Abstract
Purpose
Although Gamma Knife radiosurgery (GKR) is widely recognized as an effective and minimally invasive treatment for intractable trigeminal neuralgia, its role in glossopharyngeal neuralgia (GPN) has not yet been determined.
Methods
Between January 2002 and February 2009, 7 patients with medically intractable GPN were treated using GKR. Indication for GKR was the presence of medically intractable GPN, patient’s refusal for open surgery or contraindication to microvascular decompression. Patients underwent preoperative investigation and were evaluated postoperatively with periodic assessment of pain relief and neurological function. Seven patients, 5 males and 2 females, with mean age 62 (range 36-83) presented with symptoms for an average of 28 months (range 8-72). Four patients had a neurovascular conflict. Patients were treated with a dose ranging from 60 to 80 Gy, targeted on the cisternal segment (n=2) or glossopharyngeal meatus (GPM) (n=5).
Results
Outcome was favorable with cure of GPN in 5 of 7 patients (71%) in the short-term (3 months post GKR) and 4 of 7 (57%) patients in the long term (> 7 months, mean 16 months). One patient required 2 treatments because of a recurrence of symptoms and was treated with a maximum doses of 60 and 70 Gy, respectively. There were no neurological complications.
Conclusions
All patients with GPM as a target that received a dose greater than 75 Gy were cured at long-term follow-up. The 2 patients with cisternal segment as the target and received a dose lower than 70 Gy were not cured of their GPN. There were no neurological deficits involving the lower cranial nerves. It will be necessary to investigate the optimal radiation dose and target of GKR for GPN in order to achieve long-term pain relief.
Keywords: Gamma Knife radiosurgery, Glossopharyngeal meatus, Glossopharyngeal neuralgia
INTRODUCTION
Glossopharyngeal neuralgia (GPN), a rare disorder, is characterized by a severe and transient stabbing pain. Commonly triggered by swallowing, talking or coughing, the pain typically emanates from the root of the tongue and pharynx then radiates to the throat and/or deep ear structures.6 It often remits and relapses much like trigeminal neuralgia (TN)6 and GPN has been associated with cardiac arrest, hypotension, syncope and convulsions in rare cases.1,3,36 The annual incidence of GPN is approximately 0.7 to 0.8 per 100,000 people per year with relative frequency of 0.2 to 16.9% compared to TN.9,10,16 Two-thirds of the patients are female with the mean age at presentation of 50 years, and patients often presenting to a neurosurgeon after an average of 6 years of pain.5 The treatment strategy for GPN is similar to that of TN with the first-line medical treatment being carbamazepine with other anti-epileptic drugs such as phenytoin, oxcarbazepine, gabapentin and amitriptyline being also effective.2,4,18,27
With insufficient or no response to the medical treatment due to resistance to anticonvulsants and/or significant side effects with medications, surgical intervention can provide the cure, with microvascular decompression (MVD) being the first option since vascular compression is the main cause of the neuralgia. Various destructive procedures such as rhizotomy delivered successful pain relief at the cost of postoperative symptoms due to sacrificed nerves.7,28,29,32,33,35 However, since the first favorable results of MVD for GPN reported by Laha and Janetta in 1977,13 many investigators have confirmed the efficacy of the nondestructive surgical procedure for the treatment of drug-resistant GPN.21, 25, 30
Although uncommon but never negligible, significant morbidity or death can follow microsurgical procedures even in the modern microsurgical era. For the treatment of TN, a non-invasive alternative to MVD is Gamma Knife radiosurgery (GKR), which has become more established in the last two decades.11,15,23,24,31,37 With the GPN pathophysiology being similar to that of TN, we hypothesized that GKR could also be used to treat GPN, especially in cases where the disease is medically refractory or where the patient is refusing MVD.
Since the first case of GPN treated by GKR reported by Stieber in 2005,34 we have published two other cases of GPN treated by GKR.39 We now report a clinical series with additional cases describing our experience in Marseille.
METHODS
The Gamma Knife Unit of the Timone University Hospital in Marseille, France is a tertiary referral center for GKR. The patients in this study were referred for evaluation and subsequent treatment of GPN. Indication for GKR was the presence of medically intractable GPN, patient’s refusal for open surgery or contraindication to microvascular decompression. Patients selected for GKR were presented with detailed information concerning not only GPN but also other surgical options, including the expected risks and benefits for each treatment. Informed consent was obtained from all patients for GKR. Following the GKR, patients were evaluated postoperatively with periodic assessment of pain relief and neurological function.
Between January 2002 and February 2009, total of 7 patients (5 males and 2 females) with mean age of 62 (range 36-83) and with symptoms of medically intractable GPN were treated using GKR at the Timone University Hospital Gamma Knife Unit. All patients had normal neurological examinations and suffered from deafferentation pain. In all patients, medical therapy had been attempted and had proven unsuccessful. From the time of the failure of the medical treatment for the GPN to GKR ranged from 8 to 72 months with the mean interval being 28 months. Four patients had a neurovascular conflict. One patient had GPN secondary to a surgical procedure for laryngeal adenocarcinoma. None of the patients had undergone any previous surgical intervention for pain. Patient characteristics are summarized in Table 1.
Table 1.
Demographic, clinical, surgical and outcome data for patients with glossopharyngeal neuralgia treated with Gamma Knife radiosurgery
| Patient | Age | Sex | NV conflict | Target | Dose (Gy) | Total follow-up (months) | Result at 3 months | Result at last follow-up | Subsequent Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | F | Yes | GPM | 60 | 7 | Class I | Class V | Repeat GKR |
| GPM | 70 | 10 | Class I | Class V | TC twice | ||||
| 2 | 62 | M | No | Cistern | 70 | 24 | Class IV | Class V | CS |
| 3 | 66 | M | Yes | Cistern | 70 | 24 | Class IV | Class V | MVD |
| 4 | 49 | M | No | GPM | 75 | 32 | Class I | Class I | None |
| 5 | 71 | M | Yes | GPM | 80 | 13 | Class I | Class II | None |
| 6 | 36 | F | No | GPM | 80 | 10 | Class I | Class I | None |
| 7 | 65 | M | Yes | GPM | 80 | 8 | Class I | Class II | None |
Cistern: Cisternal segment of the glossopharyngeal nerve, Class I: Pain-free without medication, Class II: Pain-free with medication, Class IV: Pain frequency reduction between 50 to 90%, Class V: No significant reduction in pain frequency, CS: Cortical stimulator, F: Female, GKR: Gamma Knife radiosurgery, GPM: Distal part of the glossopharyngeal nerve at the glossopharyngeal meatus of the jugular foramen, Gy: Gray, M: Male, MVD: Microvascular decompression, NV: Neurovascular, TC: Thermocoagulation.
GKR was performed while the patient was under local anesthesia supplemented with inhalation of analgesic agents. Leksell stereotactic frame (Elekta Instruments AB, Sweden) was fixed to the patient’s head and the patient subsequently underwent stereotactic enhanced magnetic resonance imaging (MRI) and computed tomography (CT). For the MRI, three-dimensional (3D) constructive interference in steady-state (CISS) imaging without gadolinium and 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE) with gadolinium were routinely used.
Anatomical landmarks and the relationship between vascular structures and lower cranial nerves were visualized on Gamma-Plan (Elekta Instruments AB, Sweden), a dedicated treatment planning software, to evaluate whether there was evidence of neurovascular compromise or compression. Either the cisternal part or the distal part of the glossopharyngeal nerve at the level of the glossopharyngeal meatus (GPM) of the jugular foramen were chosen as a radiosurgical target, irrespective of the presence of neurovascular compression, to minimize the radiation dose to the brainstem and vagus nerve.19,20,26 Dose planning was performed in both the axial and coronal planes by using Gamma-Plan (Fig. 1), and the target was irradiated using Gamma Knife 4C Model (Elekta Instruments AB, Sweden) with a dose ranging from 60 to 80 Gy, targeted on the cisternal segment (n=2) or GPM (n=5) with a single 4-mm collimator.
Figure 1.
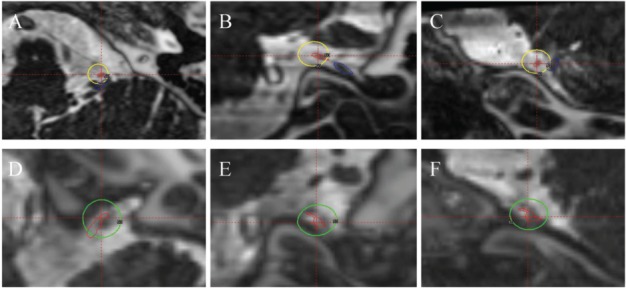
MRI CISS images fused with CT from the Gamma-Plan (Elekta Instruments AB, Sweden). A, B and C) Axial, coronal and sagittal images (respectively) of the patient treated with cisternal target. The yellow outline indicates the 50% isodose line of 70 Gy dose delivered with a single 4-mm collimator. The red outlines the glossopharyngeal nerve and the blue outlines the vagus nerve. D, E and F) Axial, coronal and sagittal images (respectively) of the patient treated with glossopharyngeal meatus target. The green outline indicates the 50% isodose line of 80 Gy dose delivered with a single 4-mm collimator. The red outlines the glossopharyngeal nerve. Here the vagus is not seen due to its distance away from the glossopharyngeal nerve.
Patients were discharged the day following the GKR and followed-up every 3 months for the first year and every 6 months thereafter with imaging and the assessment of pain. The extent of the pain relief was categorized using the following classification: Class I, pain-free without medication; Class II, pain-free with medication; Class III, pain frequency reduction greater than 90%; Class IV, pain frequency reduction between 50 to 90%; Class V, no significant reduction in pain frequency; and Class VI, pain worsening.
RESULTS
The follow-up period ranged from 7 to 32 months (mean 16 months). One patient had longer than 12 months of follow-up and 3 had longer than 24 months of follow-up. The initial pain relief at 3-month follow-up after GKR was Class I in 6 treatments for 5 patients (GPM target), which included the second treatment for Patient 1, and Class IV in two treatments (cisternal target). At the last follow-up examination, 2 patients were Class I, two were Class II, and the 3 patients (4 treatments) were Class V. All patients with GPM as the target had good initial response to GKR with Class I at the first 3-months follow-up. All the patients treated with a dose ≥ 75 Gy with GPM as the target had a good outcome of pain relief (Class I and II) and all the patients with a dose < 75 Gy had a recurrence requiring further intervention. Table 1 summarizes the clinical, surgical and outcome data for the patients.
For the 3 patients with a poor pain relief (Class V), including the one patient (Table 1, Patient 1) who underwent a second GKR treatment 7 months after the first treatment because of a recurrence of symptoms, underwent subsequent treatment to address their symptoms. Patient 1 ultimately benefited from two treatments of thermocoagulation through the jugular foramen. One patient underwent MVD and the other underwent cortical stimulation.
We did not observe any complications after GKR in these patients. All patients were without clinical signs of the motor impairment (hoarseness, dysphagia, or shoulder muscle weakness) or worsening of sensory functions of the glossopharyngeal nerve. No signal abnormalities were demonstrated in the brainstem on the follow-up MRIs after GKR in any of the patients.
DISCUSSION
Compared to TN, GPN is a relatively uncommon one of the craniofacial pain syndromes. The treatment strategy for GPN is broadly similar to TN with firstline treatment being pharmacological medical treatment. In cases that are refractory to medical treatment, surgical intervention is indicated. MVD is widely accepted as a standard procedure for cranial neuralgia, including GPN, because it is nondestructive and corrects the underlying cause.21,25,30,35 However, because GPN typically occurs in the elderly population, there are potentially significant risks related to surgery or anesthesia8,25,38 along with other medical comorbidities. In the early 1950’s, Dr. Leksell used GKR for TN,14 but it was not until the 1990s, in conjunction with the advancements in neuroimaging modalities such as MRI, that the technique became widely used. The efficacy of GKR for TN as reported in the literature is comparable to that of MVD and the interval between GKR and pain relief in GPN appears to be shorter than TN.24,37
Dose escalation in GKR for GPN
In order to minimize the morbidity involving the brainstem and the vagus nerve, the initial treatment dose and its subsequent incremental increase was selected cautiously. Because Kondziolka et al. reported a 10% rate of nerve dysfunction after applying 80 Gy,12 we therefore elected to deliver a maximum dose of 60 Gy for our first patient using a single shot with a 4-mm collimator. The doses used in this study (60, 70, 75, 80 Gy) are lower compared to those that we use in TN (80, 85 and 90 Gy).24
In our series, the initial response to the lower dose GKR (60 and 70 Gy) to the GPM target was Class I but deteriorated to Class IV requiring further intervention. The initial response to the lower dose GKR (70 Gy) to the cisternal target was Class IV which deteriorated to Class V, also requiring further intervention. Four patients who received ≥75 Gy dose to GPM target all had a initial response of Class I, of which two maintained the same pain relief level and two other had slight decrease to Class II yet did not require further intervention. Stieber and colleagues reported on a GPN patient treated with 80 Gy GKR with satisfactory results, pain slightly relapsing at 6 month but requiring no further treatment.34 This result, combined with our observation, may imply that the dose for achieving effective pain relief in GPN should be ≥75 Gy, which is similar to the dose used in GKR for TN where the maximum dose is a major predictive factor of a successful treatment.12,17,22,24
Technical nuances in GKS for GPN
For the first patient, the distal end of the nerve was targeted at the level of the glossopharyngeal meatus of the jugular foramen (GPM) in order to ensure the accuracy of radiosurgical targeting and to minimize the radiation dose to the brainstem and vagus nerve. 19,20,26 However, the long-term result was poor (Class V) prompting us to change the target to the glossopharyngeal root entry zone (REZ) in the cisternal part of the glossopharyngeal nerve. This decision was based on the fact that the myelin, produced by oligodendrocytes and Schwann cells, surrounding the axons ending at the REZ can be more radiosensitive than myelin, engendered exclusively by Schwann cells, that surrounds the trigeminal root more distally.1,9,13 This new target was difficult to identify because the glossopharyngeal nerve is more difficult to visualize clearly than the trigeminal nerve, even with their low intensity lines on CISS images, and the glossopharyngeal nerve could be difficult to distinguish from the vagus nerve.
The 2 patients we have treated with the cisternal target had poor response with the initial pain relief of Class IV, later deteriorating to Class V. Therefore, we decided to return to the first target, GPM, but with increase of dose to 75 Gy (patient 4) and 80 Gy (patient 5,6 and 7). Targeting the nerve root complex at its entry into the osseous canal of the jugular foramen (GPM) has three advantages over the previous cisternal target. First, the opening of the jugular foramen is a good landmark wellseen on the CT images. Secondly, where the nerve root complex enters into the jugular foramen through the uppermost opening (pars nervosa), a fibrous crest separates the vagus and the accessory nerves, which permits better distinction between the two nerves. Thirdly, the relative distance away from the brainstem permits the increase of the dose delivered.
With return to the GPM as the target along with increased dose (75 and 80 Gy) to the nerve permitted by the relative distance from the brainstem, the initial pain relief was Class I in all 4 patients. This level of pain relief continued for two patients and the two other had only slight deterioration to Class II, with none of the 4 patients requiring further treatment at last follow-up (8 to 32 months). The possibility of a placebo effect must be considered in these cases because this is a nonblinded study. However, the intensity and disabling nature of the pain experienced by these patients would make this hypothesis less favorable.
Currently, GKR appears to be a safe treatment modality for medically refractory GPN. Of course, MVD remains the first-line surgical treatment for GPN. However, for patients not suitable for MVD, i.e., elderly or medically at risk for an open procedure, the only alternatives are percutaneous procedure through the jugular foramen7,29 or GKR. The former alternative carries a high morbidity of neurological deficit since the destructive effect on the nerve is the cost of effective pain relief.28,33,35 The latter alternative, taking into account the experience with TN,24 has the potential to provide effective pain relief without cranial nerve injury. In spite of the small number of patients in our series, this report contributes to the understanding of the potential role of GKR in this rare disease by providing relevant information about radiation dose and painfree probability.
CONCLUSIONS
Our experience suggests there are advantages to GKR and warrants further investigation of its safety and efficacy. Although it is difficult to draw definitive conclusions due to the small number of patients and the limited follow-up period in this report, GKR would appear to be a viable alternative treatment for GPN with a low risk of neurological morbidity. The identification of the optimal GKR dose is a significant issue and a prospective study of a large number of patients with a longer follow-up duration is mandatory before the role of GKR for typical GPN can be determined.
DISCLOSURES
Jean Régis, M.D., has received support from Elekta Instrument AB for nonstudy–related clinical or research effort.
REFERENCES
- 1. Barbash GI, Keren G, Korczyn AD, Sharpless NS, Chayen M, Copperman Y, et al. : Mechanisms of syncope in glossopharyngeal neuralgia. Electroencephalogr Clin Neurophysiol 63: 231–235, 198. [DOI] [PubMed] [Google Scholar]
- 2. Dalessio DJ: Diagnosis and treatment of cranial neuralgias. Med Clin North Am 75: 605–615, 199. [DOI] [PubMed] [Google Scholar]
- 3. Ferrante L, Artico M, Nardacci B, Fraioli B, Cosentino F, Fortuna A: Glossopharyngeal neuralgia with cardiac syncope. Neurosurgery 36: 58–63, 199. [DOI] [PubMed] [Google Scholar]
- 4. Fromm GH: Clinical pharmacology of drugs used to treat head and face pain. Neurol Clin 8: 143–151, 199. [PubMed] [Google Scholar]
- 5. Giorgi C, Broggi G: Surgical treatment of glossopharyngeal neuralgia and pain from cancer of the nasopharynx. A 20-year experience. J Neurosurg 61: 952–955, 198. [DOI] [PubMed] [Google Scholar]
- 6. Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia; 24: 1 Suppl 9–160, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Isamat F, Ferran E, Acebes JJ: Selective percutaneous thermocoagulation rhizotomy in essential glossopharyngeal neuralgia. J Neurosurg 55: 575–580, 198. [DOI] [PubMed] [Google Scholar]
- 8. Kalkanis SN, Eskandar EN, Carter BS, Barker FG, II: Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery 52: 1251–1252, 200. [DOI] [PubMed] [Google Scholar]
- 9. Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT: Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945–1984. Neuroepidemiology 10: 276–281, 199. [DOI] [PubMed] [Google Scholar]
- 10. Katusic S, Williams DB, Beard CM, Bergstralh E, Kurland LT: Incidence and clinical features of glossopharyngeal neuralgia, Rochester, Minnesota, 1945–1984. Neuroepidemiology 10: 266–275, 199. [DOI] [PubMed] [Google Scholar]
- 11. Kondziolka D, Lunsford LD, Flickinger JC: Gamma knife radiosurgery as the first surgery for trigeminal neuralgia. Stereotact Funct Neurosurg 70: 1 Suppl 187–191, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Kondziolka D, Lunsford LD, Flickinger JC, Young RF, Vermeulen S, Duma CM, et al. : Stereotactic radiosurgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg 84: 940–945, 199. [DOI] [PubMed] [Google Scholar]
- 13. Laha RK, Jannetta PJ: Glossopharyngeal neuralgia. J Neurosurg 47: 316–320, 197. [DOI] [PubMed] [Google Scholar]
- 14. Leksell L: Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand 137: 311–314, 197. [PubMed] [Google Scholar]
- 15. Maesawa S, Salame C, Flickinger JC, Pirris S, Kondziolka D, Lunsford LD: Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg 94: 14–20, 200. [DOI] [PubMed] [Google Scholar]
- 16. Manzoni GC, Torelli P: Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci 26: 2 Suppl s65–s67, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Massager N, Lorenzoni J, Devriendt D, Desmedt F, Brotchi J, Levivier M: Gamma knife surgery for idiopathic trigeminal neuralgia performed using a far-anterior cisternal target and a high dose of radiation. J Neurosurg 100: 597–605, 200. [DOI] [PubMed] [Google Scholar]
- 18. Moretti R, Torre P, Antonello RM, Bava A, Cazzato G: Gabapentin treatment of glossopharyngeal neuralgia: a follow-up of four years of a single case. Eur J Pain 6: 403–407, 200. [DOI] [PubMed] [Google Scholar]
- 19. Ozveren MF, Ture U: The microsurgical anatomy of the glossopharyngeal nerve with respect to the jugular foramen lesions. Neurosurg Focus 17: 2E3, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Ozveren MF, Ture U, Ozek MM, Pamir MN: Anatomic landmarks of the glossopharyngeal nerve: a microsurgical anatomic study. Neurosurgery 52: 1400–1410, 200. [DOI] [PubMed] [Google Scholar]
- 21. Patel A, Kassam A, Horowitz M, Chang YF: Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery 50: 705–711, 200. [DOI] [PubMed] [Google Scholar]
- 22. Pollock BE, Phuong LK, Foote RL, Stafford SL, Gorman DA: High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery 49: 58–64, 200. [DOI] [PubMed] [Google Scholar]
- 23. Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL: Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg 97: 347–353, 200. [DOI] [PubMed] [Google Scholar]
- 24. Regis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille-Turc F: Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg 104: 913–924, 200. [DOI] [PubMed] [Google Scholar]
- 25. Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G: Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery 36: 64–69, 199. [DOI] [PubMed] [Google Scholar]
- 26. Rhoton AL, Jr: Jugular foramen. Neurosurgery 47: 3 Suppl S267–S285, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Rozen TD: Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin 22: 185–206, 200. [DOI] [PubMed] [Google Scholar]
- 28. Rushton JG, Stevens JC, Miller RH: Glossopharyngeal (vagoglossopharyngeal) neuralgia: a study of 217 cases. Arc. Neurol 38: 201–205, 198. [DOI] [PubMed] [Google Scholar]
- 29. Salar G, Ori C, Baratto V, Iob I, Mingrino S: Selective percutaneous thermolesions of the ninth cranial nerve by lateral cervical approach: report of eight cases. Surg Neurol 20:276–279, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Sampson JH, Grossi PM, Asaoka K, Fukushima T: Microvascular decompression for glossopharyngeal neuralgia: long-term effectiveness and complication avoidance. Neurosurgery 54: 884–890, 200. [DOI] [PubMed] [Google Scholar]
- 31. Sheehan J, Pan HC, Stroila M, Steiner L: Gamma knife surgery for trigeminal neuralgia: outcomes and prognostic factors. J Neurosurg 102: 434–441, 200. [DOI] [PubMed] [Google Scholar]
- 32. Sicard R, Robineau J: Communications et presentations: I. Algie veĺopharyngeé essentielle–Traitment chirugical. Rev Neurol (Paris) 36: 256–257, 192. [Google Scholar]
- 33. Sindou M, Henry JF, Blanchard P: [Idiopathic neuralgia of the glossopharyngeal nerve. Study of a series of 14 cases and review of the literature.] Neurochirurgie 37: 18–25, 1991. (Fr) [PubMed] [Google Scholar]
- 34. Stieber VW, Bourland JD, Ellis TL: Glossopharyngeal neuralgia treated with gamma knife surgery: treatment outcome and failure analysis. Case report. J Neurosurg 102: Suppl 155–157, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Taha JM, Tew JM, Jr: Long-term results of surgical treatment of idiopathic neuralgias of the glossopharyngeal and vagal nerves. Neurosurgery 36: 926–931, 199. [DOI] [PubMed] [Google Scholar]
- 36. Taylor PH, Gray K, Bicknell PG, Rees JR: Glossopharyngeal neuralgia with syncope. J Laryngol Otol 91: 859–868, 197. [DOI] [PubMed] [Google Scholar]
- 37. Urgosik D, Liscak R, Novotny JJ, Vymazal J, Vladyka V: Treatment of essential trigeminal neuralgia with gamma knife surgery. J Neurosurg 102: 29–33, 200. [DOI] [PubMed] [Google Scholar]
- 38. Wang HB, Fan ZM, Han J, Li KY, Fan Z: [Serious complications of the microvascular decompression in cerebellopontine angle.] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 40: 352–356, 2005. (Chn) [PubMed] [Google Scholar]
- 39. Yomo S, Arkha Y, Donnet A, Régis J: Gamma Knife surgery for glossopharyngeal neuralgia. J Neurosurg 110: 559-563, 2009 [DOI] [PubMed] [Google Scholar]


