Abstract
Background
Infectious skin disorders are not uncommon in mink. Such disorders are important as they have a negative impact on animal health and welfare as well as on the quality and value of the fur. This study presents the isolation of Arcanobacterium phocae from mink with severe skin lesions and other pathological conditions, and from wild seals and otters.
Results
In 2015, A. phocae was isolated for the first time in Denmark from outbreaks of dermatitis in mink farms. The outbreaks affected at least 12 farms. Originating from these 12 farms, 23 animals cultured positive for A. phocae. The main clinical findings were necrotizing pododermatitis or dermatitis located to other body sites, such as the lumbar and cervical regions. A. phocae could be isolated from skin lesions and in nine animals also from liver, spleen and lung, indicating a systemic spread. The bacterium was also, for the first time in Denmark, detected in dead seals (n = 9) (lungs, throat or wounds) and otters (n = 2) (throat and foot).
Conclusions
An infectious skin disorder in mink associated with A. phocae has started to occur in Danish farmed mink. The origin of the infection has not been identified and it is still not clear what the pathogenesis or the port of entry for A. phocae infections are.
Electronic supplementary material
The online version of this article (doi:10.1186/s13028-017-0342-8) contains supplementary material, which is available to authorized users.
Keywords: Arcanobacterium phocae, Arcanobacterium phocisimile, Mink, Seal, Otter
Background
Infectious skin disorders are not uncommon in mink and are therefore of significance to the fur industry. Skin disorders have an impact on animal health and welfare and therefore also on the quality and value of the fur. Well-known skin disorders are the “sticky kit syndrome” and infected bite wounds [1, 2]. The bite wounds appear sporadically, although the brown mink show more aggressiveness than other breeds and therefore have an increased prevalence of bite wounds [1]. Secondary infections of bite wounds are often associated with bacteria like Staphylococcus delphini and Streptococcus canis [3]. These bacteria are also common in several other infectious conditions in mink, such as urinary tract infections, pneumonia, and pleuritis. In 1970 severe pyoderma was for the first time reported in mink in the USA and two decades later (i.e., the mid-1990s) also reported in Canada [4], but the condition had yet to be diagnosed in Scandinavia. The findings and causative agents were at the time believed to be a mixture of predisposed factors e.g., immune incompetence combined with secondary bacterial infections such as by staphylococci and streptococci [5]. In 2007 a similar type of skin infection, Fur Animal Epidemic Necrotic Pyoderma (FENP), was diagnosed in Finland [6]. Recently, Nordgren et al. [6] reported Arcanobacterium phocae as a potential causative pathogen of FENP commonly observed on the paws and facial skin. A. phocae is a Gram positive, non-motile, catalase positive, coryneform coccobacillus, which is beta-haemolytic on blood agar [7]. A. phocae was first isolated from seals and described as a new species in 1997 on the basis of biochemical and physiological characteristics supplemented with 16S rRNA phylogenetic analysis of the genus Actinomyces [8]. The hypothesized association between A. phocae in mink and seals is a historically use of seal meat as a source of high protein feed for farmed mink. Canadian mink farmers began using seal meat for mink in the mid to late 1990s, which coincided with the first reports of pododermatitis in Canadian mink [4]. FENP was also recently reported by the Danish Fur Farming Society [9]. This type of pyoderma tends to spread within and between farms causing poor animal health and economic loss [6]. We here report the first documented Danish cases of A. phocae associated pododermatitis and additionally the first cases of A. phocae without any association to pododermatitis.
Methods
Animals
During the spring of 2015 till early winter 2015, 15 adult and eight juvenile mink (Neovison vison) carcasses, Nos. 1–23, (Table 1) originating from 12 mink farms (Fig. 1) were submitted to the National Veterinary Institute, Technical University of Denmark for laboratory examination. The animals were subjected to necropsy and follow up diagnostic examination including microbiological examination. Additionally, seven harbor seals (Phoca vitulina, Nos. 24–30), two grey seals (Halichoerus grypus, Nos. 31 and 32) and two otters (Lutra lutra, Nos. 33 and 34) were submitted to the laboratory during the fall/winter 2015/16. All submitted seals and otters were free-ranging animals, which were either found dead or had been euthanized due to animal welfare reasons.
Table 1.
Isolation of Arcanobacterium phocae in mink, seals and otters
| Lung | Liver | Thoracic cavity | Nasal cavity/throat | Foot/flipper | Skin/ulcer | |
|---|---|---|---|---|---|---|
| Neovison vison (case no.) | 5, 6, 10, 12, 13 | 11 | 1, 7, 8 | 4, 20, 21 | 3, 14, 19 | 2, 9, 15, 16, 17, 18, 22, 23 |
| Phoca vitulina (case no.) | 24 | 29 | – | 25, 28 | 30 | 24, 26, 27 |
| Halichoerus grypus (case no.) | – | – | – | 31 | 32 | – |
| Lutra lutra (case no.) | – | – | – | 34 | 34 | 33 |
Fig. 1.
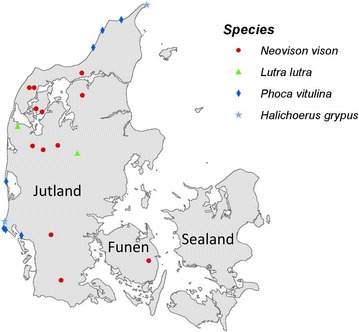
Distribution of mink, seals and otters infected with Arcanobacterium phocae. The majority of infected mink farms are located in Jutland and one farm is at the island of Funen. All seals were located on the coast of Jutland and otters were found in the countryside in Jutland
Pathological examination
The carcasses of mink, seals and otters were subjected to standard necropsy procedures. Except for three mink (Nos. 1, 5 and 6), specimens of lung, liver, spleen, duodenum, ileum and kidney were sampled for histology. Additional samples were taken from other organs with lesions e.g., skin and/or feet. All tissue samples were fixed in 10% neutral buffered formalin, processed by routine methods for histology, embedded in paraffin wax and cut in 3–5 μm sections. The sections were mounted on conventional glass slides and stained with haematoxylin and eosin for histopathological examination [10]. Seals and otters were not examined by histology.
Bacteriological examination
Material from skin and internal organs was collected for bacteriological examination. Columbia agar supplemented with 5% calf blood (SSI Diagnostica, Hillerød, Denmark) and Drigalski agar (SSI Diagnostica) were inoculated and incubated aerobically at 37 °C. For some of the samples, Columbia blood agar plates supplemented with colistin (25,000 units/mL), were used to prevent swarming growth of Proteus spp. and incubated at 37 °C in an atmosphere of 10% CO2. Plates were read after 16–20 h. In case of growth of pin-point colonies, the plates were reincubated and read again after another 24 h. All colonies of interest were subcultured on blood agar, where after they were identified by Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS). Mass spectra were obtained using an Autoflex Speed instrument (Bruker Daltonics, Bremen, Germany) calibrated with the Bruker Escherichia coli Bacterial Test Standard for Mass Spectrometry. Isolates were analysed, as described by Bizzini et al. [11], by the MALDI Biotyper RTC 3.1 software using a library of 6903 spectra using BDAL database (Bruker Daltonics) combined with verified local spectra from National Veterinary Institute. Included in the BDAL database are six different species of Arcanobacteria in which the species A. phocae is represented by 8 spectra and Arcanobacterium phocisimile is represented by 4 spectra. The MALDI Biotyper RTC 3.1 software compares the 10 closest spectra to the sample and provides a log score with a cut-off value of 2.0 for identification at species level and 1.7 for identification at genus level. These cut-off values were used as recommended by the manufacturer.
Virological examination
Suspicion of influenza led to virological examinations in two mink (Nos. 20 and 21, Additional file 1). Lungs from mink and seals were tested by real time polymerase chain reaction (RT-PCR) for influenza virus using RNeasy Mini QIAcube Kit (Qiagen, Copenhagen, Denmark) as previously described [12]. Blood samples from otters and all mink, but Nos. 1, 3, 4 and 10, were tested for Aleutian mink disease virus (AMDV) antibodies by counter-current immunoelectrophoresis at Kopenhagen Diagnostic as described by Cho and Ingram [13]. Lung samples from seals and one otter were tested for canine distemper virus by an in-house immunofluorescence test or RT-PCR according to [14].
Results
Gross pathology and histopathology
Detailed information on the pathology of individual mink, seals and otters are available in Additional file 1.
In general, the mink carcasses were in good nutritional condition with moderate amounts of subcutaneous and abdominal fat. One mink was emaciated and three were obese. In total, 14 mink had skin lesions on the feet, legs, head and/or body, mainly represented by profound, suppurative and necrotizing pododermatitis on one or all feet (n = 10), some of which also had pustular to suppurative and necrotizing dermatitis on the legs (n = 4) or the head (n = 2) (Fig. 2). Other skin lesions were profound suppurative and necrotizing dermatitis in the head (n = 1) or in the lumbar region (n = 1), and dry crusts around nares and/or pus in the nasal cavity (n = 7) (Fig. 2). Six mink had an ulcer on the tip of the tail.
Fig. 2.
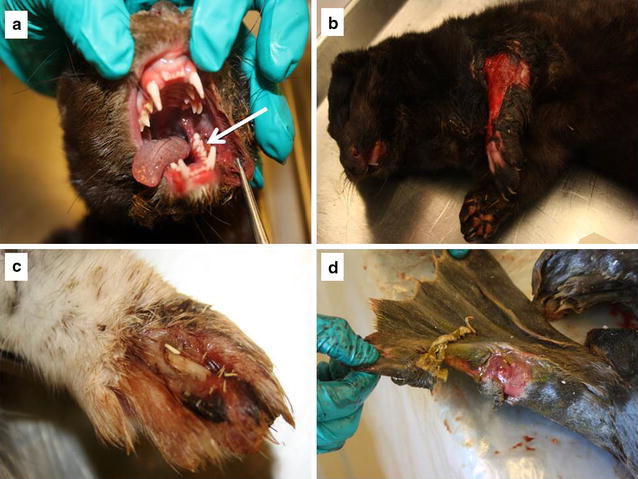
Arcanobacterium phocae associated lesions in mink and seals. a Mink with stomatitis of the buccal mucosa (arrow) and suppurative dermatitis of the cheek. A. phocae was cultured from the lesions. b Severe profound necrotizing dermatitis on the forelimb of a mink. A. phocae was cultured from pus. c Exudative pododermatitis caused by A. phocae. d Ulcer on rear flipper of a seal. A. phocae was cultured from the ulcer
Pyothorax was a frequent finding (n = 8) often accompanied by pulmonary compression atelectasis. Other findings were suppurative bronchopneumonia and multifocal non-suppurative perivasculitis. Twelve mink had hepatomegaly due to congestion and most of these also had varying degrees of hepatocellular lipidosis. Furthermore, two mink had multifocal non-suppurative periportal hepatitis. Petechial haemorrhages were seen in the kidneys of five mink and two mink had focal or multifocal non-suppurative interstitial nephritis. Splenomegaly due to congestion was seen in 13 mink and petechial haemorrhages were seen in the spleen of four mink (Nos. 10, 11, 17 and 18). Furthermore, four mink had oral lesions such as fractured canine teeth, large amounts of dental calculus, stomatitis or gingival haemorrhages, and one pregnant mink had suppurative and necrotizing endometritis and placentitis. The findings in mink No. 22 with non-suppurative interstitial nephritis and non-suppurative periportal hepatitis, were consistent with Aleutian mink disease.
Five seals and one of the otters were emaciated, whereas the remaining seals and otter were in good body condition. Three seals had skin ulcerations (Fig. 2) and one otter had an abscess on the lower jaw. Most of the seals had lungworms (Filaroides gymnurus and Otostrongylus circumlitus) (n = 6) and one of these seals also had suppurative bronchopneumonia. Examination of the liver showed disseminated white pinpoint processes in one otter.
Virological and serological examinations
Both mink tested for influenza virus were negative. Mink no. 22 tested positive for AMDV. All seals tested negative for influenza virus and canine distemper virus. Otters tested negative for AMDV and the otter, which was tested for canine distemper virus, was negative.
Bacteriological examination
Bacteriological culture from skin lesions of mink revealed growth of pin-point, beta-haemolytic, white colonies, barely visible after 16–24 h of incubation, but clearly visible after 2 days of incubation. Many samples also displayed growth indicative of beta-haemolytic streptococci and haemolytic staphylococci. Colonies were subcultured and the monocultures subjected to identification by using MALDI-TOF. The pin-point colonies were identified as A. phocae, all with a log score above 2.0, while other pathogenic bacteria were identified as S. canis and S. delphini. A. phocae was not only isolated from skin lesions, but in some mink also from lung (n = 5), liver (n = 1), thoracic empyema (n = 3), and nose or nasal swabs (n = 3) (Table 1). In the seals and the two otters, bacteriological cultures from skin lesions, pharynx, lung and liver were also identified as A. phocae (Table 1, Additional file 1) while other findings were S. canis, Streptococcus dysgalactiae and E. coli. Notably, in one of the seals, A. phocisimile was found in an infected flipper.
Discussion
Arcanobacterium phocae was for the first time in Denmark isolated from cases of dermatitis and other pathological conditions in mink, seals and otters. Furthermore the detection of A. phocae in nine seals and two otters shown here suggest the existence of a wildlife reservoir. The bacterium, A. phocae, originally isolated from lesions and internal organs in seals living in the coastal waters surrounding Scotland [7, 8], has in recent years become an important pathogen in farmed mink in both Europe [15] and Canada [3]. Hypothetically, since association with disease was originally observed in marine animals [4] and the tiny colonies could easily be overlooked, overgrown by contaminant flora, or mixed up with streptococci, A. phocae may have been present and associated with disease in mink earlier than reported. Prior to 1997 A. phocae had not been characterized and was therefore unknown as a pathogen. Historically, until the description of A. phocae as a pathogen, the bacterium has been suggested to have been misidentified as Listeria ivanovii in seals [7], which is known as a pathogen in ruminants and humans [16, 17]. In the genus Arcanobacterium another member, A. phocisimile, with close phenotypically resemblance to A. phocae was recently detected in seals from the German North Sea [18].
In the present study, A. phocae was identified in 23 farmed mink, 9 seals and 2 otters. The bacterium was isolated from both skin and internal organs (Table 1) and identified using the MALDI TOF technique. This technique has during the last decade proven to be a fast and reliable tool primarily used for diagnostics of humane pathogens. Recently, the technique has gained ground in in veterinary diagnostics in Scandinavia as well as other parts of northern Europe. The success of this technique relies on a well-equipped database containing suitable reference spectra for human and animal pathogens. A challenge for this method is however also the need for monocultures, which is a particular challenge when dealing with dermatitis due to the high risk of contamination or infection with secondary pathogens. In this study concurrent cultivation and identification of S. canis, S. dysgalactiae, S. schleiferi and S. delphini from the skin and nose (data not shown) in the majority of the mink were conducted. Also, isolation and identification of A. phocosimile from one of the Danish seals (No. 31) is reported here. A. phocosimile was identified at species level and in spite of close phenotypical resemblance to A. phocae, the MALDI Biotyper software 3.1 was able to distinguish between the two species [19].
This is the first report where A. phocae is associated with disease and death without signs of pododermatitis (Nos. 1, 6–8, 12, 13, 20 and 21) in mink. Some of the mink had been submitted for examination due to a suspicion of Aleutian mink disease and the veterinary practitioner informed of problems with pododermatitis in one specific farm; unfortunately, no animals were submitted for laboratory investigation at that time (Peter Vase Hansen, personal communication).
It is still not clear what the pathogenesis or the port of entry for A. phocae is. Pododermatitis is speculated to be a multifactorial disease and it is suggested that genetic or immune factors and age could make the animals more susceptible [20]. Many streptococci and staphylococci are known commensals of the skin but act as opportunistic pathogens in case an ulcus is formed, e.g. due to trauma. It is not known if there is an interaction between streptococci, staphylococci and A. phocae in the skin infections examined in this study, but the bacteria might act synergistically and thereby aggravate a skin lesion. Since we here present an alternative pathological expression of mink with no prior signs of pododermatis, further investigations are necessary to determine the point of entry and which predisposing factors are essential for A. phocae to establish a focus of infection. Since Denmark and other Scandinavian countries are not accustomed to use wild life animals as ingredients in mink feed, we consider wildlife as an unlikely source of infection. Likewise, as this bacterium has not been described from food producing animals or fish, it is not likely that the feed, which contains slaughter offal, was the source of infection.
Conclusions
Necrotic dermatitis on the feet and skin of Danish mink was associated to infection by A. phocae, Staphylococcus spp. and Streptococcus spp. The presence of streptococci and staphylococci in such lesions has been reported previously, but A. phocae, seems to play a major role. The findings in some mink indicate that a systemic spread of A. phocae may develop even in mink without pododermatits.
Authors’ contributions
BN carried out the MALDI-TOF MS analysis and interpretation of results, participated in analysis of all results in this study, drafting and writing the manuscript and participated in the necropsies. GL has carried out a major part of the necropsies in mink and otters as well as bacterial identification. EH carried out necropsies in both mink and seals. MSH carried out necropsies in seals and supervised the part concerning pathology. All histology was carried out by MSH. MC has been involved in drafting and revising critically contents of the manuscript. KP has been involved in drafting and design of the manuscript as well as critically revising the manuscript and given final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the technical staff at the National Veterinary Institute for excellent assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was funded by Pelsdyrafgiftsfonden (the Danish Fur Animal Levy Fund).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AMDV
Aleutian mink disease virus
- FENP
Fur Animal Epidemic Necrotic Pyoderma
- MALDI Biotyper RTC software
Matrix-assisted laser desorption/ionization Biotyper Real Time Classification software
- MALDI-TOF
Matrix-assisted laser desorption/ionization time of flight
- RT-PCR
real time polymerase chain reaction
- 16S rRNA
16 Subunit ribosomal ribonucleic acid
Additional file
Additional file 1. Data on individual animals regarding isolation of Arcanobacterium phocae and gross and microscopic pathological findings.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13028-017-0342-8) contains supplementary material, which is available to authorized users.
Contributor Information
Bettina Nonnemann, Email: benon@vet.dtu.dk.
Mariann Chriél, Email: march@vet.dtu.dk.
Gitte Larsen, Email: gitl@vet.dtu.dk.
Mette Sif Hansen, Email: mesi@vet.dtu.dk.
Elisabeth Holm, Email: ehol@vet.dtu.dk.
Karl Pedersen, Email: kape@vet.dtu.dk.
References
- 1.Hansen SW, Møller SH, Damgaard BM. Bite marks in mink—induced experimentally and as reflection of aggressive encounters between mink. Appl Anim Behav Sci. 2014;158:76–85. doi: 10.1016/j.applanim.2014.06.008. [DOI] [Google Scholar]
- 2.Englung L, Chriél M, Dietz HH, Hedlund KO. Astrovirus epidemiologically linked to pre-weaning diarrhoea in mink. Vet Microbiol. 2002;85:1–11. doi: 10.1016/S0378-1135(01)00472-2. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers G, Mclean J, Hunter DB, Brash M, Slavic D, Pearl DL, et al. Staphylococcus spp., Streptococcus canis, and Arcanobacterium phocae of healthy Canadian farmed mink and mink with pododermatitis Résumé. Can J Vet Res. 2015;79:129–135. [PMC free article] [PubMed] [Google Scholar]
- 4.Bröjer C. Pododermatitis in farmed mink in Canada. MS Thesis. Guelph, Canada: The University of Guelph; 2000.
- 5.Ihrke PJ. An overview of bacterial skin disease in the dog. Br Vet J. 1987;143:112–118. doi: 10.1016/0007-1935(87)90002-9. [DOI] [PubMed] [Google Scholar]
- 6.Nordgren H, Aaltonen K, Sironen T, Kinnunen PM, Kivistö I, Raunio-Saarnisto M, et al. Characterization of a new epidemic necrotic pyoderma in fur animals and its association with Arcanobacterium phocae infection. PLoSOne. 2014;9:10. doi: 10.1371/journal.pone.0110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SP, Jang S, Gulland FMD, Miller MA, Casper DR, Lawrence J, et al. Characterization and clinical manifestations of Arcanobacterium phocae infections in marine mammals stranded along the central California coast. J Wildl Dis. 2003;39:136–144. doi: 10.7589/0090-3558-39.1.136. [DOI] [PubMed] [Google Scholar]
- 8.Ramos CP, Foster G, Collins MD. Phylogenetic analysis of the genus Actinomyces based on 16S rRNA gene sequences: description of Arcanobacterium phocae sp. nov., Arcanobacterium bernardiae comb. nov., and Arcanobacterium pyogenes comb. nov. Int J Syst Bacteriol. 1997;47:46–53. doi: 10.1099/00207713-47-1-46. [DOI] [PubMed] [Google Scholar]
- 9.Hammer AS, Aalbæk B, Damborg P, Andresen L, Calusen T, Jensen MK, Struve T, Elbek P. Ny type hudbetændelse påvist som årsag til alvorlige sår. In: Fagblad for Danske Minkavlere. Kopenhagen Fur/Dansk Pelsdyravl. 2015. http://ipaper.ipapercms.dk7KopenhagenFur/DanskPelsdyravlfebruar2015/?Page=32. Accessed 22 Oktober 2015.
- 10.Stevens A, Wilson I. The haematoxylins and eosin. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. 4. London: Churchill Livingstone, Medical Division of Pearson Professional Limited; 1996. pp. 99–112. [Google Scholar]
- 11.Bizzini A, Durussel C, Bille J, Greub G. Prod’hom G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol. 2010;48:1549–1554. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen LE, Breum SØ, Trebbien R, Bradstad K, Nielsen LP, Chriél M, et al. Proceedings of the X International Scientific Congress in Fur Animal Production. Larsen PF, Møller SH, Clausen T, Hammer AS, Lássen TM, Nielsen VH, et al, Editors, Wageningen Academic Publishers The Netherlands; 2012. http://www.wageningenacademic.com/doi/book/10.3920/978-90-8686-760-8.
- 13.Cho HJ, Ingram DG. Antigen and antibody in Aleutian disease in mink. In: precipitation reaction by agar-gel electrophoresis. J Immunol. 1972;108:555–557. [PubMed] [Google Scholar]
- 14.Barrett T, Visser IK, Mamaev L, Goatley L, van Bressem MF, Osterhaus AD. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology. 1993;193:1010–1012. doi: 10.1006/viro.1993.1217. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Antonio R, Fraílde LD, Losada AP, López-Peña M, Vázquez S, R, Bermúdez S, Quiroga MI, and Nieto JM. Pododermatitis in farmed mink (Neovison vison) in Spain. In: Benkel B, Farid H, Hunter B, Murphy B,Rouvinen-Watt K, Harris L, editors. IX international scientific congress in fur animal production. Halifax, Canada: IFASA; 2008. p. 214.
- 16.Guillet C, Join-Lambert O, Le Monnier A, Leclercq A, Mechaï F, Mamzer-Bruneel MF, et al. Human listeriosis caused by Listeria ivanovii. Emerg Infect Dis. 2010;16:136–138. doi: 10.3201/eid1601.091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoder D, Winter P, Kareem A, Baumgartner W, Wagner M. A case of sporadic ovine mastitis caused by Listeria monocytogenes and its effect on contamination of raw milk and raw-milk cheeses produced in the on-farm dairy. J Dairy Res. 2003;70:395–401. doi: 10.1017/S0022029903006277. [DOI] [PubMed] [Google Scholar]
- 18.Hijazin M, Sammra O, Ulbegi-Mohyla H, Nagib S, Alber J, Lämmler C, et al. Arcanobacterium phocisimile sp. nov., isolated from harbour seals. Int J Syst Evol Microbiol. 2013;63:2019–2024. doi: 10.1099/ijs.0.045591-0. [DOI] [PubMed] [Google Scholar]
- 19.Sammra O, Balbutskaya A, Hijazin M, Nagib S, Alber J, Lämmler C, et al. Further studies on Arcanobacterium phocisimile : a novel species of genus Arcanobacterium. J Vet Med. 2014;2014:923592. doi: 10.1155/2014/923592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jespersen A, Hammer AS, Jensen HE, Bonde-Jensen N, Lassus MM, Agger JF, et al. Foot lesions in farmed mink (Neovison vison): pathologic and epidemiologic characteristics on 4 Danish farms. Vet Pathol. 2015 doi: 10.1177/0300985815600502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.


