Abstract
Background
Patients experiencing disuse atrophy report acute loss of skeletal muscle mass which subsequently leads to loss of strength and physical capacity. In such patients, especially the elderly, complete recovery remains a challenge even with improved nutrition and resistance exercise. This study aimed to explore the clinical potential of bimagrumab, a human monoclonal antibody targeting the activin type II receptor, for the recovery of skeletal muscle volume from disuse atrophy using an experimental model of lower extremity immobilization.
Methods
In this double‐blind, placebo‐controlled trial, healthy young men (n = 24; mean age, 24.1 years) were placed in a full‐length cast of one of the lower extremities for 2 weeks to induce disuse atrophy. After cast removal, subjects were randomized to receive a single intravenous (i.v.) dose of either bimagrumab 30 mg/kg (n = 15) or placebo (n = 9) and were followed for 12 weeks. Changes in thigh muscle volume (TMV) and inter‐muscular adipose tissue (IMAT) and subcutaneous adipose tissue (SCAT) of the thigh, maximum voluntary knee extension strength, and safety were assessed throughout the 12 week study.
Results
Casting resulted in an average TMV loss of −4.8% and comparable increases in IMAT and SCAT volumes. Bimagrumab 30 mg/kg i.v. resulted in a rapid increase in TMV at 2 weeks following cast removal and a +5.1% increase above pre‐cast levels at 12 weeks. In comparison, TMV returned to pre‐cast level at 12 weeks (−0.1%) in the placebo group. The increased adiposity of the casted leg was sustained in the placebo group and decreased substantially in the bimagrumab group at Week 12 (IMAT: −6.6%, SCAT: −3.5%). Knee extension strength decreased by ~25% in the casted leg for all subjects and returned to pre‐cast levels within 6 weeks after cast removal in both treatment arms. Bimagrumab was well tolerated with no serious or severe adverse events reported during the study.
Conclusions
A single dose of bimagrumab 30 mg/kg i.v. safely accelerated the recovery of TMV and reversal of accumulated IMAT following 2 weeks in a joint‐immobilizing cast.
Keywords: Activin type II receptor, Bimagrumab, Disuse atrophy, Inter‐muscular adipose tissue, Thigh muscle volume
Introduction
Loss of skeletal muscle mass due to inactivity, commonly known as disuse atrophy, has been described in patients experiencing short‐term immobilization of a joint (casting), prolonged bed rest, or after a hip fracture.1 The extensive loss of skeletal muscle protein due to an imbalance between anabolic and catabolic processes during disuse atrophy can precipitate muscle weakness, reduce physical capacity, and prolong functional disability.2, 3, 4 In addition to contractile protein loss, reduced physical activity has been associated with increase in inter‐muscular adipose tissue (IMAT), which can further weaken contractility and performance by multiple mechanisms, including the release of pro‐inflammatory cytokines and reduced responsiveness to natural anabolic hormones.5, 6, 7, 8, 9
Disuse atrophy is especially problematic in the elderly.9 Ageing is associated with a reduction in skeletal muscle mass, strength, and physical capacity, leading to impaired mobility, increased sedentary behaviour, and numerous adverse health effects.10 In such individuals, an acute episode of disuse atrophy (e.g. a hip fracture) can lead to disability in terms of mobility, increased hospitalization, and loss of independence, which can culminate in premature mortality.11, 12 Hence, the timely recovery of muscle mass and strength is the key goal for the physical rehabilitation of patients with disuse atrophy.
Although sufficient dietary protein intake and resistance exercise can increase skeletal muscle mass, these measures are often difficult to implement, particularly with older patients. Compliance with increased dietary protein intake and regular intensive exercise training is highly variable and typically unsustainable.1, 13, 14 In addition, the ability to recover from atrophied skeletal muscles is attenuated with age even when combined with improved nutrition and exercise.14, 15, 16 These physiological and behavioural obstacles to optimal recovery of involuntary muscle loss have prompted innovative approaches to treatment. Currently, several novel pharmacotherapeutic approaches are being explored as possible treatments for conditions of skeletal muscle wasting to promote muscle growth and improve functional recovery, particularly in elderly patients.17, 18
Research in the past decade has led to a better understanding of the critical signalling pathways regulating skeletal muscle mass.19, 20, 21 The activin type‐IIB receptor (ActRIIB) and its natural ligands such as myostatin, activin, and growth differentiation factor 11 have been identified to play a key role in modulating muscle growth and maintenance.22, 23 Bimagrumab, a human monoclonal anti‐ActRII antibody, prevents the binding of these ligands to their receptor, which promotes muscle hypertrophy, and accelerates recovery from muscle wasting conditions (e.g. glucocorticoid‐induced atrophy) in animals.24 Translation of this effect of bimagrumab to increase muscle size and function in patients could hold considerable clinical potential.
The purpose of the present study was to explore the clinical potential of bimagrumab to facilitate the full recovery of skeletal muscle mass in an established experimental model of disuse atrophy using single leg casting in healthy, young, male volunteers. Assessment of the pro‐myogenic agent focused on changes in thigh muscle volume (TMV) and adiposity [IMAT and subcutaneous adipose tissue (SCAT)] following intravenous (i.v.) administration of a single dose of bimagrumab 30 mg/kg or placebo at the time of cast removal.
Methods
Subjects
A total of 24 healthy young men aged between 18 and 40 years with a body mass index (BMI) of 18 to 32 kg/m2 participated in the study. Only men were enrolled to avoid confounding by a possible gender difference in the responses to casting, recovery, or the drug.25, 26 The key exclusion criteria were as follows: prior immobilization of the knee or ankle for >2 weeks in the past 12 months; use of testosterone, growth hormone, systemic corticosteroids, or anti‐diabetic medications in the last 3 months prior to screening; inadequate average daily intake of dietary protein (<0.6 g/kg/day) or calories (<20 kcal/kg/day) for body weight; presence of a metal implant (screws, nails, or plate) or prosthesis that would prohibit a magnetic resonance imaging (MRI) assessment; bone fracture of the lower extremities within the last 6 months; positive test result for Factor V Leiden; and a history of known bleeding disorders.
The study was conducted at the Celerion Institute, USA. This single‐site study was approved by the local institutional review board and conducted in adherence to the Declaration of Helsinki. All subjects provided written informed consent prior to study participation.
Induction of disuse atrophy
A light polyester cast was placed on the right lower limb of each subject extending from the groin to the top of the foot, fixing the knee at an angle of 20° and the ankle at ~0°.27 The casting procedure was performed by trained technicians. Subjects were required to wear the cast for two consecutive weeks during which they were domiciled at the investigational site and encouraged to ambulate with crutches.
Study medication
Following removal of the cast, the subjects were randomized in a 3:2 ratio to receive a single dose of either bimagrumab 30 mg/kg i.v. (n = 15) or placebo (n = 9) within 24 h. During the post‐cast phase (Weeks 1–12), all subjects were required to participate in a standardized recovery program of self‐limited activity and a series of exercises designed for recovery of range of motion of the hip, knee, and ankle. A schematic outline of the study design is shown in Figure 1. A block design was used with treatment assignments randomly generated prior to the start of the study. The study was conducted in a double‐blind manner, wherein the subjects, investigators, and sponsor were blinded to the treatment allocation.
Figure 1.
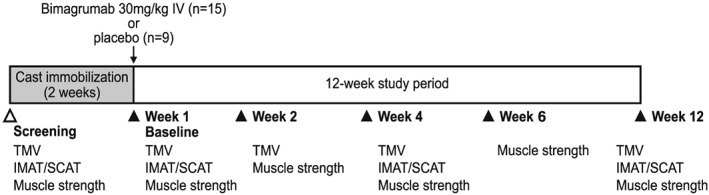
Study design.
Thigh muscle and adipose tissue volumes
Thigh muscle volume, IMAT, and SCAT data (primary endpoints) were obtained for all study participants using a 1.5 T MRI scanner and Q‐body coil. Subjects were not allowed to stand or bear weight for up to 1 h and were maintained in a horizontal position for ~30 min prior to scanning, to promote fluid equilibrium and avoid an artificial increase in TMV. Subjects were positioned carefully by placing folded pads under their buttocks and lower legs to ensure the posterior thigh remained free of compression and to minimize motion during scanning, both of which can alter the accuracy of the measurements. Following a rapid survey scan, lean TMV was assessed based on proton density images of the thigh using a two‐dimensional multi‐slice pulse sequence covering the entire thigh (knee to hip). The TMVs for both the right (casted) and left (non‐casted) lower limbs were measured before casting, after 2 weeks of casting (baseline), and at 2, 4, and 12 weeks after cast removal. The MRI scans were assessed for quality, and both muscle and adipose tissues were quantified by a trained central reader who was not affiliated with the sponsor and was blinded to the treatment assignment. The MRI images were automatically segmented into non‐overlapping class maps representing muscle volume, IMAT, and SCAT. The segmentation was reviewed by a radiologist who made adjustments, if needed, due to artefacts or inhomogeneity of the signal intensity throughout the image. Slice‐by‐slice areas were then computed and multiplied by the number of slices covering the thigh to generate the muscle and respective fat volumes. Muscle and fat mass values were determined and expressed in kilogrammes by multiplying the muscle and fat volumes by density values of 1.0597 and 0.9196 g/cm3, respectively.
Quadriceps strength
Changes in the maximum voluntary force production (strength) of a single limb knee extension from pre‐cast levels were assessed in both legs using the one‐repetition maximum technique, as described in detail elsewhere.28 The assessment was primarily used to document the changes in strength induced by casting.
Safety
Safety assessments were reported throughout the study. The safety of bimagrumab was determined based on physical examination, assessment of vital signs (body temperature, body weight, blood pressure, and heart rate), electrocardiogram (ECG), blood tests (haematology, blood chemistry, and testing for the presence of anti‐drug antibodies), urinalysis, and identification of adverse events (AEs) and serious adverse events.
Statistical analysis
Sample size calculation was based on the anticipated change in TMV from baseline (cast removal) to 4 weeks after study drug administration. The time period of 4 weeks was chosen based on the assumption that the maximum effect of bimagrumab was likely to be observed during this period based on prior human data. Analysis of covariance was used to assess differences between the treatment and placebo groups for all efficacy outcomes at each time point, with baseline values as the covariate. All data are reported as mean (±standard error) unless indicated otherwise.
Results
Subject disposition and characteristics
The subjects were recruited between May 2011 and August 2011. The study population was predominantly Caucasian (79%) with an average (±standard deviation) age of 24.1 ± 6.2 years and a BMI of 25.3 ± 3.8 kg/m2. Subject characteristics and key measures of body composition are summarized in Table 1.
Table 1.
Demographic characteristics of study participants and pre‐cast measures of the main efficacy parameters
|
Bimagrumab 30 mg/kg (n = 15) |
Placebo (n = 9) |
|
|---|---|---|
| Age (years) | 23.5 (4.9) | 25.1 (8.2) |
| Male, n (%) | 15 (100%) | 9 (100%) |
| Race, n (%) | ||
| Caucasian | 12 (80%) | 7 (78%) |
| Black | 1 (7%) | 1 (11%) |
| Other | 2 (13%) | 1 (11%) |
| Height (cm) | 177.7 (7.3) | 177.3 (8.6) |
| Weight (kg) | 79.8 (12.0) | 80.2 (17.9) |
| BMI (kg/m2) | 25.3 (3.8) | 25.3 (3.9) |
| TMV (cm3) | 5237.9 (821.2) | 5010.4 (800.6) |
| IMAT (g) | 390 (118) | 410 (139) |
| SCAT (g) | 2420 (1050) | 2560 (1200) |
Values are represented as mean (SD) unless otherwise indicated.
BMI, body mass index; IMAT, inter‐muscular adipose tissue; SCAT, subcutaneous adipose tissue; SD, standard deviation; TMV, thigh muscle volume.
Magnetic resonance imaging of the thigh
After 2 weeks of immobilization in a fixed‐joint cast, significant decreases in TMV of −4.8% ± 0.7% and −4.6% ± 0.7% were noted in the bimagrumab and placebo groups, respectively (P < 0.01). In subjects who received a single dose of bimagrumab 30 mg/kg i.v. after cast removal, TMV recovered to within −0.8% ± 0.6% of the baseline value in 2 weeks, which further increased to +1.3% ± 0.6% and +5.1% ± 0.9% from baseline at 4 and 12 weeks, respectively. In comparison, subjects who received placebo showed a mean percentage change in TMV from baseline of −4.2% ± 0.8% and −1.6% ± 1.0% at 2 and 4 weeks, respectively, recovering to the pre‐cast baseline value (−0.1% ± 1.7%) at 12 weeks (Figure 2A). Differences in the rate of TMV increase between bimagrumab‐treated and placebo‐treated subjects were statistically significant at all time points (P < 0.01).
Figure 2.
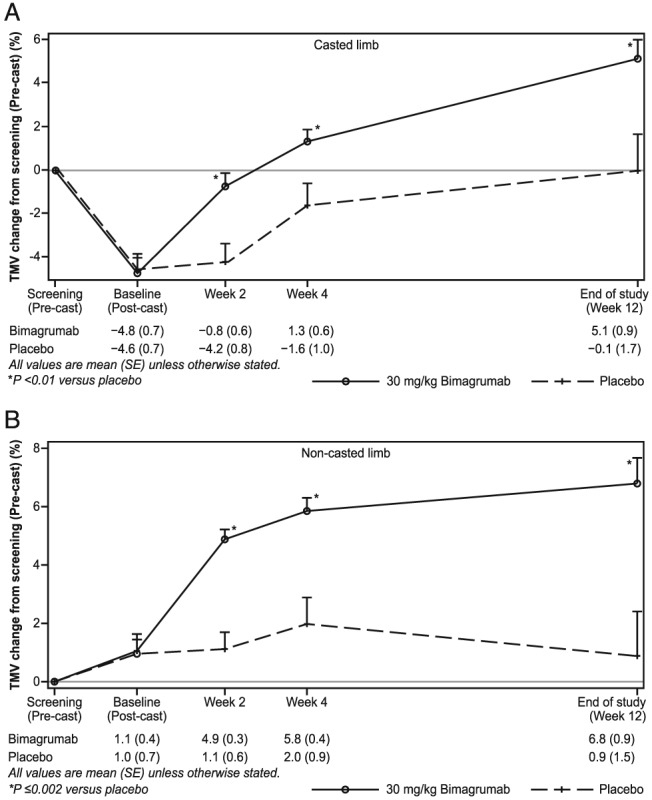
Mean per cent change in thigh muscle volume from pre‐cast level in the (A) casted and (B) non‐casted limb.
Thigh muscle volume in the non‐casted limbs of all subjects showed no significant increase during the casting period but responded differently after receiving treatment (Figure 2B). In the bimagrumab‐treated group, the increases in TMV from pre‐cast levels were +4.9% ± 0.3%, +5.8% ± 0.4%, and +6.8% ± 0.9% at 2, 4, and 12 weeks post dosing, respectively. Comparatively, subjects who received placebo demonstrated changes of +1.1% ± 0.6%, +2.0% ± 0.9%, and +0.9% ± 1.5% at the same time points.
The changes in TMV were accompanied by treatment‐specific effects on IMAT and SCAT volumes (Figure 3, Supplementary Table 1). After 2 weeks in a cast and before receiving treatment, the IMAT volume increased by +4.6% ± 1.2% and +4.8% ± 2.0% in the bimagrumab and placebo groups, respectively. Following treatment with bimagrumab, IMAT levels increased during the first 2 weeks (+7.0% ± 2.2%) followed by a marked decline to below pre‐cast levels over the subsequent weeks after dosing (Week 12, −6.6% ± 1.6%; Figure 3A). In the non‐casted limb, IMAT did not change during casting but began to decline 2 weeks after bimagrumab administration, ending at a level of −6.4% ± 1.5% at 12 weeks. In contrast, IMAT volume remained fairly constant in the placebo group throughout the 12 week follow‐up period with a slight non‐significant trend towards a net increase from pre‐casting values with large variations in individual responses (Week 12: +2.5% ± 5.3%) (Figure 3B). SCAT volume increased during casting with peaks of +3.4% ± 1.0% and +4.6% ± 1.5% in the bimagrumab and placebo groups, respectively. After cast removal and study drug administration, a clear decline was noted in the bimagrumab group starting at Week 4 and ending at −3.5 ± 2.3% at Week 12. SCAT continued to increase in the placebo group with noticeable variations in individual responses (Figure 3C). The pattern of mean changes in SCAT in the non‐casted leg was similar between groups throughout the study period (Figure 3D).
Figure 3.
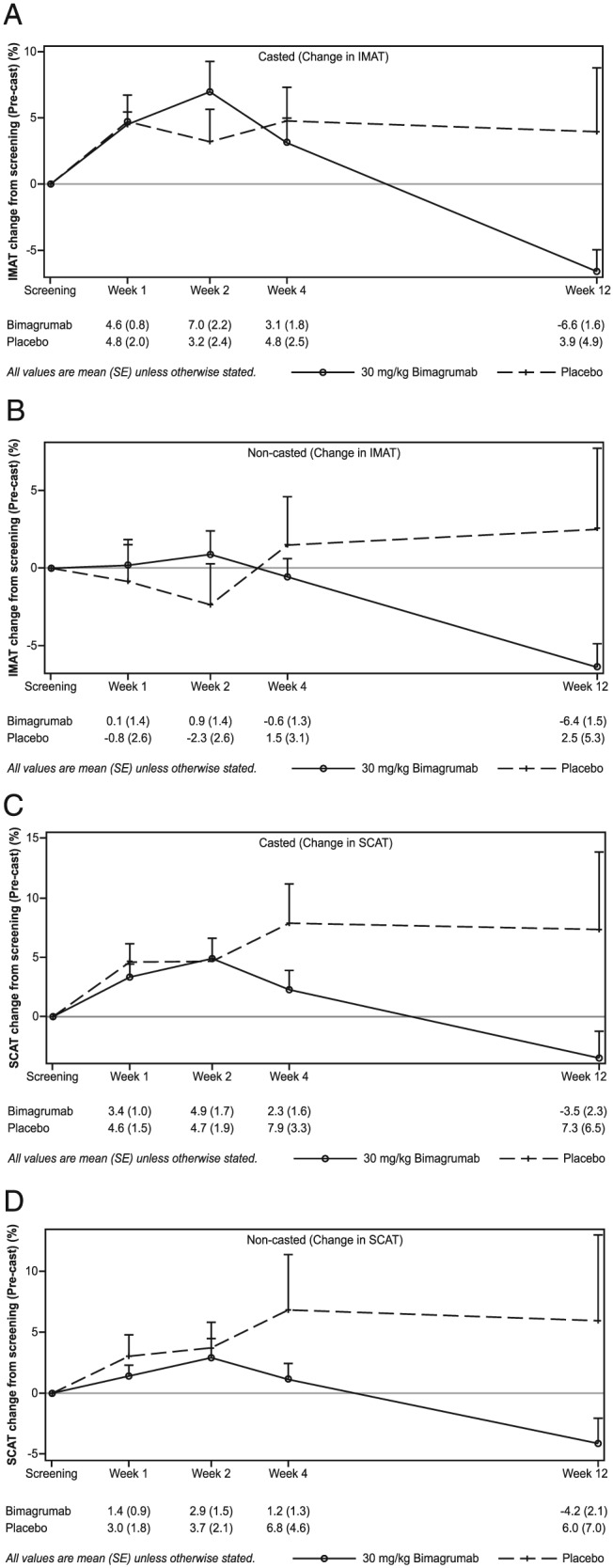
Change in inter‐muscular and subcutaneous adipose tissue from pre‐cast level in the casted and non‐casted limbs.
Quadriceps muscle strength
Immobilization resulted in a significant decline in maximum voluntary knee extension strength (−20.9% ± 4.3% and −27.8% ± 6.6% in the bimagrumab and placebo groups, respectively). Following treatment administration, the time courses of strength recovery were virtually identical in the two treatment groups (Figure 4A). The mean percentage change from pre‐cast levels indicated a close to full recovery at 4 weeks following study drug administration (−7.6% ± 5.1% and −2.2% ± 2.9% in the bimagrumab and placebo groups). As expected, no change in maximum force production was noted in the non‐casted limb, and no significant difference was observed in response to treatment in this healthy, young, cohort following a short period of disuse (Figure 4B).
Figure 4.
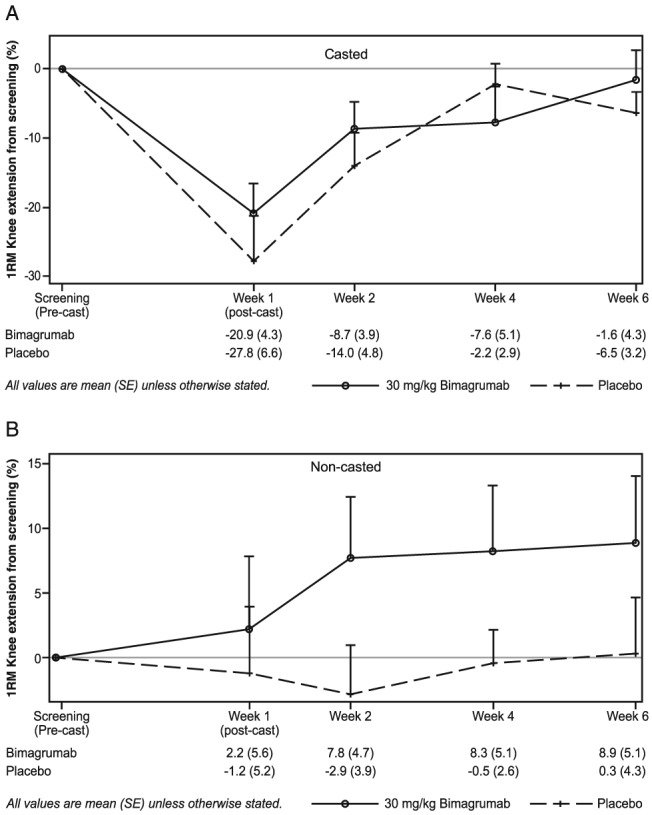
Maximum voluntary strength of single knee extension in the (A) casted and (B) non‐casted limbs assessed by one‐repetition maximum.
Safety
Of the 24 subjects, 22 (92%) experienced ≥1 AE. No serious or severe AE was reported, and no subject withdrew consent because of an AE. AEs were reported by both the bimagrumab (14/15, 93%) and placebo (8/9, 89%) groups. The most commonly reported AEs by preferred term in the bimagrumab group were dermatitis acneiform [n = 10/15 (66.7%)], muscle spasms [n = 7/15 (46.7%)], and pain in extremity [n = 3/15 (20%)]. Subjects in the placebo group reported infusion site extravasation [n = 2/9 (22%)], dermatitis acneiform [n = 1/9 (11%)], and pain in extremity [n = 1/9 (11%)]. All AEs in both treatment groups were mild in intensity and did not require treatment. Muscle spasms were transient; involuntary muscle contractions lasting ~10 to 30 s. No drug‐related, clinically significant ECG alterations or abnormalities were reported. No clinically significant changes were noted in any laboratory finding; any changes observed were judged by the investigator to be clinically insignificant, and such changes returned to normal limits during subsequent visits.
Discussion
This is the first study in humans to assess the efficacy of bimagrumab in disuse atrophy. The main finding of the study was the accelerated recovery of skeletal muscle volume from casting‐induced atrophy by a single 30 mg/kg i.v. dose of bimagrumab compared with the normal recovery time course (4 vs. 12 weeks from cast removal, respectively). In addition, the MRI data demonstrated an increase in inter‐muscular and SCAT during casting, which remained above pre‐cast levels in the placebo group after 12 weeks but decreased significantly with bimagrumab. The 2 week casting model in this population of young adult men proved to be unsuitable to assess the rate of recovery of muscle strength from the short period of disuse. The rapid recovery of skeletal muscle volume and reduction in inter‐muscular adiposity suggest the potential for short‐term use of bimagrumab in the multimodal treatment of certain health conditions with associated disuse atrophy.
The average loss of TMV in the casted limb was ~5%. This finding is similar to that previously reported in healthy young individuals following immobilization of the knee by either a cast or a brace.25, 27, 29, 30 In the study by Wall et al.,29 immobilization for 5 days induced muscle loss of ~3.5%, which increased to 8.4% when immobilization was extended to 14 days. While this magnitude of muscle loss can be recovered with little rehabilitation in healthy young men, it is difficult to overcome in elderly patients.14, 15, 16, 31
Growing evidence suggests that the structural composition of a muscle is an important factor in its function.10 With ageing, muscle strength is lost at a faster rate than lean mass, pointing to additional determinants of impaired contractility in older adults.32 Findings of appreciable increases in IMAT in the thigh of the immobilized limb are in agreement with previous observations in young5, 33 and older adults.34 Interestingly, no changes were apparent in the non‐casted limb. The increase in IMAT persisted in the casted leg of subjects for 4 weeks after returning to normal daily activities before those on bimagrumab began to lose fat volume (Figure 3A). Increased muscle adiposity may contribute to a decline in muscle performance (muscle quality) through biochemical (e.g. localized pro‐inflammatory cytokines and insulin resistance), neurological (central activation ratio) and biomechanical (altered muscle fibre pennation) mechanisms that impair contractile protein output.35, 36, 37, 38, 39 In addition, IMAT has been shown to be a significant predictor of impaired mobility in older adults.40, 41 The suggested synergistic implications of changes in muscle mass and adiposity on mobility in the frail elderly42 point to possible opportunities for drugs that act on both muscle and fat tissues.
The 25% reduction in knee extension strength following casting was similar to previous observations.15, 27, 30 Muscle weakness following immobilization by a full‐length leg cast was mainly due to the marked decrease in the maximum voluntary force (neurogenic muscle activation) rather than loss of TMV.43 This mechanism is supported by the consistent observation that the loss of voluntary muscle strength is greater than that of muscle cross‐sectional area.44, 45 Muscle strength recovered in a similar manner in both the bimagrumab and placebo groups, seemingly independent of the rate and magnitude of TMV changes. This finding underscores the possibility that the recovery of maximal force production in these healthy young men was likely driven by the simple return of normal muscle activation in the extremities (e.g. free walking) rather than the restoration of muscle volume,25 whether the rapid return of muscle mass benefits the rate of strength recovery in older adults requires a different study.
Recently published results in rodents and humans have highlighted the potential competitive advantages of ActRII involvement in the treatment of muscle wasting conditions.23, 24 The present observations add further support for a receptor antagonist by demonstrating the ability of bimagrumab to induce a rapid and marked increase in TMV in an established human experimental model of disuse atrophy. The speed and magnitude of muscle recovery are essential while considering the importance of returning to a pre‐injury level of mobility, whether for a young professional athlete or an elderly hip fracture patient.
A single dose of i.v. bimagrumab 30 mg/kg was safe and generally well tolerated by the sample group of healthy young men. There were no serious adverse events or withdrawals from the study due to AEs. The most frequently reported AEs possibly related to the drug were dermatitis acneiform and spontaneous muscle contractions. Both these AEs were mild in intensity, transient, and resolved during the study period. Spontaneous muscle contractions, mostly manifesting as painless twitches, might be the accompanying symptoms of intensive muscle remodeling in response to the pro‐myogenic stimulus, as these findings have often been described in elderly patients under intensive resistance training46 and with other medications. The increased incidence of acne in subjects treated with bimagrumab suggests the possible response of blocking ActRII on the hair follicle or another direct or indirect effect.47 There were no clinically significant ECG alterations in the group receiving bimagrumab.
The results of the present study suggest that ActRII blockade with bimagrumab provides a safe and effective method for disrupting the myostatin pathway in healthy young men with acute disuse atrophy, resulting in an expedited recovery of skeletal muscle volume. These findings suggest the potential value of this treatment approach as part of a multimodal rehabilitation of patients experiencing an acute episode of disuse atrophy (e.g. hip fracture) and populations that would benefit clinically from an accelerated recovery of skeletal muscle. Clinical trials (e.g. ClinicalTrials.gov Identifier: NCT02152761 and NCT02333331) are underway to explore the added benefits of such treatment.
Conflict of interest
Michael Bartlett, Jens Praestgaard, Didier Laurent, László B. Tankó, and Daniel S. Rooks are full‐time employees of Novartis Pharma AG. Scott Rasmussen has nothing to disclose.
Supporting information
Supplementary table 1. Mean change in TMV, IMAT and SCAT in the casted and non‐casted limb.
Acknowledgement
The authors would like to thank Preetinder Kaur and Vasundhara Pathak for providing medical writing support. The authors of this manuscript certify that they complied with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle.48
This study was funded by Novartis Institutes for BioMedical Research.
Rooks, D. S. , Laurent, D. , Praestgaard, J. , Rasmussen, S. , Bartlett, M. , and Tankó, Ló. B. (2017) Effect of bimagrumab on thigh muscle volume and composition in men with casting‐induced atrophy. Journal of Cachexia, Sarcopenia and Muscle, 8: 727–734. doi: 10.1002/jcsm.12205.
References
- 1. Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med 2006;27:94–99. [DOI] [PubMed] [Google Scholar]
- 2. Ferrando AA, Lane HW, Stuart CA, Davis‐Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Phys 1996;270:E627–E633. [DOI] [PubMed] [Google Scholar]
- 3. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 2008;586:6049–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paddon‐Jones D, Sheffield‐Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006;91:4836–4841. [DOI] [PubMed] [Google Scholar]
- 5. Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz‐Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007a;85:377–384. [DOI] [PubMed] [Google Scholar]
- 6. Chevalier S, Gougeon R, Choong N, Lamarche M, Morais JA. Influence of adiposity in the blunted whole‐body protein anabolic response to insulin with aging. J Gerontol A Biol Sci Med Sci 2006;61:156–164. [DOI] [PubMed] [Google Scholar]
- 7. Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–160. [DOI] [PubMed] [Google Scholar]
- 8. Beenakker KG, Westendorp RG, de Craen AJ, Slagboom PE, van Heemst D, Maier AB. Pro‐inflammatory capacity of classically activated monocytes relates positively to muscle mass and strength. Aging Cell 2013;12:682–689. [DOI] [PubMed] [Google Scholar]
- 9. Bodine SC. Disuse‐induced muscle wasting. Int J Biochem Cell Biol 2013;45:2200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 11. Hershkovitz A, Polatov I, Beloosesky Y, Brill S. Factors affecting mortality of frail hip‐fractured elderly patients. Arch Gerontol Geriatr 2010;51:113–116. [DOI] [PubMed] [Google Scholar]
- 12. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–1650. [DOI] [PubMed] [Google Scholar]
- 13. Miller MD, Foley A, Gunn SM, Crotty M. Progression and adherence to an individually prescribed and supervised resistance training intervention in older adults recovering in hospital from lower limb fragility fracture. Patient Prefer Adherence 2008;2:107–113. [PMC free article] [PubMed] [Google Scholar]
- 14. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short‐term disuse: implications for age‐related sarcopenia. Ageing Res Rev 2013;12:898–906. [DOI] [PubMed] [Google Scholar]
- 15. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009;107:1172–1180. [DOI] [PubMed] [Google Scholar]
- 16. Hvid LG, Suetta C, Nielsen JH, Jensen MM, Frandsen U, Ortenblad N, et al. Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp Gerontol 2014;52:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci 2010;1211:25–36. [DOI] [PubMed] [Google Scholar]
- 18. Molfino A, Amabile MI, Rossi Fanelli F, Muscaritoli M. Novel therapeutic options for cachexia and sarcopenia. Expert Opin Biol Ther 2016;16:1239–1244. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee A, Guttridge DC. Mechanisms for maintaining muscle. Curr Opin Support Palliat Care 2012;6:451–456. [DOI] [PubMed] [Google Scholar]
- 20. Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle 2012;3:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 2013;280:4294–4314. [DOI] [PubMed] [Google Scholar]
- 22. Argiles JM, Orpi M, Busquets S, Lopez‐Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov Today 2012;17:702–709. [DOI] [PubMed] [Google Scholar]
- 23. Elkina Y, von HS , Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle 2011;2:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lach‐Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol 2014;34:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex‐based differences in skeletal muscle function and morphology with short‐term limb immobilization. J Appl Physiol 2005;99:1085–1092. [DOI] [PubMed] [Google Scholar]
- 26. Clark BC, Manini TM, Hoffman RL, Russ DW. Restoration of voluntary muscle strength after 3 weeks of cast immobilization is suppressed in women compared with men. Arch Phys Med Rehabil 2009;90:178–180. [DOI] [PubMed] [Google Scholar]
- 27. Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB 2004;18:1025–1027. [DOI] [PubMed] [Google Scholar]
- 28. Levinger I, Goodman C, Hare DL, Jerums G, Toia D, Selig S. The reliability of the 1RM strength test for untrained middle‐aged individuals. J Sci Med Sport 2009;12:310–316. [DOI] [PubMed] [Google Scholar]
- 29. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014;210:600–611. [DOI] [PubMed] [Google Scholar]
- 30. Snijders T, Wall BT, Dirks ML, Senden JM, Hartgens F, Dolmans J, et al. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci (Lond) 2014;126:557–566. [DOI] [PubMed] [Google Scholar]
- 31. Nedergaard A, Jespersen JG, Pingel J, Christensen B, Sroczynski N, Langberg H, et al. Effects of 2 weeks lower limb immobilization and two separate rehabilitation regimens on gastrocnemius muscle protein turnover signaling and normalization genes. BMC Res Notes 2012;5:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age‐related decline of grip strength: cross‐sectional and longitudinal perspectives. J Gerontol 1990;45:M82–M88. [DOI] [PubMed] [Google Scholar]
- 33. Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz‐Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007b;85:377–384. [DOI] [PubMed] [Google Scholar]
- 34. Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury—a cross‐sectional study. Spinal Cord 2004;42:711–716. [DOI] [PubMed] [Google Scholar]
- 35. Verghese J, Holtzer R, Oh‐Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci 2011;66:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin‐6 and tumor necrosis factor‐alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2002;57:M326–M332. [DOI] [PubMed] [Google Scholar]
- 37. Pahor M, Kritchevsky S. Research hypotheses on muscle wasting, aging, loss of function and disability. J Nutr Health Aging 1998;2:97–100. [PubMed] [Google Scholar]
- 38. Stehno‐Bittel L. Intricacies of fat. Phys Ther 2008;1265–1278. [DOI] [PubMed] [Google Scholar]
- 39. Wei Y, Chen K, Whaley‐Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 2008;673–680. [DOI] [PubMed] [Google Scholar]
- 40. Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012;629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res 2012;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait‐speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr 2013;97:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand 1996;157:63–70. [DOI] [PubMed] [Google Scholar]
- 44. Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol 1991;70:1882–1885. [DOI] [PubMed] [Google Scholar]
- 45. Hather BM, Adams GR, Tesch PA, Dudley GA. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol 1992;72:1493–1498. [DOI] [PubMed] [Google Scholar]
- 46. Kent‐Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 2002;93:1813–1823. [DOI] [PubMed] [Google Scholar]
- 47. Nakamura M, Matzuk MM, Gerstmayer B, et al. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J 2013;17:497–499. [DOI] [PubMed] [Google Scholar]
- 48. von Haehling S , Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Mean change in TMV, IMAT and SCAT in the casted and non‐casted limb.


