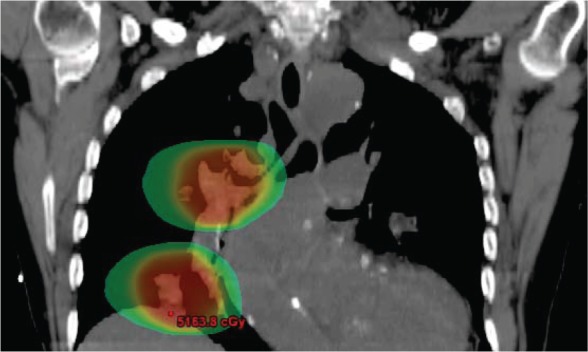
A treatment plan for a patient with multiple primary lung cancers treated with stereotactic body radiotherapy. Dose is shown in color wash with a 20 Gy threshold level.
After completion of treatment, a review of each patient’s medical record was performed to assess for local control, disease progression, treatment toxicity, and overall survival. Local control failure was defined as any discussion of disease progression in any provider’s clinical notes, any mention of disease progression in any radiologic report, or as progressive lesion expansion on CT or PET scan without other clinical explanation. Treatment toxicity was assessed by reviewing all clinical notes for discussion of toxicity or new symptoms during or after treatment.
3. RESULTS
Outcomes are summarized in Table II for the 36 lesions treated in these 18 patients. The lesions treated with SBRT ranged from 0.9 to 7.2cm (mean = 2.7cm) in greatest dimension. The lesions treated with SBRT were central in 7 cases and peripheral in 20 cases. All patients completed SBRT as prescribed. Six patients had synchronous MPLC lesions diagnosed. Treatment with SBRT was administered as sequential courses in 3 of these patients and as a simultaneous course in 3 patients. The median follow-up time from initial SBRT was 20 months. At the time of this analysis 12 of the patients were still living and 6 had died. Among those that died, 3 of the 6 cases had progressive metastatic disease after SBRT. The actuarial 2-year overall survival was 62%. Of the 27 lesions treated with SBRT, 22 (81%) showed local control at last follow-up.
Table 2.
Outcomes
| Patient Data | |
|---|---|
| Follow-up time from initial SBRT, median | 20 months |
| Total radiation dose, median (range) (n=27) | 50 Gy (48-56Gy) |
| Number of SBRT fractions, median (range) (n=27) | 5 fractions (4-13 fractions) |
| Crude local control of lesions treated by SBRT (n=27) | 81% |
| Actuarial 2-year overall survival | 62% |
| Development of distant metastases, by patient (n =18) | 28% |
| Acute toxicity, grade 2 or more (n = 18) | 0% |
| Late toxicity, grade 2 or more (n =18) | 17% (all pneumonitis) |
There was no significant acute toxicity. Three of the patients experienced clinical radiation pneumonitis as late toxicity after completion of RT; at 3, 4 and 8 months after SBRT. All developed cough and there was evidence of pneumonitis on chest CT. Two of these three patients had pairs of central lesions treated by SBRT and required a short course of steroid medication. All of the patients had complete resolution of symptoms from radiation pneumonitis.
4. DISCUSSION
The proportion of patients with multiple primary lung cancers is relatively small among all cases of NSCLC, but this diagnosis may be increasing in frequency with more sensitive imaging like PET CT scans and early detection with CT imaging. Results from the National Lung Cancer Screening Trial (NLST) also suggest a benefit of low dose CT screening in a high risk population[19], which in turn may lead to increased diagnosis of MPLCs. Beyreuther [20] first described the occurrence of multiple lung tumors in 1924. Since then, several series have been published describing therapeutic experiences in treating MPLCs, primarily by surgery [7-11].
A challenging clinical question is the differentiation between multiple primary lung cancers versus a solitary lung cancer with intraparenchymal metastasis. The criteria by Martini and Melamed [2] are the most widely used published criteria for making this distinction. These criteria was based on surgical specimens and autopsy results where full pathologic evaluation was available. Histopathologic examination of tissue along with immunohistochemistry analysis has led to increased accuracy in differentiating MPLCs from intraparenchymal metastases [21-25], and certainly metastatic disease from non-lung primary cancers.
However, the histologic comparison of multiple lesions pre-operatively is not always clinically attainable in the pre-treatment diagnostic setting due to patients’ intolerance of procedures and the diagnostic yield of biopsy. In fact, the very reason many patients are considered for primary SBRT of lung cancer rather than surgery is due to poor pulmonary function and the risk of procedures such as a biopsy or resection. Imaging patterns particularly by PET CT scan are useful additional data. The majority of patients evaluated for radiotherapy in this situation are medically inoperable and also may not be suitable for biopsy of multiple lesions. Therefore, in this patient population it is not always possible to pathologically confirm the diagnosis of NSCLC in all visible lesions due to the risks involved. As such, the diagnosis of MPLCs must often be based on clinical and imaging criteria alone, as was the case with many of the patients in our cohort.
The use of combined clinical, radiographic, pathologic and molecular/genetic characterization can improve accuracy of differentiation between MPLCs, intraparenchymal lung metastases, or non-lung metastatic disease. As an example, consider a case of two small peripheral unilateral or bilateral lesions with no radiologic evidence of nodal disease in the mediastinum or any extra-thoracic disease. With corroborating PET-CT findings and no present or past evidence of any other primary cancer, these findings are assumed to be consistent with synchronous lung primary cancers. We advocate for obtaining biopsy confirmation in this situation whenever clinically feasible.
Although surgical resection remains the mainstay of treatment for these patients, a substantial proportion of patients are not suitable for surgical procedures such as pneumonectomy or bilobectomy due to associated medical comorbidities and poor lung reserve. Stereotactic body radiotherapy has been successfully and safely used for early stage non-small cell lung cancer, but there is minimal experience using this emerging technology for multiple primary lung lesions. In general there is a paucity of literature on the use of radiotherapy for multiple primary lung cancers, particularly stereotactic body radiotherapy.
Several patients in the present series had SBRT for a second primary NSCLC after a previous surgically-resected NSCLC. These patients were felt to be poor candidates for additional surgery due to limited pulmonary reserve. Additionally, several patients in this series had received previous fractionated RT for NSCLC or SCLC prior to SBRT for a second primary NSCLC. In both of the above scenarios SBRT appears to be an effective option with acceptable risk for treatment of early stage second primary MPLC lesions.
Sinha & McGarry [18] reported ten patients treated with SBRT for MPLC. All patients were found to have independent malignant pulmonary nodules at initial workup or during the course of their disease. Every patient had biopsy proven non-small-cell lung carcinoma (primarily in one of the lesions), and all were poor candidates for surgery. The patients underwent SBRT with radiation doses were between 4800-6600cGy in 3 to 4 fractions, mostly sequentially. With a median follow-up of 21 months there was 80% progression free survival and no grade 3 or higher toxicity. Another study by Kelly et al., [14] described SBRT for lung cancer in patients who had previously fractionated thoracic radiotherapy. Grade 3 radiation pneumonitis occurred in 28% of the cases. The local control was 92% in this series and the 2 year actuarial survival was 59%. These previous series demonstrate that SBRT is a safe and potentially effective treatment option for patients with MPLCs. The results of our present data closely parallel the outcomes of the report by Sinha & McGarry [18] and Kelly et al. [14].
Interestingly, two of the three patients who developed pneumonitis in the present study were treated with SBRT for pairs of central lesions. It’s possible that SBRT of central lesions increases the risk of radiation pneumonitis, although this has not been a significant problem in other series of hypofractionated RT [26]. Milano et al. [26] reviewed the role of hypofractionated SBRT in fifty-three cases of central thoracic cancers. Stereotactic body radiotherapy was found to have good local control and safety for multiple lung lesions in the setting of oligometastatic disease. A subset of these patients were treated with multiple courses of SBRT for new or recurrent lesions. Of the 33 patients with oligometastatic disease, 2-year survival rate was 50%. Importantly, the patients who received the hypofractionated SBRT showed minimal lung toxicity, with no grade 3-5 pneumonitis seen, and 4 patients suffering ≤ grade 2 pneumonitis.
SBRT for multiple primary lung cancers appears to be a viable treatment for patients who are not operative candidates or who refuse surgery. It is generally a safe and effective treatment in this scenario based on the present data and prior reports. In our series with a median follow-up after initial SBRT of 20 months, the actuarial overall survival rate at 2 years was 62% and we observed a local control in 81% of lesions treated. SBRT was relatively well tolerated in this cohort with no acute toxicity and a 17% late toxicity rate of temporary pneumonitis. Further large, randomized prospective trials would be beneficial to authenticate results presented here and elsewhere, which could help refine treatment recommendations for patients with MPLCs.
5. ACKNOWLEDGEMENTS
There were no conflicts of interest, financial or otherwise. There was no financial sponsorship for this study.
REFERENCES
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74-108. [DOI] [PubMed] [Google Scholar]
- 2. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70: 606-612. [PubMed] [Google Scholar]
- 3. Ferguson MK. Synchronous primary lung cancers. Chest 1993; 103: 398S-400S. [DOI] [PubMed] [Google Scholar]
- 4. Antakli T, Schaefer RF, Rutherford JE, Read RC. Second primary lung cancer. Ann Thorac Surg 1995; 59: 863-866. [DOI] [PubMed] [Google Scholar]
- 5. Trousse D, Barlesi F, Loundou A, Tasei AM, Doddoli C, Giudicelli R, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007; 133: 1193-1200. [DOI] [PubMed] [Google Scholar]
- 6. Verhagen AF, Tavilla G, van de Wal HJ, Cox AL, Lacquet LK. Multiple primary lung cancers. Thorac Cardiovasc Surg 1994; 42: 40-44. [DOI] [PubMed] [Google Scholar]
- 7. Finley DJ, Yoshizawa A, Travis W, Zhou Q, Seshan VE, Bains MS, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010; 5: 197-205. [DOI] [PubMed] [Google Scholar]
- 8. Adebonojo SA, Moritz DM, Danby CA. The results of modern surgical therapy for multiple primary lung cancers. Chest 1997; 112: 693-701. [DOI] [PubMed] [Google Scholar]
- 9. Carretta A, Ciriaco P, Melloni G, Bandiera A, Libretti L, Puglisi A, et al. Surgical treatment of multiple primary adenocarcinomas of the lung. Thorac Cardiovasc Surg 2009; 57: 30-34. [DOI] [PubMed] [Google Scholar]
- 10. Chang YL, CT Wu, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007; 134: 630-637. [DOI] [PubMed] [Google Scholar]
- 11. Rea F, Zuin A, Callegaro D, Bortolotti L, Guanella G, Sartori F. Surgical results for multiple primary lung cancers. Eur J Cardiothorac Surg 2001; 20: 489-495. [DOI] [PubMed] [Google Scholar]
- 12. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004; 101: 1623-1631. [DOI] [PubMed] [Google Scholar]
- 13. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly P, Balter PA, Rebueno N, Sharp HJ, Liao Z, Komaki R, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys 2010; 78: 1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad E, Sandhu AP, Fuster MM, Messer K, M Pu, Nobiensky P, et al. Hypofractionated radiotherapy as definitive treatment of stage I non-small cell lung cancer in older patients. Am J Clin Oncol 2011; 34: 254-258. [DOI] [PubMed] [Google Scholar]
- 16. Sandhu AP, Messer K, Fuster MM, Ahmad E, M Pu, Bazhenova L, et al. Definitive radiation therapy for stage I non-small-cell lung carcinoma: institutional experience with contemporary conformal planning. Clin Lung Cancer 2009; 10: 433-437. [DOI] [PubMed] [Google Scholar]
- 17. Nath SK, Sandhu AP, Kim D, Bharne A, Nobiensky PD, Lawson JD, et al. Locoregional and distant failure following imageguided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol 2011; 99: 12-17. [DOI] [PubMed] [Google Scholar]
- 18. Sinha B, McGarry RC. Stereotactic body radiotherapy for bilateral primary lung cancers: the Indiana University experience. Int J Radiat Oncol Biol Phys 2006; 66: 1120-1124. [DOI] [PubMed] [Google Scholar]
- 19. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beyreuther H. Multiplicitat von Carcinomen bei einem Fall von sog: “Schneeberger” Lungenkrebs mit tuberkulose. Virchows Arch 1924; 250: 230-243. [Google Scholar]
- 21. Girard N, Deshpande C, Azzoli CG, Rusch VW, Travis WD, Ladanyi M, et al. Use of epidermal growth factor receptor/ Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest 2010; 137: 46-52. [DOI] [PubMed] [Google Scholar]
- 22. Girard N, Deshpande C, Lau C, Finley D, Rusch V, Pao W, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009; 33: 1752-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girard N, Ostrovnaya I, Lau C, Park B, Ladanyi M, Finley D, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res 2009; 15: 5184-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nonami Y, Ohtuki Y, Sasaguri S. Study of the diagnostic difference between the clinical diagnostic criteria and results of immunohistochemical staining of multiple primary lung cancers. J Cardiovasc Surg (Torino) 2003; 44: 661-665. [PubMed] [Google Scholar]
- 25. van Rens MT, Eijken EJ, Elbers JR, Lammers JW, Tilanus MG, Slootweg PJ. p53 mutation analysis for definite diagnosis of multiple primary lung carcinoma. Cancer 2002; 94: 188-196. [DOI] [PubMed] [Google Scholar]
- 26. Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009; 91: 301-306. [DOI] [PubMed] [Google Scholar]


