Abstract
The spectrum of activity of vitamin D goes beyond calcium and bone homeostasis, and growing evidence suggests that vitamin D contributes to maintain musculoskeletal health in healthy subjects as well as in patients with chronic kidney disease (CKD), who display the combination of bone metabolism disorder, muscle wasting, and weakness. Here, we review how vitamin D represents a pathway in which bone and muscle may interact. In vitro studies have confirmed that the vitamin D receptor is present on muscle, describing the mechanisms whereby vitamin D directly affects skeletal muscle. These include genomic and non‐genomic (rapid) effects, regulating cellular differentiation and proliferation. Observational studies have shown that circulating 25‐hydroxyvitamin D levels correlate with the clinical symptoms and muscle morphological changes observed in CKD patients. Vitamin D deficiency has been linked to low bone formation rate and bone mineral density, with an increased risk of skeletal fractures. The impact of low vitamin D status on skeletal muscle may also affect muscle metabolic pathways, including its sensitivity to insulin. Although some interventional studies have shown that vitamin D may improve physical performance and protect against the development of histological and radiological signs of hyperparathyroidism, evidence is still insufficient to draw definitive conclusions.
Keywords: Bone, Chronic kidney disease, Muscle, Physical performance, Vitamin D
Introduction
Beyond the well‐described functions of vitamin D in mineral bone metabolism and calcium–phosphate homeostasis, there is growing evidence of its role on muscle health and function.1, 2, 3 Vitamin D deficiency is common in patients with chronic kidney disease (CKD),4 a population in whom muscle wasting and weakness are also highly prevalent.5, 6, 7 Observational studies have shown that circulating 25‐hydroxyvitamin D [25(OH)D] levels are reduced in parallel to the severity of muscle symptoms.8 Similarly, emerging evidence suggests that vitamin D receptor (VDR) is expressed in muscle and that VDR regulates gene expression and modulates the uptake of 25(OH)D in skeletal muscle cells, which may also act as a storage site for this vitamin D.9, 10 There are also evidences that hypovitaminosis D affects both contractile muscle function and muscle metabolism via disturbing insulin sensitivity.11 These observations collectively imply an integrated role of vitamin D for bone and muscle health. Such a role may have substantial clinical implications, especially for CKD patients, in which musculoskeletal alterations and their complications, including muscle pain and weakness, sarcopenia, fatigability, reduced exercise tolerance, fractures, and falls, adversely affect quality of life and survival.12, 13, 14, 15, 16, 17
In this review, we discuss the bidirectional actions of vitamin D in bone and muscle, arguing on the potential benefits of vitamin D supplementation as a strategy to tackle the musculoskeletal problems of patients with CKD.
Vitamin D physiology
Vitamin D and bone‐mineral homeostasis
Natural (frequently referred as well as ‘native’) vitamin D is produced at the skin following sunshine exposure and is not totally required from the diet. The difference between natural vitamin D2 and vitamin D3 lies on their origin (vegetal or animal) and on the structure of their side chains.18 Vitamin D is absorbed through the proximal segments of the small intestine.19 As a hydrophobic molecule, vitamin D circulates in the bloodstream mostly (88–90%) bound with high affinity to the vitamin D binding protein (DBP). Less than 0.05% of calcidiol [25(OH)D or calcifediol] circulates free in plasma. To become fully active, vitamin D needs to be transformed twice.20 A first hydroxylation occurs in the liver microsomes by the 25‐hydroxylase (CYP2R1) enzyme to form 25(OH)D. There is a second hydroxylation in the proximal tubule by the 1α‐hydroxylase (CYP27B1) to form 1,25‐dihydroxyvitamin D [1,25(OH)2D], also called calcitriol. In contrast to liver hydroxylation, renal hydroxylation is highly regulated by several factors including calcium, phosphate, parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23), which is produced by osteocytes and osteoblasts in bone.21
There is a feedback loop between FGF23 and vitamin D, whereby FGF23 inhibits 1α‐hydroxylase activity and stimulates 24,25‐hydroxylase, and simultaneously vitamin D stimulates FGF23 production, which can still exacerbate the high circulating FGF23 levels already existing in CKD and impact bone metabolism.21, 22 Indirect effects of FGF23 include the increase of renal excretion of phosphate, affecting the amount of phosphate available for mineralization at the bone surfaces.23 Direct effects of FGF23 on bone metabolism include the modulation of bone mineralization via the tissue non‐specific alkaline phosphatase through the fibroblast growth factor receptor 3. FGF23 inhibits tissue non‐specific alkaline phosphatase, and consequently, FGF23 increases extracellular concentration of pyrophosphate, reduces the amount of inorganic phosphate, and indirectly stimulates osteopontin gene expression, a known mineralization inhibitor.24
1,25(OH)2D that passes into the bloodstream is also bound to DBP, binding to the VDR in several tissues, including parathyroid cells, bone, and intestine.25, 26 The VDR‐1,25(OH)2D complex acts as heterodimer with the retinoic X receptor (RXR) to control transcriptional activity of target genes, after binding to special DNA sequences called vitamin D response elements. Circulating 1,25(OH)2D also exerts non‐genomic effects through the binding in some tissues to membrane proteins with subsequent modification of the intra‐cellular calcium flux and stimulation of tyrosine kinases (Figure 1).20, 27, 28 As a result of these processes, 1,25(OH)2D maintains calcium and phosphate homeostasis, stimulating their intestinal absorption and bone resorption.29 To accomplish this, the 1,25(OH)2D‐VDR‐RXR complex binds to the vitamin D response elements in the small intestinal cells, increasing the expression of the epithelial calcium channel. It permits more calcium to enter the cell, which is translocated into the circulation, ensuring the availability of sufficient calcium and phosphate for adequate mineralization of the newly formed bone matrix to avoid rickets/osteomalacia. 1,25(OH)2D induces skeletal anabolism and couples the activity of osteoblasts and osteoclasts through the regulation of several genes including osteopontin, osteocalcin, and the Wnt receptor LRP5.30, 31 Indeed, vitamin D stimulates the expression of LRP5, which, together with sclerostin, Dkk1, and frizzled, constitutes the Wnt pathway, a critical process for skeletal mineralization that is tissue specific. Unlike in bone, Vitamin D inhibits Wnt signals in the vessels and the kidney, ameliorating the effect of Wnt activation on the vascular calcification and kidney progression.32, 33
Figure 1.
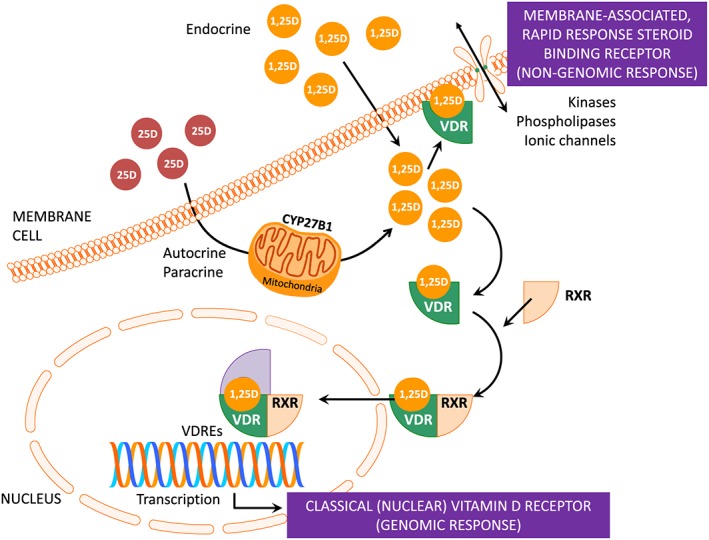
Vitamin D receptor (VDR)‐mediated actions of vitamin D: genomic and non‐genomic (rapid response) cellular signalling. 1,25(OH)2D interacts with caveolae‐associated VDR to activate second messengers systems, including protein kinase C, phosphatidylinositol phosphate kinase, phospholipase C, or opening of the voltage‐gated chloride channels or calcium channels, to generate non‐genomic responses. In the genomic pathway, 1,25(OH)2D associates with the retinoic acid receptor (RXR) and the trimeric complex (1,25(OH)2D‐VDR‐RXR) binds to the DNA in special sites called ‘vitamin D responsive elements’ (VDRE) to stimulate or inhibit the transcription of various genes. 1,25(OH)2D can locally be produced in an auto‐paracrine or paracrine way.
Besides this anabolic effect on bone, direct effects of vitamin D in osteoblasts may have the opposite effect, stimulating bone resorption through osteoclastogenesis to increase bone calcium mobilization.34 To do this, Vitamin D interacts with the VDR in osteoblasts to induce the expression of the plasma membrane protein receptor activator of NF‐κB ligand (RANKL). The RANK on the plasma membrane of preosteoclasts binds RANKL, which induces the maturation of preosteoclasts to osteoclasts. The mature osteoclast releases collagenases and hydrochloric acid to dissolve bone and release its calcium and phosphate stores into the bloodstream. Therefore, the ‘classical’ physiologic function of vitamin D is to maintain blood levels of calcium and phosphate within the normal physiologic range to support most metabolic functions, neuromuscular transmission, and bone mineralization.35
The VDR is also present in other tissues (including skeletal muscle) that are not involved in mineral and bone metabolism, where 1,25(OH)2D can locally be produced in an auto‐paracrine or paracrine way (Figure 1), what results in the so‐called ‘non‐classical’ vitamin D effects. Table 1 summarizes the main functions of vitamin D.29, 36
Table 1.
Effects and functions of vitamin D
| Endocrine effects | Non‐calcaemic and non‐skeletal effects |
|---|---|
|
1. Increase intestinal absorption of calcium and phosphate 2. Down‐regulate expression of PTH mRNA in the parathyroid glands 3. Induce mature osteoclastic activity, which releases calcium and phosphate into the bloodstream ↓ ‘Classical’ functions To maintain normal blood levels of calcium and phosphate in order to support: 1. Bone mineralization 2. Metabolic functions 3. Neuromuscular function |
1. Maintain normal cell proliferation and differentiation. 2. Decrease renal production of renin 3. Stimulate pancreatic production of insulin 4. Immunomodulation ↓ ‘Non‐classical’ functions To modulate human health by metabolic imprinting during the pre‐natal and neo‐natal periods that may influence chronic disease susceptibility to cancer, autoimmune, and cardiovascular diseases, soon after birth as well as later in life. |
mRNA, messenger ribonucleic acid; PTH, parathyroid hormone.
Vitamin D and skeletal muscle weakness
In addition to the endocrine effects on calcium homeostasis that are essential for muscle function, in vitro and in vivo studies, along with changes in muscle morphology and metabolism observed in subjects with hypovitaminosis D, have allowed the elucidation of novel pathways by which vitamin D might act directly on skeletal muscle. These include genomic and non‐genomic (rapid) effects (Figure 2).37, 38 Genomic effects are delayed and include the gene expression of contractile proteins and myogenic transcription factors after interacting vitamin D with the VDR in skeletal muscle cells, which regulate muscle development and metabolism. Several studies confirm that VDR is expressed in muscle cells.9, 39 Although VDR is expressed at low levels in resting adult muscle, markedly VDR expression and 1α‐hydroxylase have been observed in neonatal muscle or following muscle injury, supporting the muscle capacity for local production of 1,25(OH)2D, and a developmental and regenerative role for vitamin D in this tissue.36, 40, 41
Figure 2.
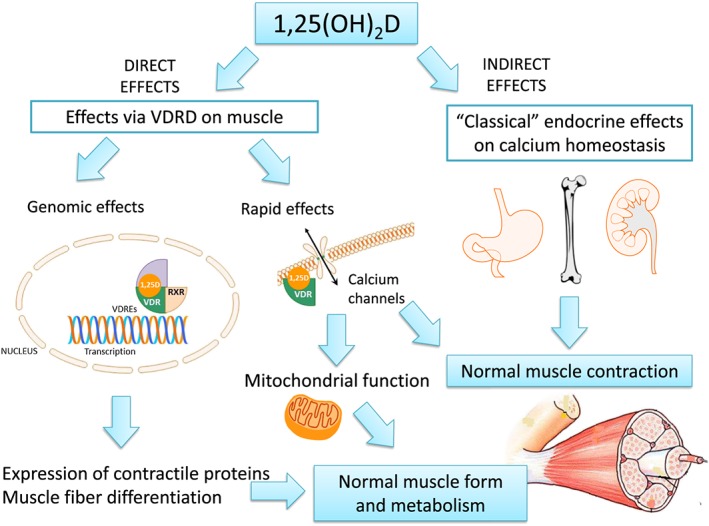
Plausible effects of vitamin D on muscle cells. Adapted from Girgis et al.35
Vitamin D may also interact with the VDR in muscle cells by non‐genomic effects, which are independent of the intra‐nuclear transcription process. They involve the rapid regulation of membrane calcium channels, suggesting a role for vitamin D in the calcium‐mediated muscle functions, such as muscle contraction and mitochondrial function, which leads to an adequate insulin signalling and muscle substrate metabolism.42 All these findings may clarify the relationship between low vitamin D status and muscle weakness,37, 43 intramuscular fat deposition,44 and resistance to insulin,45 which is related to cardiovascular risk and increased skeletal muscle breakdown.46 Of note, skeletal muscle may also act as a storage site for vitamin D, as recently described.10
In addition to changes in muscle metabolic pathways, the impact of vitamin D deficiency on skeletal muscle also concerns muscle morphology. Subjects with mutations of the VDR or severe vitamin D deficiency show generalized muscle atrophy, even before biochemical signs of bone disease appear.36, 47 Changes in muscle morphology include derangement of the intermyofibrillar network, increases in intramuscular lipids, and atrophy of the fast‐twitch white (type 2) fibres,11, 44, 48, 49 which are the first to be recruited when preventing a fall. All these changes seem to be reversible,50 supporting co‐ordinated effects of vitamin D in musculoskeletal physiology.51, 52, 53, 54
Integrated pathway of the vitamin D, bone, and muscle interplay
It is well known that sarcopenia and osteopenia occur simultaneously in vitamin D‐deficient patients, whereas muscle weakness and falls have been associated to vitamin D deficiency are suggested as responsible for the high fracture rate in this population.37, 40, 55 Observational data have revealed that 25(OH)D levels predict the decline in bone mineralization and physical performance when 25(OH)D falls below 8 and 20 ng/mL (20 and 50 nmol/L), respectively.8, 56 Although the underlying mechanisms remain to be elucidated, vitamin D may represent a pathway by which bone and muscle may work together, enabling cross‐talk between these tissues (Figure 3).1, 37, 57 In vitro studies have reported that vitamin D reduces myostatin in cultured muscle cells, a hormone released from the muscle that inhibits muscle growth.58, 59 A reduction in myostatin levels is also associated with increases in bone mass.60 Vitamin D stimulates the muscle production of vascular endothelial growth factor and insulin‐like growth factor‐1 (IGF‐1), which are involved in muscle regeneration after injury, as well as in bone growth and density.61, 62, 63 This may explain how the administration of vitamin D improves the recovery of skeletal muscle strength due to intense exercise.64 1,25(OH)2D also brings the expression of osteoglycin, another bone anabolic factor that is produced by muscle tissues.65
Figure 3.
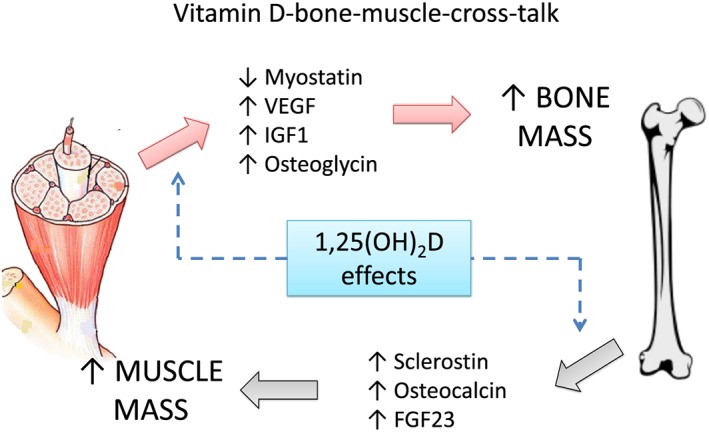
Integrative bone‐muscle‐cross‐talk mediated by vitamin D. Adapted from Girgis et al.38
Potential bone factors that affect muscle metabolism and are regulated by vitamin D include sclerostin, osteocalcin, and FGF23.1 Sclerostin is secreted by mature osteocytes, inhibiting the Wnt signalling pathway that leads to decreased bone formation and increased muscle differentiation.66 Osteocalcin is a hormone produced by osteoblasts that reduces sensitivity to insulin and enhances the exercise capacity.67, 68 FGF23 induces hypertrophy at least on cardiac muscle, although its effects on skeletal muscle are not fully understood.69 In addition, the effect of vitamin D on decreasing serum levels of PTH may positively affect muscle function, given that PTH induces proteolysis and reduces creatine phosphate and inorganic phosphate in muscle cell.70
Vitamin D metabolism in chronic kidney disease
The kidney is the main site for conversion of 25(OH)D to circulating 1,25(OH)2D. Although decreased 1,25(OH)2D synthesis has been classically related to CKD, the circulating concentration of both metabolites, 25(OH)D and 1,25(OH)2D, begins to decrease from the earliest stages of CKD.71 Several factors are associated to this phenomenon including reduced renal mass, dietary restrictions and nutritional deficiencies, reduced sunlight exposure, skin hyperpigmentation, diabetes mellitus, obesity, accumulation of uremic toxins, impaired skin synthesis of cholecalciferol, proteinuria, and increased FGF23.72, 73 In addition, vitamin D is transported in conjugation with DBP and filtered through the glomerulus. Tubular reabsorption of vitamin D bound to DBP is facilitated by the multi‐ligand receptor megalin.74 In proteinuric CKD subjects, megalin is occupied by an extensive albumin load, and therefore fewer receptors are available to uptake 25(OH)D‐DBP, which contributes to vitamin D deficiency.75
In addition to 25(OH)D, 1,25(OH)2D levels are also reduced in CKD.4 Renal 1α‐hydroxylase activity reduces as the renal mass decreases. Other down‐regulating factors that are present in CKD patients include low availability of 25(OH)D, hyperphosphatemia, metabolic acidosis, and uraemia itself. Additionally, elevated FGF23 activates the enzyme 24‐hydroxylase (CYP24), hydroxylating both 25(OH)D and 1,25(OH)2D. 24‐hydroxylase limits the amount of 1,25(OH)2D in target tissues both by producing 24,25(OH)2D (thus decreasing the availability of 25(OH)D for 1 hydroxylation) or by accelerating the catabolism of 1,25(OH)2D to 1,24,25(OH)3D resulting in calcitroic acid, which is biologically inactive.76, 77 CKD is also considered as a state of vitamin D resistance, because VDR expression in bone cells and in nodular parathyroid glands is reduced.78 Low 1,25(OH)2D levels also impair its binding to the VDR‐RXR complex.79, 80 The role of VDR and its interaction with DNA has been comprehensively reviewed recently from regular physiology to the systemic effects of CKD.25
The combination of vitamin D and/or 1,25(OH)2D insufficiency and end‐organ resistance to vitamin D contribute to the development of CKD‐MBD. Additional mechanisms include the impairment of vitamin D‐dependent osteocalcin production81 and the altered Wnt signalling in osteoblasts and osteocytes observed in CKD,82 which is associated with bone loss and vascular calcification.83, 84 As vitamin D inhibits the adverse TGFβ/Smad action on bone cells, a normal vitamin D status might provide protection against Wnt signalling‐related bone loss in CKD.85
Bone and musculoskeletal abnormalities in chronic kidney disease patients
Alteration of bone mass in chronic kidney disease
Patients with CKD exhibit considerable skeletal fragility, which results from the large spectrum of CKD‐related bone diseases, in addition to a variety of other factors including age‐related osteoporosis and a significant number of non‐specific therapeutic approaches directly affecting bone metabolism such as the use of glucocorticosteroids, intestinal phosphate binders, vitamin D compounds, bisphosphonates, and calcimimetics. The measurement of bone mineral density (BMD) is the usual method to assess bone quantity in these patients. However, the assessment of bone quality is uncommon and difficult, involving others factors such as remodelling rate, bone geometry, and the extracellular matrix properties.86
In addition, in CKD, the relation between BMD values, fragility of bone, and fracture risk is not always so clear. Bone loss is site specific, predominating at the mid‐radius, with a greater loss of cortical rather than cancellous, which is related to hyperparathyroidism, as opposite to the post‐menopausal osteoporosis where the loss is mainly due to cancellous bone from the axial skeleton.87 Moreover, CKD patients show different patterns of bone loss. Whereas some patients have a minimal bone loss, others show rapid bone losses.86 Additionally, the presence of aorta calcification and spinal osteoarthritis may bias BMD measurement,88 being the hip and the radius better sites for the BMD assessment. Interestingly, it has been shown in a population of 2754 elderly subjects, including 587 subjects with CKD, that lower BMD was a risk factor associated with skeletal fractures.89 Several other recent papers have shown that low BMD actually predicts fracture in dialysis and renal transplant patients.90, 91 The use of micro‐computerized tomography might also be a useful tool for the estimation of bone loss and micro‐architectural changes; however, they need further evaluation in CKD.92 Overall, although there have been uncertainties concerning the utility of BMD in CKD,93 BMD measure may become useful in this population. This issue is currently under review,94 and it is likely that BMD testing could be suggested in CKD patients with evidence of CKD‐MBD and/or risk factors for osteoporosis, if results may affect treatment decisions.95, 96
Although bone histomorphometry is not routinely recommended or executed in uremic patients, it is the gold standard and the only way to evaluate the type of renal osteodystrophy in CKD‐MBD.97, 98 The bone histologic findings in CKD range from low to high bone turnover, mineralization troubles, and changes in bone volume. Each of these histological patterns can appear isolated or co‐exist; and none of them can be properly discriminated by using imaging tools or circulating bone biomarkers. It must be stressed here that because the prevalence of renal osteodystrophy in CKD‐MBD is high, the presence of osteoporosis is often a diagnosis of exclusion. In spite of this, the KDIGO working group recommended to use in CKD the same World Health Organisation osteoporosis definition applied to the general population. It defines osteoporosis as a bone disorder resulting in decreased bone strength and increased risk of fracture, which is a broad definition that can be appropriately used for diagnostic and management purposes at least in CKD stages 1 to 4.
Alterations of muscle in chronic kidney disease
Severity and prevalence of myopathy in CKD develops already at an eGFR <25 mL/min/1.73 m2 and increases concurrently with the decline in GFR, concerning to more than half of dialysis patients.99, 100, 101 The diagnosis of uremic myopathy is based on clinical features, including weakness (defined as a failure to generate force) and wasting (sarcopenia), which affect predominantly the proximal lower limbs.102 Whereas muscle enzymes levels and electromyographical studies are usually normal, muscle biopsies show atrophy of the fast‐twitch white (type 2) fibres.103, 104 These morphological features are similar from those found in patients with vitamin D deficiency.11, 48
The aetiology of uremic myopathy is multifactorial (Table 2), including physical inactivity, reduced protein intake, vitamin D deficiency, hyperparathyroidism, metabolic acidosis, electrolyte disorder, low serum levels of testosterone, resistance to growth hormone and insulin, accumulation of uremic toxins, and carnitine deficiency, which can lead to mitochondrial dysfunction.105, 106 Observational studies have shown an inverse correlation between muscle mass and blood levels of IL‐6 and C reactive protein in CKD patients,12, 107, 108 postulating inflammation as an additional cause of muscle wasting in this population.109, 110, 111 Although the process by which inflammation produces sarcopenia has not yet been identified, several mechanisms have been described, including activation of NF‐κβ and angiotensin II pathways,112, 113, 114 and the ATP‐dependent ubiquitin–proteasome system, which has been identified as the most important pathway for muscle wasting.115 Excellent reviews on muscle wasting and dysfunction in patients with CKD have been recently published.102, 106
Table 2.
Causes of muscle wasting in chronic kidney disease
|
1. Physical inactivity 2. Reduced protein intake 3. Protein‐energy wasting 4. Hormonal disorders: ‐Vitamin D deficiency ‐Low testosterone ‐Hyperparathyroidism ‐Resistance to growth hormone ‐Resistance to insulin ‐Increased Angiotensin II 5. Metabolic disorders: ‐Metabolic acidosis ‐Electrolyte disorder ‐Uremic toxins accumulation 6. Inflammation 7. Myostatin overexpression 8. Low carnitine |
Vitamin D status in chronic kidney disease: data from observational studies
Vitamin D status, bone mineral density, and fractures
A limited number of studies have looked at the relationship between 25(OH)D levels and bone histology, BMD, and fractures in CKD patients (Table 3). An observational study of 104 dialysis patients who underwent a trans‐iliac bone biopsy showed that patients with vitamin D insufficiency [25(OH)D ≤ 15 ng/mL] had lower trabecular mineralization surface and bone formation rate regardless of levels of 1,25(OH)2D and PTH.116 Other studies have shown that CKD patients with low circulating 25(OH)D have an increased risk of reduced BMD and of skeletal fractures,117, 118, 119 as well as of radiologic features of secondary hyperparathyroidism.120 In contrast, a more recent study that included 59 dialysis patients did not show significant differences in T‐scores and trabecular bone scores among patients according to their 25(OH)D levels.121 This apparent discrepancy may be explained by the currently available treatments for CKD‐MBD disorders that could alter the classical pathologic findings of the bones in CKD and their relation to 25(OH)D levels. Collectively, it seems clear that low vitamin D status is associated with osteomalacia and fractures, presumably because of mineralization defects. However, the data are less robust in CKD than in the general population.122 In addition, the increased osteoclastic activity due to secondary hyperparathyroidism also removes matrix and minerals, exacerbating low bone mass and osteoporosis. It is the combination of mineralization defect and low bone mass that likely increases risk for fractures.123, 124
Table 3.
Studies investigating the association between circulating 25(OH)D levels and skeletal outcomes in chronic kidney disease patients
| Reference | Year | N | CKD stage | Study design | Outcome | Results |
|---|---|---|---|---|---|---|
| Coen et al. 116 | 2005 | 104 | HD | Retrospective | Renal osteodystrophy assessed by transiliac bone biopsy |
A mineralization defect and high bone turnover was found with serum 25(OH)D < 15–20 ng/mL Serum 25(OH)D > 40 ng/mL were accompanied by a reduction of bone turnover. The optimal circulating level of 25(OH)D appeared to be between 20 and 40 ng/mL |
| Ambrus et al. 117 | 2011 | 130 | HD | Cross‐sectional | Bone densitometry of the lumbar spine, femoral neck, and distal radius |
Patients with low‐trauma fractures (n = 21) had lower serum 25(OH)D levels (6.3 ng/mL vs. 12.0; p = 0.029) 25(OH)D < 8 ng/mL was independently associated with bone fractures [OR 11.2 (95% CI: 1.3–94.8); p = 0.026] |
| Elder et al. 118 | 2006 | 242 |
Stage 5 CKD (5D, 85% |
Cross‐sectional | Prevalent spinal fracture assessed by X‐ray and BMD by DXA | 25(OH)D correlated positively with Z‐scores of BMD at the lumbar spine (r = 0.24, p = 0.0005), femoral neck (r = 0.23, p < 0.001), and wrist (r = 0.22, p < 0.01). |
| Mucsi et al. 119 | 2005 | 69 | HD | Cross‐sectional | Bone densitometry and quantitative bone ultrasound | 25(OH)D concentration was positively correlated with BMD measured at the radius (r = 0.424, p < 0.01) and with attenuation on quantitative bone ultrasound (beta = 0.262, P < 0.05). |
| Ghazali et al. 120 | 1999 | 113 | HD | Cross‐sectional | X‐rays of the hands and pelvis were obtained for evaluation of sub‐periosteal resorption and Looser's zones | 25(OH)D was significantly lower in the groups with isolated sub‐periosteal resorption (17.6 vs. 22.8 ng/mL; p < 0.05) and with the combination of resorption with Looser's zones (10.4 vs. 22.8 ng/mL; p < 0.004) than in the normal X‐ray group. |
| Brunerová et al. 121 | 2016 | 59 | HD | Cross‐sectional | Bone densitometry, including trabecular bone score | Similar T‐scores and trabecular bone scores among patients according to their serum 25(OH)D levels |
CKD, chronic kidney disease; HD, haemodialysis.
Vitamin D status, falls, muscle mass, and muscle function
Although several studies have described the association between low 25(OH)D levels with lower muscle strength and mass, increased body instability and falls, worse physical performance and frailty in vitamin D‐deficient older adults,125, 126, 127 only a few studies have been undertaken in CKD patients (Table 4). Gordon et al.128 observed a relationship between 1,25(OH)2D levels, and physical performance and muscle size in non‐dialysis CKD patients. Further, Zahed et al.129 showed that 25(OH)D levels were positively associated with muscle strength of the lower extremities in haemodialysis patients, suggesting altogether a plausible role of vitamin D supplementation for improving muscle health in this population.
Table 4.
Studies investigating the association between circulating 25(OH)D levels, muscle strength, and physical performance in chronic kidney disease patients
| Reference | Year | N | CKD stage | Study Design | Outcome | Results |
|---|---|---|---|---|---|---|
| Gordon et al. 128 | 2012 | 26 | CKD stage 3 or 4. | Cross‐sectional | Gait speed, 6 min walk, sit‐to‐stand time, 1‐legged balance, and thigh MCSA, measured by MRI. |
Serum 25(OH)D levels were associated with normal gait speed only (r = 0.41, P = 0.04). Normal and fast gait speed, the distance walked in 6 min, and sit‐to‐stand time were best explained by 1,25OH2D values. Variance in MCSA was best explained by a model containing 1,25OH2D values. |
| Zahed et al. 129 | 2014 | 135 | HD | Cross‐sectional | Muscle strength estimated using a micro manual muscle tester | Lower serum 25(OH)D levels were observed in the group with less muscle strength in lower extremities |
CKD, chronic kidney disease; HD, haemodialysis; MCSA, muscle cross‐sectional area; MRI, magnetic resonance imaging.
Interventional studies on vitamin D for improving musculoskeletal health in chronic kidney disease
Effect of vitamin D supplementation on bone mineral density, renal osteodystrophy, and fractures in chronic kidney disease
Multiple randomized trials have been conducted to examine the effect of active vitamin D metabolites as well as nutritional vitamin D supplements on bone biochemical markers in CKD and end‐stage renal disease. Most of these studies have been summarized in some meta‐analysis,130, 131, 132 demonstrating the ability of vitamin D for lowering PTH, although treatment was associated with clinical elevations in serum phosphate and calcium. However, data are lacking in terms of patient‐level skeletal outcomes such as fractures, BMD, bone pain, or histomorphometric analysis of bone biopsies.133 Table 5 summarizes studies that investigated the impact of vitamin D on skeletal health in CKD.134, 135, 136, 137, 138, 139, 140, 141, 142 Although vitamin D appeared to protect against the development of histological evidence of osteitis fibrosa and radiological signs of hyperparathyroidism, most published studies have multiple methodological limitations including small sample size and insufficient follow‐up to appropriately ascertain these outcomes. To date, no clear benefit on skeletal outcomes can be concluded from the vitamin D administration in renal populations.133, 143 Fortunately, a new meta‐analysis will conduct a systematic review of nutritional vitamin D supplementation and health‐related outcomes including fracture in end‐stage renal disease patients.144
Table 5.
Studies investigating the effects of vitamin D supplementation on skeletal health in chronic kidney disease
| Reference | Year | N | CKD stage | Study design | Duration of study | Vitamin D regimen | Endpoint | Results |
|---|---|---|---|---|---|---|---|---|
| Fournier et al. 134 | 1979 | 10 | HD | Open‐label interventional | 6 months |
Oral alfacalcidol (1–2 mcg/d) vs. oral calcifediol (50–100 mcg/d) |
Bone matrix mineralization evaluated by histomorphometry | Calcifediol induced more effectively bone mineralization |
| Memmos et al. 135 | 1981 | 57 | HD | RCT | 1–2 years |
Oral 1,25(OH)2D (0.25–0.50 mcg/d) vs. placebo |
Radiological signs of hyperparathyroidism |
1,25(OH)2D prevented radiological signs of secondary hyperparathyroidism in patients with normal radiographs 1,25(OH)2D arrested or reversed radiological signs of secondary hyperparathyroidism in patients with abnormal radiographs |
| Morinière et al. 136 | 1985 | 27 | HD | RCT | 6 months |
Oral alfacalcidol (0.3–1.0 mcg/d) + CaCO3 (3 g/d) vs. CaCO3 (9 ± 5 g/d) |
Development of bone pain | No differences between groups. |
| Baker et al. 137 | 1986 | 76 | HD | RCT | 5 years |
Oral 1,25(OH)2D (0.25–1.00 mcg/d) vs. placebo |
Bone biopsy Fracture risk |
1,25(OH)2D appeared to protect against the development of histological evidence of osteitis fibrosa but not of osteomalacia, but accumulation of aluminium in bone occurred during the study No differences on fracture risk between groups |
| Baker et al. 138 | 1989 | 13 | Stage 3–4 CKD | RCT | 1 year |
Oral 1,25(OH)2D (0.25–0.50 mcg/d) vs. placebo |
Bone biopsy | 1,25(OH)2D ameliorated histological signs of secondary hyperparathyroidism |
| Llach et al. 139 | 1998 | 35 | HD | RCT | 4 weeks |
Intravenous paricalcitol (0.04–0.24 mcg/kg three times weekly) vs. Placebo |
Development of bone pain | No differences between groups. |
| Watson et al. 140 | 1998 | 12 |
CAPD (children) |
RCT | 6 months |
Oral alfacalcidol (10–20 ng/kg/d) vs. no treatment |
Bone biopsy Radiological signs of secondary hyperparathyroidism |
Significant reduction in osteoid index and seam in alfacalcidol group. More patients developed sub‐periosteal erosions on radiography in the no treatment group. |
| Delmez et al. 141 | 2000 | 15 | HD | RCT | 1 year |
Intravenous 1,25(OH)2D (0.5–2.0 mcg) plus CaCO3 vs. CaCO3 alone (control) |
Fracture risk | No differences between groups. |
| Mager et al. 142 | 2016 | 60 | Stage 1–4 CKD | RCT | 6 months |
Oral cholecalciferol (2000 IU/d) vs. oral cholecalciferol (40,000 IU/month) |
Bone mineral density |
No differences between groups. Patients with 25(OH)D ≥ 30 ng/mL was associated with significant improved physical functioning (secondary outcome). |
CAPD, continuous ambulatory peritoneal dialysis; CKD, chronic kidney disease; HD, haemodialysis; RCT, randomized controlled trial.
Effect of vitamin D supplementation on risk of falls, muscle mass and strength, and physical performance in chronic kidney disease
Although extensive literature has shown that supplementation with vitamin D in the general population has a positive effect on skeletal muscle dysfunction including falls, strength, and athletic performance,40, 50, 145, 146 there is not enough evidence to address the role of vitamin D on musculoskeletal outcomes in CKD population.147 Musculoskeletal outcomes have not usually been considered in most of existing trials. Although it may be argued that intervention time was too short, in a recent randomized trial providing oral cholecalciferol vs. placebo to haemodialysis patients, no difference in the frequency of falls was noted after 6 months.148 Similarly, only few small studies have addressed the effect of vitamin D on muscle metabolic pathways in renal population.149, 150 Whereas in general vitamin D does not seem to have any additional benefit on glucose homeostasis and insulin sensitivity,151 repletion with ergocalciferol may assist in improving glycaemic control in CKD patients.150
Any vitamin D benefit on muscle strength is likely to occur in patients with severe vitamin D deficiency. In an interventional study that included both non‐dialysis CKD stage 3–4 and peritoneal dialysis patients with severe vitamin D deficiency [mean 25(OH)D < 7 ng/mL (17.5 nmol/L)], vitamin D supplementation was found to improve physical performance significantly, evaluated by the time to up and go test, gait speed test, the timed chair stand test, and the stair climb test.152 However, no definite conclusions can be yet drawn from this emerging evidence and the question of whether vitamin D supplementation is effective for muscle outcomes remains unanswered.
Controversies in the definition of vitamin D insufficiency in chronic kidney disease
The optimal levels of 25(OH)D and the definition of vitamin D insufficiency remain controversial both for the general population and for patients with CKD.153, 154 Whereas KDIGO and the US Society of Endocrinology favour maintaining 25(OH)D levels between 30 to 50 ng/mL (75 to 125 nmol/L),154, 155 the Institute of Medicine and the World Health Organisation favour the range 20 to 40 ng/mL (50 to 100 nmol/L).156, 157 Differences in these recommended target ranges are attributed to controversies regarding 25(OH)D intestinal calcium absorption, maximal suppression of PTH, or optimal levels to prevent a clinical end‐point such as fracture or death:
Adequate intestinal calcium absorption. The adequate 25(OH)D levels to guarantee sufficient substrate for its conversion to 1,25(OH)2D and ensure optimal calcium absorption has been estimated to be >4.4 ng/mL (11 nmol/L).158 However, this definition may be unsuitable for CKD patients, in whom calcium absorption and 1,25(OH)2D production are impaired.159
Maximal suppression of PTH. Based on the inflexion point at which PTH secretion is suppressed to a minimum in its relation to 25(OH)D levels in the general population,160 KDIGO guidelines suggest to maintain serum 25(OH)D levels >30 ng/mL (75 nmol/L) in CKD patients.154 Other experts, however, estimate that 25(OH)D > 20 ng/mL (50 nmol/L) are adequate to suppress PTH.157 Although there is also an inverse relationship between 25(OH)D and PTH levels in CKD patients,161, 162 this pathophysiological definition is possibly inappropriate in these patients, given that PTH secretion is influenced by several factors related to the uremic state (such as hypocalcaemia or hyperphosphatemia), independently of 25(OH)D levels.163
Fracture prevention. In non‐CKD population, vitamin D supplementation to achieve the 25(OH)D target concentration of 28 to 40 ng/mL (70 to 100 nmol/L) lowered fracture risk.164, 165, 166 However, cross‐sectional studies do not agree on the 25(OH)D threshold level needed to maximize BMD and even suggest that BMD may not improve with vitamin D supplementation once baseline levels of 25(OH)D are >20 ng/mL.153 Moreover, chronic 25(OH)D levels >40 ng/mL (100 nmol/L) after a single annual dose of 500 000 IU of cholecalciferol increased the risk of fractures.167 Interventional data are lacking in CKD patients, and the optimal 25(OH)D concentration for fracture risk reduction may only be inferred from observational studies.133 In a small cross‐sectional study including 130 patients on haemodialysis, 25(OH)D < 8 ng/mL (20 nmol/L) was independently associated with increased risk for bone fractures.117
Death prevention. Observational studies in both dialysis and non‐dialysis patients have examined the prognostic value of 25(OH)D levels. Wolf et al. showed that among incident haemodialysis patients, those with 25(OH)D levels < 10 ng/mL (25 nmol/L) were at increased risk of 90 day mortality, compared with subjects with 25(OH)D > 30 ng/mL (75 nmol/L). The risk for cardiovascular‐related mortality was also higher for patients with 25(OH)D between 10 to 30 ng/mL (25 to 75 nmol/L).168 Similar data have been reported for non‐dialysis patients in two prospective studies of small sample size.169, 170 We have recently examined the prognostic value of 25(OH)D levels among 470 non‐dialysis 3–5 stage CKD patients, and observed consistent associations between 25(OH)D levels and the risk of death, kidney progression, and hospitalization, with the respective concentrations of 17.4 ng/mL (43.4 nmol/L), 18.6 ng/mL (46.4 nmol/L), and 19.0 ng/mL (47.4 nmol/L), denoting the highest risk prediction sensitivity and specificity.171
There are currently insufficient data to determine the safe upper limit of serum 25(OH)D.153 Although the safety margin to minimize the risk of hypercalcaemia as 25(OH)D equal to 100 ng/mL (250 nmol/L), there are some concerns at serum 25(OH)D levels above 50 ng/mL (125 nmol/L). These concerns are based upon conflicting observational studies describing an increased risk for fractures, ischaemic cardiopathy, and some cancers, with levels above 30 to 48 ng/mL (75 to 120 nmol/L).167, 172, 173, 174, 175 Based on a recent analysis from the 2007–2010 National Health and Nutrition Examination Survey, proposals for lowering the cut‐off for vitamin D deficiency to 12.5 ng/mL (31.2 nmol/L) have emerged.176 CKD patients may be at special risk of overscreening and overtreatment of vitamin D, and vitamin D excess may be also a risk contributor for vascular calcifications.177
Using randomized clinical trials from the general population as the main guideline, we conclude that levels below 20 ng/mL (50 nmol/L) are likely suboptimal for skeletal health, which is in agreement with current experts' recommendations.154, 155, 156, 157 The recommendation of targeting 25(OH)D levels of 30 ng/mL (75 nmol/L) may be beneficial for skeletal and extraskeletal health in CKD patients, but we acknowledge that this statement is based on observational studies and warrants consensus and confirmation.153 Although future trials will guide us to determine the optimal 25(OH) levels for dialysis patients, currently available data suggest that vitamin D administration may confer a survival benefit.178
Conclusion
In addition to control bone metabolism and calcium homeostasis, growing evidence suggests that vitamin D plays a key role for muscle function and metabolism in health and CKD. Mechanistically, vitamin D exerts both genomic and rapid effects on bone and muscle metabolism. Furthermore, vitamin D may represent a pathway by which bone and muscle may work together, enabling cross‐talk between these tissues. Observational studies have shown that CKD patients with vitamin D deficiency have an increased risk of reduced BMD and of skeletal fractures, presumably due to mineralization defects, although the evidence is less strong in CKD than in the general population. Likewise, the clinical symptoms and muscle morphological changes observed in CKD patients correlate with 25(OH)D levels, similarly to that observed in subjects with hypovitaminosis D of other origin. Lastly, although some interventional studies have shown that vitamin D supplementation may improve physical performance and bone health in CKD patients, the limited evidence does not allow a certain conclusion about the definitive role of vitamin D supplementation on musculoskeletal outcomes in this population. However, this lack of evidence does not necessarily indicate that vitamin D supplementation has no effect on musculoskeletal health. Moreover, given that vitamin D supplementations is safe and cost‐effective, it can be considered to improve muscle strength and physical performance in CKD patients, especially those who have 25(OH)D levels below 20 ng/mL (50 nmol/L).
Conflict of interest
P.M. has received speaking honoraria from Vifor‐Pharma‐Fresenius Medical Care and Abbott Nutrition. J.J.C. has received speaking honoraria from Abbott Nutrition and Baxter Healthcare; institutional grants from AstraZeneca and Vifor‐Pharma. J.B. has received speaking honoraria from Abbvie, Amgen, and Shire; fees as a consultant for Abbvie, Amgen, Vifor/Fresenius‐Pharma, Chugai, Medice, Genzyme/Sanofi, and Sanifit. P.C. acknowledges speaker honoraria from Fresenius Kabi and Vifor Pharma. S.M. has received speaker honoraria from Abbvie and Amgen. P.U.T. has received personal fees and grants from Abbie, Amgen, Astellas, Genzyme‐Sanofi, Hemotech, and Vifor‐Pharma‐Fresenius Medical Care.
Acknowledgements
The European Renal Nutrition (ERN) and the Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD) working groups are initiatives of and supported by the European Renal Association‐European Dialysis Transplant Association (ERA‐EDTA). The authors thank to the website http://www.free‐anatomy‐quiz.com/ for sharing free images used on the figures of this article.
The following are the board members of the ERN working group: Denis Fouque (France); Juan J Carrero (Sweden); Daniel Teta (Switzerland); Vincenzo Bellizi (Italy); Christoph Wanner (Germany); Peter Ter Wee (Netherlands); Siren Sezer (Turkey); Lina Johansson (United Kingdom); Philippe Chauveau (France); and Pablo Molina (Spain).
The following are the board members of the CKD‐MBD working group: Mario Cozzolino (Italy); Marc Vervloet (Netherlands); Vincent Brandenburg (Germany); Jordi Bover (Spain); Adrian Covic (Romania); Pieter Evenepoel (Belgium); David Goldsmith (United Kingdom); Ziad Massy (France); Sandro Mazzaferro (Italy); and Pablo Ureña‐Torres (France).
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.179
Molina, P. , Carrero, J. J. , Bover, J. , Chauveau, P. , Mazzaferro, S. , Torres, P. U. , and for the European Renal Nutrition (ERN) and Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD) Working Groups of the European Renal Association–European Dialysis Transplant Association (ERA‐EDTA) (2017) Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. Journal of Cachexia, Sarcopenia and Muscle, 8: 686–701. doi: 10.1002/jcsm.12218.
References
- 1. Gunton JE, Girgis CM, Baldock PA, Lips P. Bone muscle interactions and vitamin D. Bone 2015;80:89–94. [DOI] [PubMed] [Google Scholar]
- 2. Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int 2015;2015:953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanner SB, Harwell SA. More than healthy bones: a review of vitamin D in muscle health. Ther Adv Musculoskelet Dis 2015;7:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Górriz JL, Molina P, Bover J, et al. Characteristics of bone mineral metabolism in patients with stage 3‐5 chronic kidney disease not on dialysis: results of the OSERCE study. Nefrologia 2013;33:46–60. [DOI] [PubMed] [Google Scholar]
- 5. Kittiskulnam P, Carrero JJ, Chertow GM, Kaysen GA, Delgado C, Johansen KL. Sarcopenia among patients receiving hemodialysis: weighing the evidence. J Cachexia Sarcopenia Muscle 2016. https://doi.org/10.1002/jcsm.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalantar‐Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 2013;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar‐Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2011;2:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 2007;92:2058–2065. [DOI] [PubMed] [Google Scholar]
- 9. Girgis CM, Mokbel N, Cha KM, et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25‐hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014;155:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abboud M, Puglisi DA, Davies BN, et al. Evidence for a specific uptake and retention mechanism for 25‐hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 2013; 154:3022–3030. [DOI] [PubMed] [Google Scholar]
- 11. Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care 2009;12:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carrero JJ, Chmielewski M, Axelsson J, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008;27:557–564. [DOI] [PubMed] [Google Scholar]
- 13. Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014;9:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kutner NG, Zhang R, Huang Y, Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US Renal Data System special study. Am J Kidney Dis 2015;66:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stenvinkel P, Carrero JJ, von Walden F, Ikizler TA, Nader GA. Muscle wasting in end‐stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant 2016;31:1070–1077. [DOI] [PubMed] [Google Scholar]
- 16. Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar‐Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2011;2:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein‐energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77–90. [DOI] [PubMed] [Google Scholar]
- 18. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014;21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr 1985;42:644–649. [DOI] [PubMed] [Google Scholar]
- 20. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 21. Jüppner H. Phosphate and FGF‐23. Kidney Int Suppl 2011;121:S24–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kolek OI, Hines ER, Jones MD, et al. 1alpha,25‐Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal‐gastrointestinal‐skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 2005;289:G1036–G1042. [DOI] [PubMed] [Google Scholar]
- 23. Prié D, Ureña Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int 2009;75:882–889. [DOI] [PubMed] [Google Scholar]
- 24. Murali SK, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben RG. FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho‐independent manner. J Bone Miner Res 2016;31:129–142. [DOI] [PubMed] [Google Scholar]
- 25. Bover J, Ruiz CE, DaSilva M, Diaz M, Guillen E. In Urena Torres P, Cozzolino M, Vervloet, eds. Vitamin D receptor and interaction with DNA: from physiology to chronic kidney disease. Vitamin D in Chronic Kidney Disease: Cham, Switzerland: Springer; 2016. [Google Scholar]
- 26. Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Jurutka PW. Nuclear Vitamin D Receptor: Natural Ligands, Molecular Structure‐Function, and Transcriptional Control of Vital Genes. In David Felman JW Pike, Adams JS, eds. Vitamin D San Diego, CA: Elsevier; 2011. p137–169. [Google Scholar]
- 27. Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 2010;9:709–715. [DOI] [PubMed] [Google Scholar]
- 28. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)‐mediated actions of 1α,25(OH)₂vitamin D₃: genomic and non‐genomic mechanisms. Best Pract Res Clin Endocrinol Metab 2011;25:543–559. [DOI] [PubMed] [Google Scholar]
- 29. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116:2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panda DK, Miao D, Bolivar I, et al. Inactivation of the 25‐hydroxyvitamin D 1alpha‐hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 2004. 16;279:16754–16766. [DOI] [PubMed] [Google Scholar]
- 31. Goltzman D. Use of genetically modified mice to examine the skeletal anabolic activity of vitamin D. J Steroid Biochem Mol Biol 2007;103:587–591. [DOI] [PubMed] [Google Scholar]
- 32. He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β‐catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 2011;22:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al‐Aly Z. Arterial calcification: a tumor necrosis factor‐alpha mediated vascular Wnt‐opathy. Transl Res 2008;151:233–239. [DOI] [PubMed] [Google Scholar]
- 34. Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77–98. [DOI] [PubMed] [Google Scholar]
- 35. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79:362–371. [DOI] [PubMed] [Google Scholar]
- 36. Kaludjerovic J, Vieth R. Relationship between vitamin D during perinatal development and health. J Midwifery Womens Health 2010;55:550–560. [DOI] [PubMed] [Google Scholar]
- 37. Girgis CM, Clifton‐Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev 2013;34:33–83. [DOI] [PubMed] [Google Scholar]
- 38. Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports 2010;20:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int 2013;92:151–162. [DOI] [PubMed] [Google Scholar]
- 40. Girgis CM, Clifton‐Bligh RJ, Turner N, Lau SL, Gunton JE. Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf) 2014;80:169–181. [DOI] [PubMed] [Google Scholar]
- 41. Srikuea R, Zhang X, Park‐Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol 2012;303:C396–C405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab 2013;98:E509–E513. [DOI] [PubMed] [Google Scholar]
- 43. Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D. Myopathy in chronic renal failure. Q J Med 1974;43:509–524. [PubMed] [Google Scholar]
- 44. Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab 2010;95:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gagnon C, Lu ZX, Magliano DJ, et al. Serum 25‐hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population‐based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 2011;34:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non‐diabetic chronic hemodialysis patients. Kidney Int 2007;71:146–152. [DOI] [PubMed] [Google Scholar]
- 47. Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 2000;66:419–424. [DOI] [PubMed] [Google Scholar]
- 48. Yoshikawa S, Nakamura T, Tanabe H, Imamura T. Osteomalacic myopathy. Endocrinol Jpn 1979;26:65–72. [DOI] [PubMed] [Google Scholar]
- 49. Tagliafico AS, Ameri P, Bovio M, et al. Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol 2010;194:728–734. [DOI] [PubMed] [Google Scholar]
- 50. Ceglia L, Niramitmahapanya S, da Silva MM, et al. A randomized study on the effect of vitamin D₃ supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 2013;98:E1927–E1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sørensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha‐hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:157–161. [DOI] [PubMed] [Google Scholar]
- 52. Sato Y, Iwamoto J, Kanoko T, Satoh K. Low‐dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis 2005;20:187–192. [DOI] [PubMed] [Google Scholar]
- 53. Cipriani C, Pepe J, Piemonte S, Colangelo L, Cilli M, Minisola S. Vitamin d and its relationship with obesity and muscle. Int J Endocrinol 2014;2014:841248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int. 2014;2014:121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Visser M, Deeg DJ, Lips P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–5772. [DOI] [PubMed] [Google Scholar]
- 56. Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 2008;23:1859–1863. [DOI] [PubMed] [Google Scholar]
- 57. Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem 2015;116:687–695. [DOI] [PubMed] [Google Scholar]
- 58. Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011;152:2976–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. White TA, LeBrasseur NK. Myostatin and sarcopenia: opportunities and challenges ‐ a mini‐review. Gerontology 2014;60:289–293. [DOI] [PubMed] [Google Scholar]
- 60. Elkasrawy MN, Hamrick MW. Myostatin (GDF‐8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 2010;10:56–63. [PMC free article] [PubMed] [Google Scholar]
- 61. Garcia LA, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)(2)vitamin D(3) enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C(2)C(12) skeletal muscle cells. J Steroid Biochem Mol Biol 2013;133:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF‐1 directly regulate bone growth and density. J Clin Invest 2002;110:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 2002;99(15):9656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barker T, Schneider ED, Dixon BM, Henriksen VT, Weaver LK. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr Metab (Lond) 2013;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun 2014;450:482–487. [DOI] [PubMed] [Google Scholar]
- 66. Kaji H. Interaction between muscle and bone. J Bone Metab 2014;21:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012;50:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 2016;23:1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haroon M, FitzGerald O. Vitamin D deficiency: subclinical and clinical consequences on musculoskeletal health. Curr Rheumatol Rep 2012;14:286–293. [DOI] [PubMed] [Google Scholar]
- 71. Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD‐related metabolic complications. J Am Soc Nephrol 2009;20:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacob AI, Sallman A, Santiz Z, Hollis BW. Defective photoproduction of cholecalciferol in normal and uremic humans. J Nutr 1984;114:1313–1319. [DOI] [PubMed] [Google Scholar]
- 73. Ureña‐Torres P, Metzger M, Haymann JP, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis 2011;58:544–553. [DOI] [PubMed] [Google Scholar]
- 74. Christensen EI, Willnow TE. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol 1999;10:2224–2236. [DOI] [PubMed] [Google Scholar]
- 75. Sato KA, Gray RW, Lemann J Jr. Urinary excretion of 25‐hydroxyvitamin D in health and the nephrotic syndrome. J Lab Clin Med 1982;99:325–330. [PubMed] [Google Scholar]
- 76. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am 2010;39:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y. Decreased 1,25‐dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 1993;92:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hsu CH, Patel SR. Altered vitamin D metabolism and receptor interaction with the target genes in renal failure: calcitriol receptor interaction with its target gene in renal failure. Curr Opin Nephrol Hypertens 1995;4:302–306. [DOI] [PubMed] [Google Scholar]
- 80. Patel SR, Ke HQ, Vanholder R, Koenig RJ, Hsu CH. Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J Clin Invest 1995;96:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton. Cell 2011;144:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf‐1 in renal osteodystrophy. Clin J Am Soc Nephrol 2011;6:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sabbagh Y, Graciolli FG, O'Brien S, et al. Repression of osteocyte Wnt/β‐catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 2012;27:1757–1772. [DOI] [PubMed] [Google Scholar]
- 84. Evenepoel P, D'Haese P, Brandenburg V. Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 2015;88:235–240. [DOI] [PubMed] [Google Scholar]
- 85. Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013;153:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ureña P, Bernard‐Poenaru O, Ostertag A, et al. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant 2003;18:2325–2331. [DOI] [PubMed] [Google Scholar]
- 87. Riggs BL, Wahner HW, Seeman E, et al. Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest 1982;70:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ott SM. Review article: bone density in patients with chronic kidney disease stages 4‐5. Nephrology (Carlton) 2009;14:395–403. [DOI] [PubMed] [Google Scholar]
- 89. Yenchek RH, Ix JH, Shlipak MG, et al. Health, aging, and body composition study. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 2012;7:1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Limori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single‐center cohort study. Nephrol Dial Transplant 2012;27:345–351. [DOI] [PubMed] [Google Scholar]
- 91. Naylor KL, Leslie WD, Hodsman AB, Rush DN, Garg AX. FRAX predicts fracture risk in kidney transplant recipients. Transplantation 2014;97:940–945. [DOI] [PubMed] [Google Scholar]
- 92. Nickolas TL. The utility of circulating markers to predict bone loss across the CKD spectrum. Clin J Am Soc Nephrol 2014;9:1160–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis 2014;64:290–304. [DOI] [PubMed] [Google Scholar]
- 94. www.kdigo.org
- 95. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease‐mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int 2015;87:502–528. [DOI] [PubMed] [Google Scholar]
- 96. Goldenstein PT, Jamal SA, Moysés RM. Fractures in chronic kidney disease: pursuing the best screening and management. Curr Opin Nephrol Hypertens 2015;24:317–323. [DOI] [PubMed] [Google Scholar]
- 97. Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–1953. [DOI] [PubMed] [Google Scholar]
- 98. Torres PU, Bover J, Mazzaferro S, de Vernejoul MC, Cohen‐Solal M. When, how, and why a bone biopsy should be performed in patients with chronic kidney disease. Semin Nephrol 2014;34:612–625. [DOI] [PubMed] [Google Scholar]
- 99. Campistol JM. Uremic myopathy. Kidney Int 2002;62:1901–1913. [DOI] [PubMed] [Google Scholar]
- 100. Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol 2009;5:542–551. [DOI] [PubMed] [Google Scholar]
- 101. Patel SS, Molnar MZ, Tayek JA, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross‐sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013;4:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrology Dialysis Transplantation 2014;29:1655–1665. [DOI] [PubMed] [Google Scholar]
- 103. Diesel W, Emms M, Knight BK, et al. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis 1993;22:677–684. [DOI] [PubMed] [Google Scholar]
- 104. Fahal IH, Bell GM, Bone JM, Edwards RH. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 1997;12:119–127. [DOI] [PubMed] [Google Scholar]
- 105. Souza VA, Dd O, Mansur HN, Fernandes NM, Bastos MG. Sarcopenia in chronic kidney disease. J Bras Nefrol 2015;37:98–105. [DOI] [PubMed] [Google Scholar]
- 106. Carrero JJ, Johansen KL, Lindholm B, et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016;90:53–66. [DOI] [PubMed] [Google Scholar]
- 107. Kaizu Y, Ohkawa S, Odamaki M, et al. Association between inflammatory mediators and muscle mass in long‐term hemodialysis patients. Am J Kidney Dis 2003;42:295–302. [DOI] [PubMed] [Google Scholar]
- 108. Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014;9:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stenvinkel P, Alvestrand A. Inflammation in end‐stage renal disease: sources, consequences, and therapy. Semin Dial 2002;15:329–337. [DOI] [PubMed] [Google Scholar]
- 110. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF‐kappaB‐induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 2000;289:2363–2366. [DOI] [PubMed] [Google Scholar]
- 111. Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein‐energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77–90. [DOI] [PubMed] [Google Scholar]
- 112. Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen‐Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor‐kappaB. FASEB J 2001;15:1169–1180. [DOI] [PubMed] [Google Scholar]
- 113. Mitch WE, Du J, Bailey JL, Price SR. Mechanisms causing muscle proteolysis in uremia: the influence of insulin and cytokines. Miner Electrolyte Metab 1999;25:216–219. [DOI] [PubMed] [Google Scholar]
- 114. Zhang L, Du J, Hu Z, et al. IL‐6 and serum amyloid A synergy mediates angiotensin II‐induced muscle wasting. J Am Soc Nephrol 2009;20:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin‐proteasome pathway. N Engl J Med 1996;335:1897–1905. [DOI] [PubMed] [Google Scholar]
- 116. Coen G, Mantella D, Manni M, et al. 25‐hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int 2005;68:1840–1848. [DOI] [PubMed] [Google Scholar]
- 117. Ambrus C, Almasi C, Berta K, et al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol 2011;43:475–482. [DOI] [PubMed] [Google Scholar]
- 118. Elder GJ, Mackun K. 25‐Hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res 2006;21:1778–1784. [DOI] [PubMed] [Google Scholar]
- 119. Mucsi I, Almási C, Deák G, et al. Serum 25(OH)‐vitamin D levels and bone metabolism in patients on maintenance hemodialysis. Clin Nephrol 2005;64:288–294. [DOI] [PubMed] [Google Scholar]
- 120. Ghazali A, Fardellone P, Pruna A, et al. Is low plasma 25‐(OH)vitamin D a major risk factor for hyperparathyroidism and Looser's zones independent of calcitriol? Kidney Int 1999;55:2169–2177. [DOI] [PubMed] [Google Scholar]
- 121. Brunerová L, Ronová P, Verešová J, et al. Osteoporosis and impaired trabecular bone score in hemodialysis patients. Kidney Blood Press Res 2016;41:345–354. [DOI] [PubMed] [Google Scholar]
- 122. Moorthi RN, Moe SM. Recent advances in the noninvasive diagnosis of renal osteodystrophy. Kidney Int 2013;84:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Leonard MB. A structural approach to skeletal fragility in chronic kidney disease. Semin Nephrol 2009;29:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol 2013;9:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res 2002;17:891–897. [DOI] [PubMed] [Google Scholar]
- 126. Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 2002;75:611–615. [DOI] [PubMed] [Google Scholar]
- 127. Ensrud KE, Blackwell TL, Cauley JA, et al. Circulating 25‐hydroxyvitamin D levels and frailty in older men: the osteoporotic fractures in men study. J Am Geriatr Soc 2011;59:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gordon PL, Doyle JW, Johansen KL. Association of 1,25‐dihydroxyvitamin D levels with physical performance and thigh muscle cross‐sectional area in chronic kidney disease stage 3 and 4. J Ren Nutr 2012;22:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zahed N, Chehrazi S, Falaknasi K. The evaluation of relationship between vitamin D and muscle power by micro manual muscle tester in end‐stage renal disease patients. Saudi J Kidney Dis Transpl 2014;25:998–1003. [DOI] [PubMed] [Google Scholar]
- 130. Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta‐analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med 2007;147:840–853. [DOI] [PubMed] [Google Scholar]
- 131. Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev 2009;4:CD005633. [DOI] [PubMed] [Google Scholar]
- 132. Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta‐analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 2011;6:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease‐mineral bone disease (CKD‐MBD). Bonekey Rep 2014;3:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fournier A, Bordier P, Gueris J, et al. Comparison of 1 alpha‐hydroxycholecalciferol and 25‐hydroxycholecalciferol in the treatment of renal osteodystrophy: greater effect of 25‐hydroxycholecalciferol on bone mineralization. Kidney Int 1979;15:196–204. [DOI] [PubMed] [Google Scholar]
- 135. Memmos DE, Eastwood JB, Talner LB, et al. Double‐blind trial of oral 1,25‐dihydroxy vitamin D3 versus placebo in asymptomatic hyperparathyroidism in patients receiving maintenance haemodialysis. Br Med J (Clin Res Ed) 1981;282:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Morinière P, Fournier A, Leflon A, et al. Comparison of 1 alpha‐OH‐vitamin D3 and high doses of calcium carbonate for the control of hyperparathyroidism and hyperaluminemia in patients on maintenance dialysis. Nephron 1985;39:309–315. [DOI] [PubMed] [Google Scholar]
- 137. Baker LR, Muir JW, Sharman VL, et al. Controlled trial of calcitriol in hemodialysis patients. Clin Nephrol 1986;26:185–191. [PubMed] [Google Scholar]
- 138. Baker LR, Abrams L, Roe CJ, et al. 1,25(OH)2D3 administration in moderate renal failure: a prospective double‐blind trial. Kidney Int 1989;35:661–669. [DOI] [PubMed] [Google Scholar]
- 139. Llach F, Keshav G, Goldblat MV, et al. Suppression of parathyroid hormone secretion in hemodialysis patients by a novel vitamin D analogue: 19‐nor‐1,25‐dihydroxyvitamin D2. Am J Kidney Dis 1998;32:S48–S54. [DOI] [PubMed] [Google Scholar]
- 140. Watson AR, Kooh SW, Tam CS, Reilly BJ, Balfe JW, Vieth R. Renal osteodystrophy in children on CAPD: a prospective trial of 1‐alpha‐hydroxycholecalciferol therapy. Child Nephrol Urol 1988. ‐1989;9:220–227. [PubMed] [Google Scholar]
- 141. Delmez JA, Kelber J, Norwood KY, Giles KS, Slatopolsky E. A controlled trial of the early treatment of secondary hyperparathyroidism with calcitriol in hemodialysis patients. Clin Nephrol 2000;54:301–308. [PubMed] [Google Scholar]
- 142. Mager DR, Jackson ST, Hoffmann MR, Jindal K, Senior PA. Vitamin D3 supplementation, bone health and quality of life in adults with diabetes and chronickidney disease: Results of an open label randomized clinical trial. Clin Nutr 2016; pii: S0261‐5614(16)30108‐X. [DOI] [PubMed] [Google Scholar]
- 143. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mehrotra A, Leung WY, Joson T. Nutritional vitamin D supplementation and health‐related outcomes in hemodialysis patients: a protocol for a systematic review and meta‐analysis. Syst Rev 2015;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2011;96:2997–3006. [DOI] [PubMed] [Google Scholar]
- 146. Morley JE, Argiles JM, Evans WJ, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 2010;11:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kalantar‐Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1529–1539. [DOI] [PubMed] [Google Scholar]
- 148. Miskulin DC, Majchrzak K, Tighiouart H, et al. Ergocalciferol supplementation in hemodialysis patients with vitamin d deficiency: a randomized clinical trial. J Am Soc Nephrol 2016;27:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. de Boer IH, Sachs M, Hoofnagle AN, et al. Paricalcitol does not improve glucose metabolism in patients with stage 3‐4 chronic kidney disease. Kidney Int 2013;83:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Blair D, Byham‐Gray L, Lewis E, McCaffrey S. Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr 2008;18:375–382. [DOI] [PubMed] [Google Scholar]
- 151. Seida JC, Mitri J, Colmers IN, et al. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2014;99:3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol 2011;76:110–116. [DOI] [PubMed] [Google Scholar]
- 153. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: a critical analysis on the basis of evidence‐based medicine. J Clin Endocrinol Metab 2013;98:E1283–E1304. [DOI] [PubMed] [Google Scholar]
- 154. Kidney Disease: Improving Global Outcomes (KDIGO) CKD‐MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD). Kidney Int Suppl 2009;S1–130. [DOI] [PubMed] [Google Scholar]
- 155. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 156. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium: Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/. [PubMed] [Google Scholar]
- 157. WHO Scientific Group on the Prevention and Management of Osteoporosis 2003 Prevention and management of osteoporosis: report of a WHO scientific group. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/10665/42841/1/WHO_TRS_921.pdf (last accessed on 26th March 2017)
- 158. Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 2008;23:1859–1863. [DOI] [PubMed] [Google Scholar]
- 159. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low and high calcium diets. Kidney Int 2012;81:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Vieth R, Ladak Y, Walfish PG. Age‐related changes in the 25‐hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 2003;88:185–191. [DOI] [PubMed] [Google Scholar]
- 161. González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 2004;24:503–510. [DOI] [PubMed] [Google Scholar]
- 162. Molina P, Górriz JL, Molina MD, et al. The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant 2014;29:97–109. [DOI] [PubMed] [Google Scholar]
- 163. Metzger M, Houillier P, Gauci C, et al. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: quest for a threshold. J Clin Endocrinol Metab 2013;98:2922–2928. [DOI] [PubMed] [Google Scholar]
- 164. Dawson‐Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670–676. [DOI] [PubMed] [Google Scholar]
- 165. Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 2002;13:257–264. [DOI] [PubMed] [Google Scholar]
- 166. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815–1822. [DOI] [PubMed] [Google Scholar]
- 168. Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007;72:1004–1013. [DOI] [PubMed] [Google Scholar]
- 169. Barreto DV, Barreto FC, Liabeuf S, et al. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 2009;75:88–95. [DOI] [PubMed] [Google Scholar]
- 171. Molina P, Górriz JL, Molina MD, et al. What is the optimal level of vitamin D in non‐dialysis chronic kidney disease population? World J Nephrol 2016;5:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dror Y, Giveon SM, Hoshen M, Feldhamer I, Balicer RD, Feldman BS. Vitamin D levels for preventing acute coronary syndrome and mortality: evidence of a nonlinear association. J Clin Endocrinol Metab 2013;98:2160–2167. [DOI] [PubMed] [Google Scholar]
- 173. Stolzenberg‐Solomon RZ, Vieth R, Azad A, et al. A prospective nested case‐control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 2006;66:10213–10219. [DOI] [PubMed] [Google Scholar]
- 174. Stolzenberg‐Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25‐hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ahn J, Peters U, Albanes D, et al. Serum vitamin D concentration and prostate cancer risk: a nested case‐control study. J Natl Cancer Inst 2008;100:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency ‐ is there really a pandemic? N Engl J Med 2016;375:1817–1820. [DOI] [PubMed] [Google Scholar]
- 177. Drüeke TB, Massy ZA. Role of vitamin D in vascular calcification: bad guy or good guy? Nephrol Dial Transplant 2012;27:1704–1707. [DOI] [PubMed] [Google Scholar]
- 178. Cheng S, Coyne D. Vitamin D and outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 2007;16:77–82. [DOI] [PubMed] [Google Scholar]
- 179. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle J Cachexia Sarcopenia Muscle. 2015;4:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


