Abstract
This review evaluates the reliability and validity of ultrasound to quantify muscles in older adults. The databases PubMed, Cochrane, and Cumulative Index to Nursing and Allied Health Literature were systematically searched for studies. In 17 studies, the reliability (n = 13) and validity (n = 8) of ultrasound to quantify muscles in community‐dwelling older adults (≥60 years) or a clinical population were evaluated. Four out of 13 reliability studies investigated both intra‐rater and inter‐rater reliability. Intraclass correlation coefficient (ICC) scores for reliability ranged from −0.26 to 1.00. The highest ICC scores were found for the vastus lateralis, rectus femoris, upper arm anterior, and the trunk (ICC = 0.72 to 1.000). All included validity studies found ICC scores ranging from 0.92 to 0.999. Two studies describing the validity of ultrasound to predict lean body mass showed good validity as compared with dual‐energy X‐ray absorptiometry (r 2 = 0.92 to 0.96). This systematic review shows that ultrasound is a reliable and valid tool for the assessment of muscle size in older adults. More high‐quality research is required to confirm these findings in both clinical and healthy populations. Furthermore, ultrasound assessment of small muscles needs further evaluation. Ultrasound to predict lean body mass is feasible; however, future research is required to validate prediction equations in older adults with varying function and health.
Keywords: Ultrasonography, Muscles, Sarcopenia, Body composition, Muscular atrophy
Introduction
Globally, the proportion of older people within the worldwide population is increasing. It is estimated that, in 2050, approximately 400 million people will be aged 80 years and older.1 During ageing, body composition changes with a 1–2% loss of muscle mass per year after the age of 50.2, 3, 4, 5 This loss, together with impaired physical performance, is referred to as sarcopenia.2, 6 Sarcopenia is associated with development of functional disability, such as slow walking speed, and may lead to a lower quality of life and dependency.2, 7, 8, 9, 10, 11 The prevalence of sarcopenia in healthy older adults (mean age (SD) = 74.4 (3.2) years) is estimated to be between 0% and 15%.12 In community‐dwelling older adults with mobility problems (mean age (SD) = 80.5 (7.0) years), the prevalence is higher, with estimates between 2% and 34%. Differences in cut‐off values, operational criteria, and differences in assessment methods may possibly explain the large variation in prevalence rates. For instance, prevalence rates of 33% to 34% in community‐dwelling older adults of sarcopenia were found when only low muscle mass or low handgrip strength was used as diagnostic criteria for sarcopenia. When applying the diagnostic criteria of the European Working Group on Sarcopenia in Older People, the prevalence of sarcopenia in community‐dwelling older adults with mobility problems is approximately 25%.12
Muscle mass depletion is an important characteristic of sarcopenia. Traditionally, computed tomography (CT) and magnetic resonance imaging (MRI) are considered gold standards for assessing muscle mass.13, 14 However, both methods are not feasible for the assessment of muscle mass in daily practice. CT uses ionizing radiation and therefore is not performed on a routine basis, and MRI is expensive and has limited availability. Dual‐energy X‐ray absorptiometry (DXA) is also a widely used technique to determine muscle mass in a research setting; however, DXA also has limited availability. Ultrasound is potentially a good alternative for CT, MRI, and DXA, as it is a non‐ionizing imaging technique that provides dynamic assessment of soft tissue structures, is portable, and also highly accessible. Furthermore, ultrasound has been shown to be reliable for assessing selected foot structures, which suggests that ultrasound has the potential to accurately assess (small) muscle groups.15
Currently, it is difficult to diagnose sarcopenia in daily practice because there is a lack of valid and/or feasible tools for the assessment of muscle mass. Ultrasound might play an important role in the diagnosis of sarcopenia, because it may offer an objective measure of the amount of muscle mass. Previous reviews concluded that ultrasound is valid for measuring muscle size in a younger population compared with measurement instruments such as MRI and CT.16, 17 Ultrasound is also a reliable measure of muscle size in healthy individuals.18 However, until now, it is unclear whether ultrasound is a reliable and valid technique to assess muscle size in older adults. Furthermore, the use of ultrasound to predict whole body muscle mass in older adults has not been previously reviewed. Therefore, this systematic review aims to evaluate the reliability and validity of ultrasound for assessing muscle size in older adults. Moreover, this study evaluates the validity of ultrasound‐derived equations for the prediction of muscle mass in older adults.
Methods
We systematically searched the PubMed, Cochrane, and Cumulative Index to Nursing and Allied Health Literature databases for studies in English, German, and Dutch. Studies were searched up until 20 January 2016. Outcomes of interest were conclusions about the reliability, concurrent validity, or feasibility of ultrasound to quantify muscles. In the search strategy, a combination of terms related to sarcopenia, older adults, and ultrasonography was used: (i) sarcopenia: muscular atroph*, muscle atroph*, muscle mass*, muscle size*, muscle diameter*, muscle volume*, muscle thickness*, muscle wasting; (ii) older adults: aged, aging, older adult, elder*, older person*, older people, senior*, ageing; (iii) ultrasonography: ultrasound, ultraso* imaging, medical sonography, echography. The complete search strategy is available from the author. In addition to the search in the databases of Cumulative Index to Nursing and Allied Health Literature, PubMed, and Cochrane, other relevant studies were selected using backward citation tracking.
Study eligibility criteria
Studies evaluating the reliability, validity, and/or feasibility of ultrasound to assess muscle mass of the limbs and abdomen in the older population (mean age ≥ 60 years, or inclusion criteria ≥60 years and older) were eligible for inclusion in this study. Animal studies, studies using cadaver specimens, and (systematic) reviews were excluded.
Study appraisal and synthesis methods
Refworks (ProQuest LLC 2016) was used to insert the search hits from the databases. After deleting duplicates, titles and abstracts were independently screened by two authors (W. N. and A. S.). On the basis of the inclusion and exclusion criteria, studies were independently scored as relevant or not relevant. Disagreements regarding the relevance of the studies were solved by consensus. Both assessors (W. N. and A. S.) subsequently and independently assessed the included full text studies. The methodological quality of the included studies was assessed using two checklists: one checklist for the reliability and validity studies17 and one checklist for the studies on the validity of ultrasound‐derived prediction equations.19 The methodological quality of the reliability and validity studies was assessed using a checklist developed by Pretorius and Keating.17 The checklist contains 10 items focusing on the reliability and validity of ultrasound to measure muscles (Appendix S1). A higher score signified higher methodological quality.17 The methodological quality of the validity of ultrasound‐derived prediction equations was assessed by the consensus‐based standards for the selection of health status measurement instruments (COSMIN) checklist. The COSMIN checklist consists of nine boxes; each box entails one measurement property, for example, reliability and criterion validity. Each box consists of 5 to 18 criteria, which can be used to assess methodological quality. Eventually, a quality score was determined by taking the lowest rating of each criterion in a box. The quality score was defined to be poor, fair, good, or excellent.19
In all of the steps of the selection procedure and during assessment of methodological quality, agreement between the two independent reviewers was calculated using the Cohen's kappa coefficient.20 A score of <0.40 is regarded as poor, 0.40–0.75 as fair to good, and a score >0.75 as an excellent agreement between both observers.21
Results
An overview of the process of study selection is shown in Figure 1. After screening by title and abstract, 50 studies were assessed for eligibility. The inter‐rater agreement regarding title and abstract screening was fair to good (Cohen's kappa = 0.60 (95% confidence interval = 0.48–0.72)). From the 50 studies, 16 studies fulfilled the eligibility criteria. Inter‐rater agreement of assessment of full text studies was fair to good (Cohen's kappa = 0.68 (95% confidence interval = 0.48–0.88)). The included studies were categorized as reliability studies (n = 13), validity studies (n = 6), and ultrasound‐derived prediction equation studies (n = 2).
Figure 1.
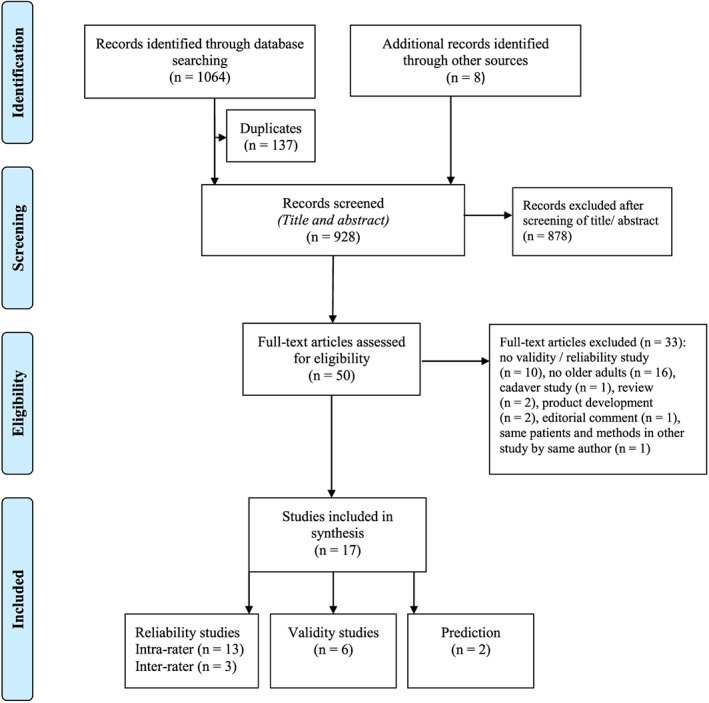
Preferred reporting items for systematic reviews and meta‐analyses flowchart showing selection procedure.
Methodological quality
The quality of the included reliability and validity studies was good, with quality scores ranging between 7 and 10. Overall, more than 7 out of the 10 questions scored ‘yes’ for all of the studies. The most consistent shortcomings were missing data on the composition of the sample and insufficient information on the scanning procedure of ultrasound (Table 1). The quality of the two ultrasound‐derived prediction equations scored as good. The reference used can be considered as a reasonable criterion method for the assessment of muscle mass, both studies used good sample sizes (both studies n = 77), and appropriate statistical analyses were performed in the studies.
Table 1.
Quality assessment of the included studies
| Study | Blinding | Sample | Reproducibility | Study procedures | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blind assessor | Data ≥80% of cohort reported | Representative sample | Sufficient information reported | Data analyses clearly defined | Proper time frame | Instructions on muscle state | Scanning point clearly described | Minimization of contact pressure | Perpendicular position of transducer | ||
| Agyapong et al., 201426 | x | – | – | x | x | x | x | x | – | x | 7 |
| Bemben, 200227 | x | x | x | x | x | – | x | x | x | x | 9 |
| Berger et al., 201536 | x | x | – | x | x | x | x | x | x | x | 9 |
| Cho et al., 201422 | x | x | x | x | x | x | x | x | x | x | 10 |
| English et al., 201118 | x | – | x | x | x | x | x | x | x | x | 9 |
| Hammond et al., 201423 | x | – | x | x | x | x | x | x | x | x | 9 |
| MacGillivray et al., 200924 | x | x | x | – | x | x | x | x | – | x | 8 |
| Raj et al., 201228 | x | x | x | x | x | x | – | x | x | – | 8 |
| Reeves et al., 200429 | x | x | x | x | x | x | x | – | x | x | 9 |
| Sions et al., 201425 | x | x | x | x | x | x | x | x | x | x | 10 |
| Sipila and Suominen, 199337 | x | x | x | x | x | – | x | x | – | – | 7 |
| Staehli et al., 201032 | x | x | x | x | x | x | x | x | x | x | 10 |
| Stetts et al., 200930 | x | x | x | x | x | – | x | x | – | x | 8 |
| Strasser et al., 201331 | x | x | x | x | x | – | x | x | x | x | 9 |
| Thomaes et al., 201233 | x | – | x | x | x | x | x | x | x | x | 9 |
| Transducer | A device that generates and receives the ultrasound waves. |
| Linear transducer | A transducer in which the width of the image is the same at all tissue levels. Therefore, a linear transducer has good near‐field resolution and is most often used for small, superficial structures, for example, muscles. |
| Curved transducer | A transducer in which the width of the image increases with deeper penetration. Therefore, a curved transducer is most often used for deep scanning. |
| Scanning plane | The direction in which the scan is generated. The two scanning planes used in this manuscript are (i) sagittal, which refers to longitudinal orientation and (ii) transverse, which refers to the axial orientation. |
| Muscle dimension | The dimension in which the muscle is measured; thickness (in millimeter or centimeter), cross‐sectional area (in cm2) or volume (in cm3). |
Reliability
As listed in Table 2, 13 studies investigated the reliability of ultrasound. Of these, four studies reported data on both the intra‐rater and the inter‐rater reliability.22, 23, 24, 25 Eight studies involved healthy older adults,24, 25, 26, 27, 28, 29, 30, 31 two studies involved stroke patients,18, 22 and three studies involved patients with chronic conditions, such as chronic obstructive pulmonary disease, osteoarthritis, and coronary artery disease.23, 32, 33 Two studies explicitly stated that the markings on the skin were removed prior to the second scan, to prevent bias in the measurement.18, 25 Out of the 13 studies, four studies used a curved‐array transducer.23, 25, 30, 34 Included studies reported different outcome measures; one study assessed muscle volume,24 three studies assessed cross‐sectional area,23, 27, 29 and nine studies assessed muscle thickness (MT).18, 22, 25, 26, 28, 30, 31, 32, 33
Table 2.
Overview of the included reliability studies
| Study | Demographicsa | Interval in days | Transducer type | Scanning plane | Muscles | Muscle dimension | Reliability estimatesb |
|---|---|---|---|---|---|---|---|
| Intra‐rater reliabilityc | |||||||
| Agyapong et al., 201426 | Community‐dwelling older adults | 7 | Linear | Transverse | Anterior thigh muscles | Thickness | ICC = 0.88 (0.77–0.94) |
| n = 32 (NR:NR) | |||||||
| age = NR (NR) | |||||||
| SEM = 2.11 mm | |||||||
| Bemben, 200227 | Postmenopausal women | 0 | Linear | Transverse | Rectus femoris | CSA | Rectus femoris: |
| n = 38 (0:38) | Biceps brachii | ||||||
| ICC = 0.88 (NR) | |||||||
| age = 58.9 (0.7) | |||||||
| SEM = 0.13 cm2 | |||||||
| Biceps brachii: | |||||||
| Older adults | ICC = 0.99 (NR) | ||||||
| n = 85 (34:51) | |||||||
| age = 65.0 (0.4) | SEM = 0.16 cm2 | ||||||
| Rectus femoris: | |||||||
| ICC = 0.72 (NR) | |||||||
| SEM = 0.12 cm2 | |||||||
| Inter‐rater reliabilityc | |||||||
| Cho et al., 201422 | Post‐stroke patients | 7 | Linear | Sagittal | Medial gastrocnemius | Thickness | Rater 1: |
| ICC = 0.982 (0.968–0.991) | |||||||
| n = 30 (15:15) | |||||||
| age = 64.7 (5.7) | |||||||
| Rater 2: | |||||||
| ICC = 0.992 (0.986–0.996) | |||||||
| English et al., 201118 | Acute stroke patients | 0 | Linear | Transverse | Anterior upper arm | Thickness | ICCs ranging from −0.26 to 0.95 (NR) |
| n = 29 (21:8) | Posterior upper arm | ||||||
| age = 64.0 (16.8) | |||||||
| Lateral forearm | Upper LOA ranging from 2.73 to 26.01 mm. | ||||||
| Abdomen | |||||||
| Anterior thigh | |||||||
| Posterior thigh | Lower LOA ranging from −2.93 to −27.69 mm. | ||||||
| Anterior lower leg | |||||||
| Posterior lower leg | |||||||
| Hammond et al., 201423 | Ambulatory COPD patients | 2–14 | Curved | Transverse | Rectus femoris | CSA | Rater 1: |
| n = 17 (NR:NR) | ICC = 0.971 (NR) | ||||||
| age = 66.0 (NR) | |||||||
| LOA = −1.10 to 1.36 cm2 | |||||||
| Rater 2: | |||||||
| ICC = 0.942 (NR) | |||||||
| LOA = −1.75 to 1.59 cm2 | |||||||
| MacGillivray et al., 200924 | Community‐dwelling older adults | NR | Linear | Sagittal | Rectus femoris | Volume | ICC = 0.997 (NR) |
| n = 11 (NR:NR) | |||||||
| SEM = 0.00 cm3 | |||||||
| median age = 79 | |||||||
| Raj et al., 201228 | Community‐dwelling older adults | 7–14 | Linear | Sagittal | Vastus lateralis | Thickness | Vastus lateralis: |
| Medial gastrocnemius | |||||||
| n = 21 (11:10) | |||||||
| age = 68.1 (5.2) | ICC = 0.96 (0.90–0.98) for both sites 1 and 2 | ||||||
| 95% ratio LOA = 17.25% (site 1) and 10.59% (site 2) | |||||||
| Medial gastrocnemius: | |||||||
| ICC = 0.97 (0.75–0.96) 95% ratio | |||||||
| LOA = 12.56% | |||||||
| Reeves et al., 200429 | Healthy adults | NR | Linear | Transverse | Vastus lateralis | CSA | ICCs between 0.997 and 0.999 for scans 1 to 10 |
| n = 6 (3:3) | |||||||
| age = 76.8 (3.2) | |||||||
| SEM = from 0.15 to 0.40 cm2 | |||||||
| Sions et al., 201425 | Community‐dwelling older adults | 10 | Curved | Transverse | Multifidus muscle | Thickness | Rater 1: |
| ICC = 0.92 (0.83–0.96) | |||||||
| n = 30 (8:22) | |||||||
| age = 71.8 (NR) | SEM = 0.21 cm | ||||||
| Rater 2: | |||||||
| ICC = 0.90 (0.78–0.95) | |||||||
| SEM = 0.22 cm | |||||||
| Staehli et al., 201032 | Patients with osteoarthritis: | 3–10 | Linear | Sagittal | Vastus lateralis | Thickness | ICC = 0.888 (0.778–0.945) |
| preoperative | |||||||
| n = 10 (NR:NR) | |||||||
| SEM = 0.09 cm | |||||||
| age = 59.6 (6.0) | |||||||
| postoperative | |||||||
| n = 20 (NR:NR) | |||||||
| age = 61.5 (5.3) | |||||||
| Stetts et al., 200930 | Community‐dwelling older adults | 0 | Curved | Transverse | Transversus abdomius | Thickness | Intra‐image: |
| Internal oblique | |||||||
| n = 12 (3:9) | ICCs ranging from 0.95 to 1.00 | ||||||
| External oblique | |||||||
| age = 72.0 (9.36) | |||||||
| SEM = from 0.02 to 0.08 cm | |||||||
| Inter‐image: | |||||||
| ICCs ranging from 0.77 to 0.97 | |||||||
| SEM = 0.01 to 0.03 cm | |||||||
| Strasser et al., 201331 | Community‐dwelling older adults | 1 | Curved | Transverse | Rectus femoris | Thickness | Rectus femoris: |
| ICC = 0.876 (NR) | |||||||
| Strasser et al., 201331 | n = 26 (NR:NR) | 1 | Curved | Transverse | Vastus medialis | Thickness | Vastus intermedius: |
| age = 67.8 (4.8) | Vastus intermedius | ||||||
| Vastus lateralis | |||||||
| ICC = 0.928 (NR) | |||||||
| Vastus lateralis: | |||||||
| ICC = 0.852 (NR) Vastus medialis: | |||||||
| ICC = 0.949 (NR) | |||||||
| Thomaes et al., 201233 | Older coronary artery disease patients without cardiovascular incident in the last year | 2 | Linear | Transverse | Rectus femoris | Thickness | ICC = 0.97 (0.92–0.99) |
| SEM = 0.02 cm | |||||||
| n = 25 (NR) | |||||||
| age = 68.6 (4.6) | |||||||
| Cho et al., 201422 | Post‐stroke patients | 7 | Linear | Sagittal | Medial gastrocnemius | Thickness | ICC = 0.967 (0.932–0.984) |
| n = 30 (15:15) | |||||||
| age = 64.7 (5.7) | |||||||
| Hammond et al., 201423 | Ambulatory COPD patients | NR | Curved | Transverse | Rectus femoris | CSA | ICC = 0.998 (NR) |
| n = 15 (NR:NR) | |||||||
| LOA = −0.17 to 0.30 cm2 | |||||||
| age = NR (NR) | |||||||
| MacGillivray et al., 200924 | Community‐dwelling older adults | NR | Linear | Sagittal | Rectus femoris | Volume | ICC = 0.982 |
| n = 11 (NR:NR) | SEM = −0.13 cm3 | ||||||
| Median age = 79 | |||||||
| Sions et al., 201425 | Community‐dwelling older adults | 10 | Curved | Transverse | Multifidus muscle | Thickness | Inter‐examiner measurement reliability: |
| n = 30 (8:22) | |||||||
| age = 71.8 (NR) | |||||||
| ICC = 0.98 (0.97–0.99) | |||||||
| SEM = 0.08 cm | |||||||
| Within‐day procedural reliability: | |||||||
| ICC = 0.88 (0.74–0.94) | |||||||
| SEM = 0.26 cm | |||||||
| Between‐day procedural reliability: | |||||||
| ICC = 0.86 (0.70–0.93) | |||||||
| SEM = 0.29 cm | |||||||
COPD, chronic obstructive pulmonary disease; CSA, cross‐sectional area; ICC, intraclass correlation coefficient; LOA, limits of agreement; NR, not reported; SEM, standard error of measurement.
Studies are arranged in type of study and in alphabetical order.
n = sample size of the study (Male:Female). Mean age is reported. Value in parentheses is the standard deviation.
Findings are reported in ICC values, except where otherwise specified. Values in parentheses are 95% confidence intervals.
Intra‐rater reliability is defined as all types of reliability measures within observer; inter‐rater reliability is defined as all types of reliability measures between observers.
Intra‐rater reliability
The intra‐rater reliability of ultrasound was investigated in 13 studies. The majority of the studies measured the muscle in the transverse plane.18, 23, 25, 26, 27, 29, 30, 31, 33 The interval between repeated measurements varied from several minutes18, 30 to 14 days.23, 28 Nine out of 13 studies evaluated thigh muscles.23, 24, 26, 27, 28, 29, 31, 32, 33, 35 Calf muscles,18, 22, 28 abdominal muscles,18, 30 and spinal muscles25 were also evaluated. Overall, reliability estimates ranged from −0.26 to 1.00. The highest intraclass correlation coefficient (ICC) scores were found for the vastus lateralis (ICC = 0.852 to 0.999), the rectus femoris (ICC = 0.72 to 0.997), the upper arm anterior (ICC = 0.81 to 0.99), and the trunk (0.73 to 1.00).
Inter‐rater reliability
Four studies investigated both intra‐rater and inter‐rater reliability.22, 23, 24, 25 One study assessed both measurement and procedural reliability. Reliability estimates for measurement reliability was higher than procedural reliability (ICC = 0.98 and ICC = 0.86, respectively).25 The four studies evaluated different muscles: medial gastrocnemius,22 rectus femoris,23, 24 and the lumbar multifidus muscle.25 Two studies measured the muscle in the transverse plane.23, 25 Reliability estimates ranged from 0.88 to 0.998.
Validity
All of the included studies evaluated concurrent validity with DXA,36 MRI,24, 29 CT,33, 37 or ultrasound23 used as criterion methods (Table 3). The same construct was measured with ultrasound and the reference methods, except for one study, which compared muscle size with body composition parameters.36 All of the studies evaluated thigh muscles with a linear transducer. Only one study measured thigh muscle volume in the sagittal plane.24 The other studies assessed muscle thickness33, 36, 37 or cross‐sectional area23, 29, 37 in the transverse plane. All studies found that ultrasound is valid for the assessment of muscles, with ICC scores ranging from 0.92 to 0.999,23, 24, 29, 33, 36 and r = 0.761 to r = 0.911.37
Table 3.
Overview of the included validity studies
| Study | Demographicsa | Reference method | Scanning plane | Muscle | Muscle dimension | Validity estimatesb |
|---|---|---|---|---|---|---|
| Berger et al., 201536 | Community‐dwelling older adults | DXA | Transverse | Rectus femoris | Thickness | Right: r = 0.9687 |
| n = 51 (25:26) | ||||||
| age (females) = 72.5 (5.8) | Left: r = 0.9667 | |||||
| age (males) = 74.5 (6.5) | ||||||
| Hammond et al., 201423 | Ambulatory COPD patients | Ultrasound linear transducer | Transverse | Rectus femoris | CSA | ICC = 0.982 (NR) |
| n = 15 (NR:NR) | ||||||
| age = NR (NR) | ||||||
| MacGillivray et al., 200824 | Community‐dwelling older adults | MRI | Sagittal | Rectus femoris | Volume | ICC = 0.997 (NR) |
| n = 11 (NR:NR) median | ||||||
| age = 79 | ||||||
| Reeves et al., 200429 | Healthy adults | MRI | Transverse | Vastus lateralis | CSA | ICCs between 0.998 and 0.999 for scans 6 to 10 |
| n = 6 (3:3) | ||||||
| age = 76.8 (3.2) | ||||||
| Sipila and Suominen, 199337 | Older adults n = 36 (0:36) | CT | Transverse | Quadriceps | Thickness, CSA | Thickness |
| Trained athletes | r = 0.761 | |||||
| n = 21 (0:21) | CSA | |||||
| age = 73.7 (5.6) | r = 0.911 | |||||
| Healthy controls | ||||||
| n = 15 (0:15) | ||||||
| age = 73.6 (2.9) | ||||||
| Thomaes et al., 201233 | Older coronary artery disease patients without cardiovascular incident in the last year | CT | Transverse | Rectus femoris | Thickness | ICC = 0.92 (0.81–0.97) |
| n = 20 (NR) | ||||||
| age = 68.3 (7.3) |
CSA, cross‐sectional area; CT, computed tomography; DXA, dual‐energy X‐ray absorptiometry; ICC, intraclass correlation coefficient; NR, not reported; MRI, magnetic resonance imaging;
Studies are arranged in type of study alphabetical order and in alphabetical order.
n = sample size of the study (Male:Female). Mean age is reported. Value in parentheses is the standard deviation.
Findings are reported in ICC values, except where otherwise specified. Values in parentheses are 95% confidence intervals.
Validity of ultrasound‐derived prediction equations
Two studies evaluated the validity of ultrasound to predict muscle mass in older adults as compared with DXA.38, 39 One study specifically focused on the prediction of leg muscle mass. That study was conducted with 52 healthy adults of which 22 were men (mean age 62.1 ± 8.6 years) and 30 were women (mean age 66.3 ± 5.9 years). The proposed prediction equation included the sum of four MT: thigh anterior and posterior and lower leg anterior and posterior (leg muscle mass = 0.01464 × (MTsum × length of segment) − 2.767). The results indicated a good validity of ultrasound for predicting leg muscle mass compared with DXA (r 2 = 0.96).38 The second study was conducted in 77 healthy older adults (mean age = 64.8 ± 7.2 years). Two prediction equations were proposed in that study. Equation 1 (muscle mass = (sex (female = 0, male = 1) × 7.217) + (MTthigh anterior × 1.985) + (MTthigh posterior × 2.355) + (MTlower leg anterior × 3.633) + (MTlower leg posterior × 2.670) − 6.759) included MT of the thigh (anterior and posterior) and the lower leg (anterior and posterior). The results showed good validity of the ultrasound‐derived prediction equation (r 2 = 0.929, standard error of the estimate = 2.5 kg). Equation 2 utilized the product of MT and limb length (LL) to predict muscle mass.
In this equation, the following sites were included: upper arm anterior, thigh anterior, thigh posterior, and lower leg posterior (muscle mass = (sex (female = 0, male = 1) × 5.233) + ((MT × LL)upper arm anterior × 0.006630) + ((MT × LL)thigh anterior × 0.05153) + ((MT × LL)thigh posterior × 0.05579) + ((MT × LL)lower leg posterior × 0.07097) + 1.774). The validity of Equation 2 was good compared with DXA (r 2 = 0.955, standard error of the estimate = 2.0 kg).39
Discussion
The main finding of this systematic review is that ultrasound is a reliable and valid tool for the assessment of muscle size in older adults. However, the validity of ultrasound‐derived prediction equations for the estimation of muscle mass in older adults cannot be established, because only two studies examined the validity of ultrasound‐derived prediction equations in older adults.
Our finding that ultrasound is reliable for the measurement of muscle size in older adults extends the conclusion of a previous review in a younger population.35 We found that the reliability of ultrasound in older adults is comparable with reliability estimates found in younger adults. Furthermore, we also found that ultrasound is a reliable tool in a clinical population, for example, chronic obstructive pulmonary disease, coronary artery disease, and post‐stroke and acute stroke patients, a finding in contrast to previous literature.35 Reliability estimates in a clinical population appear to be equal to reliability estimates in healthy older adults. These estimates were not only similar for intra‐rater and inter‐rater reliability but also across different body sites. Even though we found high ICC scores for the reliability of ultrasound in clinical populations, ultrasound imaging in a clinical population may be more challenging because of increased echogenicity, that is, the reflectance of the emitted ultrasound signal, and decreased definitions of bone and muscle. Therefore, work on the feasibility of ultrasound in a clinical and ageing population is warranted.
The included studies concluded that ultrasound is a reliable tool for the assessment of muscle size. Important to acknowledge is that this conclusion is based on the assessment of large muscle groups like the Musculus quadriceps. Low ICC scores were found in the assessment of relatively small muscles, such as lateral forearm and lower limb muscles.18 The low ICC scores can possibly be explained by the fact that evaluating small muscles with ultrasound might be challenging because of limited spatial resolution.40 Hence, the reliability of ultrasound for the assessment of small muscles needs further evaluation. We found that ultrasound also showed good validity for the assessment of muscle size compared with DXA, MRI, and CT. A remarkable finding of this review, however, was the lack of studies examining the validity of ultrasound‐derived prediction equations for whole body muscle mass in older adults. To the best of our knowledge, one previous review was published on the validity of ultrasound for the assessment of muscles.17 That review, however, did not specifically focus on older adults and did not include studies on the validity of ultrasound for predicting lean body mass. We found that ultrasound is a valid tool to assess muscle mass in older adults in clinical practice. Only one study showed good validity of ultrasound‐derived prediction equations compared with DXA. We found three other studies regarding the validity of ultrasound‐derived prediction equations for muscle mass, but we excluded these from this systematic review because of the age of the study sample. Nevertheless, these studies found good validity for ultrasound‐derived prediction equations for the assessment of muscle mass.14, 41, 42 This adds to the evidence for high validity of ultrasound for the prediction of muscle mass. However, for ultrasound to become a valid alternative for DXA or bioelectrical impedance analysis for diagnosing sarcopenia, cross‐validation of the proposed prediction equations in older adults is warranted.
Despite high scores on methodological quality, we found that information regarding the scanning procedure was unclear in most of the reliability studies. In particular, information was lacking on the scanning position and marking of the skin. For that reason, the findings in this review might be an overestimation of the true reliability of ultrasound. Nevertheless, we included one study that investigated both measurement and procedural reliability of ultrasound. That study found high ICC scores for both measurement and procedural reliability (ICC = 0.98 and ICC = 0.86, respectively).18 Nonetheless, it is of utmost importance to investigate the reliability of the entire ultrasound scanning procedure, as this reflects the assessment procedure in clinical practice. Therefore, the reliability of the ultrasound procedure to evaluate muscle size in older adults requires further evaluation.
All of the included studies used ICC scores to assess the agreement between raters or devices, which is considered to be the preferred statistical method to assess reliability.43, 44, 45 However, we found that 4 out of 13 reliability studies did not provide any information on the type of ICC used in the study. It is important to report complete information on the type of ICC used in the study as this influences the results and is needed for an appropriate interpretation of the results.46 Furthermore, in addition to type of ICC used in the study, data on the magnitude of the error, for example, standard error of the measurement and limits of agreement, should be reported.
The findings in this systematic review should be considered in the light of some limitations. First, although we used a comprehensive tool for the assessment of methodological quality of the ultrasound‐derived prediction equation studies, this instrument was originally developed to assess methodological quality of health measurement instruments. Therefore, some items were not applicable for specific studies. Nevertheless, the quality of the included studies is expected to be adequate because the items regarding methodological quality were scored as good. Second, because of the lack of information on the type of ICC used in the studies, a meta‐analysis could not be conducted. Finally, given the strict inclusion criteria, studies that did not mention (an equivalent of) muscle mass were excluded from this review.
In conclusion, this systematic review shows that ultrasound is a reliable and valid tool for the assessment of muscle size in older adults. However, more high‐quality research is needed to confirm these findings in both clinical as well as healthy populations. Furthermore, more research is required to validate prediction equations in older adults with varying function and health. This further validation is required to investigate whether use of ultrasound in the screening and diagnosis of sarcopenia is feasible.
Conflict of interest
W.N., A.S., H.J.‐W., H.H., and C.v.d.S. declare that they have no conflicts of interest.
Supporting information
Appendix S1. Checklist.
Acknowledgement
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.47
Nijholt, W. , Scafoglieri, A. , Jager‐Wittenaar, H. , Hobbelen, J. S. M. , and van der Schans, C. P. (2017) The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. Journal of Cachexia, Sarcopenia and Muscle, 8: 702–712. doi: 10.1002/jcsm.12210.
References
- 1. United Nations . Department of Economic and Social Affairs. Population Division (2015) 2015; World Population Ageing 2015 (ST/ESA/SER.A/390). [Google Scholar]
- 2. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 3. Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol 2008;43:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 2003;58:M911–M916. [DOI] [PubMed] [Google Scholar]
- 5. Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, et al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev 2015;24:304–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenberg IH. Summary comments. Am J Clin Nutr 1989;50:1231–1233. [Google Scholar]
- 7. Landi F, Cruz‐Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013;42:203–209. [DOI] [PubMed] [Google Scholar]
- 8. Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, et al. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med 2015;31:367–374. [DOI] [PubMed] [Google Scholar]
- 9. Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community‐dwelling older men: the Concord Health and Ageing in Men Project. J Am Med Dir Assoc 2015;16:607–613. [DOI] [PubMed] [Google Scholar]
- 10. Rizzoli R, Reginster J, Arnal J, Bautmans I, Beaudart C, Bischoff‐Ferrari H, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013;93:101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reijnierse EM, Trappenburg MC, Leter MJ, Blauw GJ, Sipila S, Sillanpaa E, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology 2015;61:491–496. [DOI] [PubMed] [Google Scholar]
- 13. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 14. Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 2006;96:24–31. [DOI] [PubMed] [Google Scholar]
- 15. Crofts G, Angin S, Mickle KJ, Hill S, Nester C. Reliability of ultrasound for measurement of selected foot structures. Gait Posture 2014;39:35–39. [DOI] [PubMed] [Google Scholar]
- 16. Koppenhaver SL, Hebert JJ, Parent EC, Fritz JM. Rehabilitative ultrasound imaging is a valid measure of trunk muscle size and activation during most isometric sub‐maximal contractions: a systematic review. Australian Journal of Physiotherapy 2009;55:153–169. [DOI] [PubMed] [Google Scholar]
- 17. Pretorius A, Keating J. Validity of real time ultrasound for measuring skeletal muscle size. Physical Therapy Reviews 2008;13:415–426. [Google Scholar]
- 18. English CK, Thoirs KA, Fisher L, McLennan H, Bernhardt J. Ultrasound is a reliable measure of muscle thickness in acute stroke patients, for some, but not all anatomical sites: a study of the intra‐rater reliability of muscle thickness measures in acute stroke patients. Ultrasound Med Biol 2012;38:368–376. [DOI] [PubMed] [Google Scholar]
- 19. Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2012;21:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 21. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions, 2nd ed. New York: John Wiley & Sons; 1981. 38–46. [Google Scholar]
- 22. Cho KH, Lee HJ, Lee WH. Reliability of rehabilitative ultrasound imaging for the medial gastrocnemius muscle in poststroke patients. Clin Physiol Funct Imaging 2014;34:26–31. [DOI] [PubMed] [Google Scholar]
- 23. Hammond K, Mampilly J, Laghi FA, Goyal A, Collins EG, McBurney C, et al. Validity and reliability of rectus femoris ultrasound measurements: comparison of curved‐array and linear‐array transducers. J Rehabil Res Dev 2014;51:1155–1164. [DOI] [PubMed] [Google Scholar]
- 24. MacGillivray TJ, Ross E, Simpson HA, Greig CA. 3D freehand ultrasound for in vivo determination of human skeletal muscle volume. Ultrasound Med Biol 2009;35:928–935. [DOI] [PubMed] [Google Scholar]
- 25. Sions JM, Velasco TO, Teyhen DS, Hicks GE. Ultrasound imaging: intraexaminer and interexaminer reliability for multifidus muscle thickness assessment in adults aged 60 to 85 years versus younger adults. J Orthop Sports Phys Ther 2014;44:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agyapong‐Badu S, Warner M, Samuel D, Narici M, Cooper C, Stokes M. Anterior thigh composition measured using ultrasound imaging to quantify relative thickness of muscle and non‐contractile tissue: a potential biomarker for musculoskeletal health. Physiol Meas 2014;35:2165–2176. [DOI] [PubMed] [Google Scholar]
- 27. Bemben MG. Use of diagnostic ultrasound for assessing muscle size. J Strength Cond Res 2002;16:103–108. [PubMed] [Google Scholar]
- 28. Raj IS, Bird SR, Shield AJ. Reliability of ultrasonographic measurement of the architecture of the vastus lateralis and gastrocnemius medialis muscles in older adults. Clin Physiol Funct Imaging 2012;32:65–70. [DOI] [PubMed] [Google Scholar]
- 29. Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 2004;91:116–118. [DOI] [PubMed] [Google Scholar]
- 30. Stetts DM, Freund JE, Allison SC, Carpenter G. A rehabilitative ultrasound imaging investigation of lateral abdominal muscle thickness in healthy aging adults [corrected] [published erratum appears in J GERIATR PHYS THER 2009;32(3):110]. J Geriatr Phys Ther 2009;32:16–22. [PubMed] [Google Scholar]
- 31. Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013;35:2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staehli S, Glatthorn JF, Casartelli N, Maffiuletti NA. Test–retest reliability of quadriceps muscle function outcomes in patients with knee osteoarthritis. J Electromyogr Kinesiol 2010;20:1058–1065. [DOI] [PubMed] [Google Scholar]
- 33. Thomaes T, Thomis M, Onkelinx S, Coudyzer W, Cornelissen V, Vanhees L. Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD‐patients. BMC Med Imaging 2012;12: 7‐2342‐12‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013;35:2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. English C, Fisher L, Thoirs K. Reliability of real‐time ultrasound for measuring skeletal muscle size in human limbs in vivo: a systematic review. Clin Rehabil 2012;26:934–944. [DOI] [PubMed] [Google Scholar]
- 36. Berger J, Bunout D, Barrera G, de la Maza MP, Henriquez S, Leiva L, et al. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch Gerontol Geriatr 2015;61:33–38. [DOI] [PubMed] [Google Scholar]
- 37. Sipila S, Suominen H. Muscle ultrasonography and computed tomography in elderly trained and untrained women. Muscle Nerve 1993;16:294–300. [DOI] [PubMed] [Google Scholar]
- 38. Takai Y, Ohta M, Akagi R, Kato E, Wakahara T, Kawakami Y, et al. Validity of ultrasound muscle thickness measurements for predicting leg skeletal muscle mass in healthy Japanese middle‐aged and older individuals. J Physiol Anthropol 2013;32, 12‐6805‐32‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takai Y, Ohta M, Akagi R, Kato E, Wakahara T, Kawakami Y, et al. Applicability of ultrasound muscle thickness measurements for predicting fat‐free mass in elderly population. J Nutr Health Aging 2014;18:579–585. [DOI] [PubMed] [Google Scholar]
- 40. Mickle KJ, Nester CJ, Crofts G, Steele JR. Reliability of ultrasound to measure morphology of the toe flexor muscles. Journal of Foot And Ankle Research 2013;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abe T, Kawakami Y, Kondo M, Fukunaga T. Comparison of ultrasound‐measured age‐related, site‐specific muscle loss between healthy Japanese and German men. Clin Physiol Funct Imaging 2011;31:320–325. [DOI] [PubMed] [Google Scholar]
- 42. Miyatani M, Kanehisa H, Kuno S, Nishijima T, Fukunaga T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol 2002;86:203–208. [DOI] [PubMed] [Google Scholar]
- 43. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 1998;26:217–238. [DOI] [PubMed] [Google Scholar]
- 44. Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 2000;30:1–15. [DOI] [PubMed] [Google Scholar]
- 45. Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil 1998;12:187–199. [DOI] [PubMed] [Google Scholar]
- 46. Weir JP. Quantifying test–retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005;19:231–240. [DOI] [PubMed] [Google Scholar]
- 47. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Checklist.


