Abstract
Introduction:
Alcohol withdrawal delirium (AWD) or delirium tremens (DT) is associated with severe complications and high mortality. Prospectively identifying patients with increased risk of developing DT would have important preventive and therapeutic implications. Thus, the present study aimed to identify clinical risk factors predicting the development of DT.
Materials and Methods:
The study was a cross-sectional quasi-experimental one with equivalent control group, conducted at a tertiary hospital from August 2014 to May 2015. Forty adult male inpatients, diagnosed with DT, were compared with forty age- and sex-matched inpatients in alcohol withdrawal state without delirium. Assessments were done using confusion assessment method, Clinical Institute Withdrawal Assessment of Alcohol Scale, and Mini–Mental Status Examination. For group comparisons, Pearson's Chi-square test and independent sample t-test were used; logistic regression was applied to identify predictors followed by receiver operating characteristic curve analysis.
Results:
Heavy drinking (P = 0.005; odds ratio [OR]: 1.17, confidence interval [CI]: 1.05–1.31), continuous pattern of drinking (P = 0.027; OR: 4.67, CI: 1.19–18.33), past history of delirium (P = 0.009; OR: 552.8, CI: 4.88–625.7), alcohol-induced psychosis (P = 0.002; OR: 74.6, CI: 4.68–1190), and presence of cognitive deficits (P = 0.044; OR: 12.5, CI: 1.07–147.3) emerged as strong predictors of AWD.
Conclusion:
The risk factors found can be easily evaluated in a clinical setting for physicians to readily identify patients at risk for developing DT and plan intensive therapies for them. At a neurobiological level, patients with preexisting brain neurotransmitter disturbances are at greater risk for developing DT.
Key words: Alcohol withdrawal delirium, delirium tremens, predictors, risk factors
INTRODUCTION
Alcohol withdrawal delirium (AWD), also known as delirium tremens (DT), is a severe complication of alcohol withdrawal leading to high mortality.[1,2] Early identification and systematic use of benzodiazepines at appropriate doses are the mainstay of treatment. Poor prognosis in DT is seen when it is associated with comorbid infection and other medical complications such as head injury and seizures.[3,4] After the development of DT treatment is to be initiated promptly, preferably in an intensive care unit.[5] Thus, identification of risk factors for developing DT is necessary for early treatment in patients with severe alcohol withdrawal syndrome (SAWS) so that delirium can be prevented in such patients.
Various studies have shown that past history of delirium,[6] tachycardia,[7] elevated blood pressure,[8,9] and thrombocytopenias[10] are persistent risk factors for the development of AWD. However, studies have methodological differences, and thus a common consensus has not been arrived at as yet. The present study attempted to identify the risk factors in predicting AWD in a population of patients with heavy consumption of alcohol as well as to corroborate our findings with the existing ones. This will lead to early identification of patients likely to develop delirium during withdrawal of alcohol and help to formulate the dose of benzodiazepines in treating those patients.
MATERIALS AND METHODS
The study was a quasi-experimental design with equivalent control group conducted in the Department of Psychiatry at Mahatma Gandhi Medical College and Research Institute, a tertiary hospital in Puducherry, from August 2014 to July 2015. The study was approved by the Institutional Ethical Committee. All male patients aged 18–64 years admitted in the Department of Psychiatry and diagnosed by consultants as AWD (complicated withdrawal) according to International Classification of Diseases-10[11] was taken up for the study. All other cases of delirium were excluded after detailed investigations, namely, blood investigations for dyselectrolytemia and infections and neuroimaging. Any cases of mixed delirium where alcohol withdrawal was one of the contributing factors along with other factors that might have contributed to delirium were also excluded from the study. Confusion assessment method[12] was applied as an adjuvant tool to diagnose delirium. A total of 58 patients were screened. Forty patients fulfilling the inclusion criteria were enrolled into the study. Forty age- and sex-matched inpatient cases of alcohol dependence syndrome without DT were included as controls. The control group was followed up for 2 weeks after admission to look for any emerging signs of delirium. Patients in the control group who developed delirium in due course were taken up in the AWD group. Those patients who were eligible for inclusion into the study were provided the salient details of the research project, and informed consent was obtained. Around 80% of patients in the DT group had hyperactive or mixed subtype of delirium. A structured pro forma was used to record information regarding the sociodemographic and clinical profile of patients. Clinical Institute Withdrawal Assessment of Alcohol Scale-Revised (CIWA-Ar)[13] was also applied to rate the severity of withdrawal symptoms in both the groups. Mini–Mental Status Examination (MMSE)[14] was administered in both groups to determine cognitive functioning. A cutoff score of 24 was kept for cognitive dysfunction. This scale was applied when both groups of patient were stable and could comprehend the instructions. All analyses were performed using Statistical Package for the Social Sciences (SPSS for Windows, Version 16.0. SPSS Inc., Chicago, IL, USA). Normality of data was examined using histograms and Shapiro–Wilk test. Patients having AWD (DT) were “androgen deprivation therapy (ADT) with DT group” and another group as “ADT without DT group.” The between-group comparison on categorical variables (withdrawal fits, presence of alcohol-induced psychosis, etc.) was done using Pearson's Chi-square test, while for continuous/numerical variables (age of the patient, duration of dependence, etc.), the independent sample t-test was applied. Binary logistic regression analysis was done to find out the predictors of DT with the significant variables contributing to group differences. A receiver operating characteristic (ROC) curve analysis was done to find out the model's ability to differentiate patients having delirium from those not having delirium. P < 0.05 was accepted as statistically significant.
RESULTS
The mean age of patients in the AWD group was 42.85 years (standard deviation [SD] 10.92 years), whereas in the control group, i.e., the alcohol withdrawal without delirium group was 40.45 years (SD 10.27 years) [Table 1]. Majority of patients in the delirium group were primary educated (57.5%) and came from lower socioeconomic status (62.5%), and most of them were employed (67.5%). In the control group, proportion of rural population was more than urban (60%), but most of them belonged to middle socioeconomic status (52.5%) and majority (80%) of them were employed [Table 2]. The age of onset in the delirium group was, however, slightly later than the control group (25.02 years [SD: 8.71 years] vs. 21.65 years [SD: 4.00 years]) and thus their duration of dependence was also less than the control group (10.75 years [SD: 6.47 years] vs. 14.07 years [SD 8. 26 years]), but the quantity of alcohol consumed in units of alcohol per day was much higher than the control group (18.15 units [SD: 1.27 units]/day vs. 14.75 units [SD: 4.89]/day). Thus, there was a statistically significant difference between the two groups in terms of quantity of alcohol consumed per day (t = −2.99; P = 0.004) [Table 1].
Table 1.
Distribution and comparison of sociodemographic and clinical characteristics across the groups (numerical)
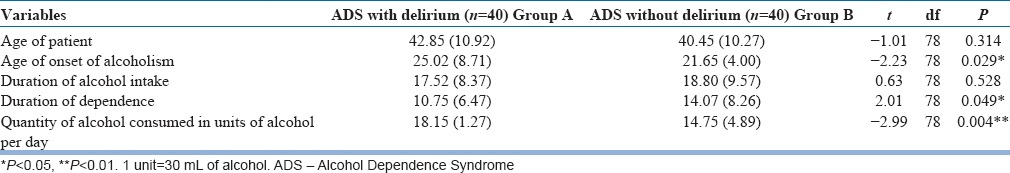
Table 2.
Distribution and comparison of sociodemographic and clinical characteristics across the groups (categorical)
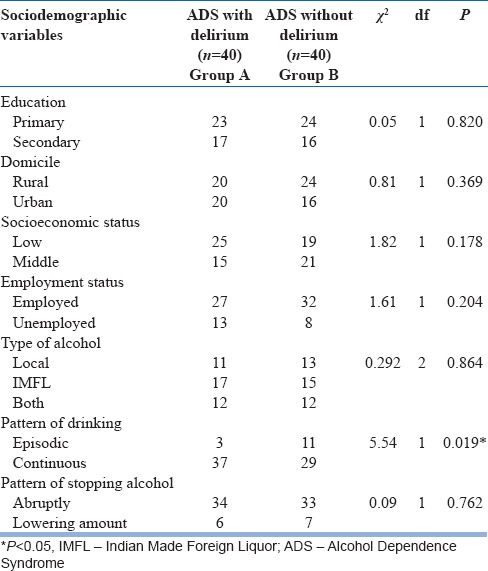
Apart from the amount of alcohol consumed, the two groups significantly differed in the pattern of drinking alcohol (χ2 = 5.54; P = 0.019). The delirium group and the control group were matched in terms of their type of alcohol consumed and the pattern in which they stopped drinking alcohol. Few patients had episodic drinking pattern (27.5%) in the control group, but most of the patient in the delirium group had a continuous pattern of drinking (92.5%).
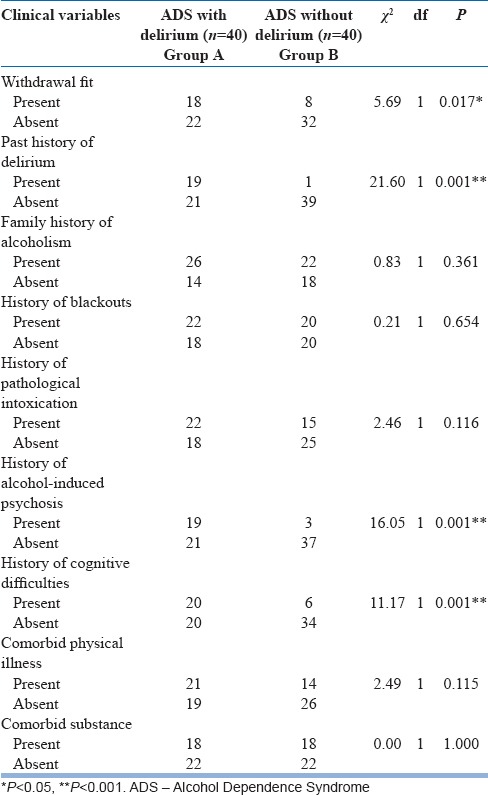
Bivariate analysis of clinical variables directly related to alcohol use such as presence of withdrawal fits (χ2 = 5.69; P = 0.017), past history of AWD (χ2 = 21.60; P < 0.001), history of alcohol-induced psychotic disorder in the past (χ2 = 16.05; P < 0.001), and presence of cognitive deficits (χ2 = 11.17; P = 0.001), showed a strong significant association between these variables and development of delirium [Table 3].
Table 3.
Distribution and comparison of clinical characteristics related to alcohol use across the groups (categorical)
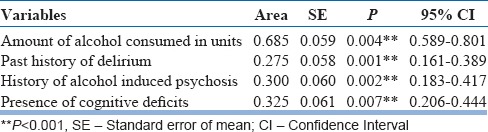
After the bivariate analysis, the statistically significant variables identified were further taken for binary logistic regression analysis to assess the contribution of each variable in developing delirium. The model explained 61.8%–82.3% of the variance in this sample; hence, it can be applied to explain the risk factors. Table 4 shows the predictors of delirium in this population. A heavy pattern of drinking (P = 0.005; odds ratio [OR]: 1.17, confidence interval [CI]: 1.05–1.31), continuous alcohol drinking pattern (P = 0.027; OR: 4.67, CI: 1.19–18.33), presence of past history of delirium (P = 0.009; OR: 552.8, CI: 4.88–625.7), presence of alcohol-induced psychosis in the past (P = 0.002; OR: 74.6, CI: 4.68–1190), and presence of cognitive deficits (P = 0.044; OR: 12.5, CI: 1.07–147.3) emerged as strong predictors of AWD. The area under ROC curve was 0.275 for the past history of delirium, 0.300 for the presence of alcohol-induced psychosis, 0.325 for the presence of cognitive deficits, and 0.685 for the amount of alcohol consumed in units [Figure 1 and Table 5].
Table 4.
Stepwise logistic regression showing the predictors of alcohol withdrawal delirium
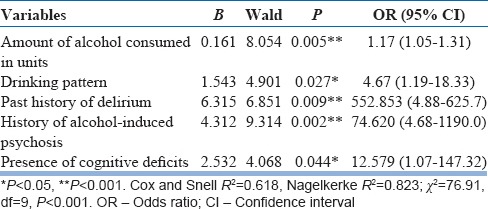
Figure 1.
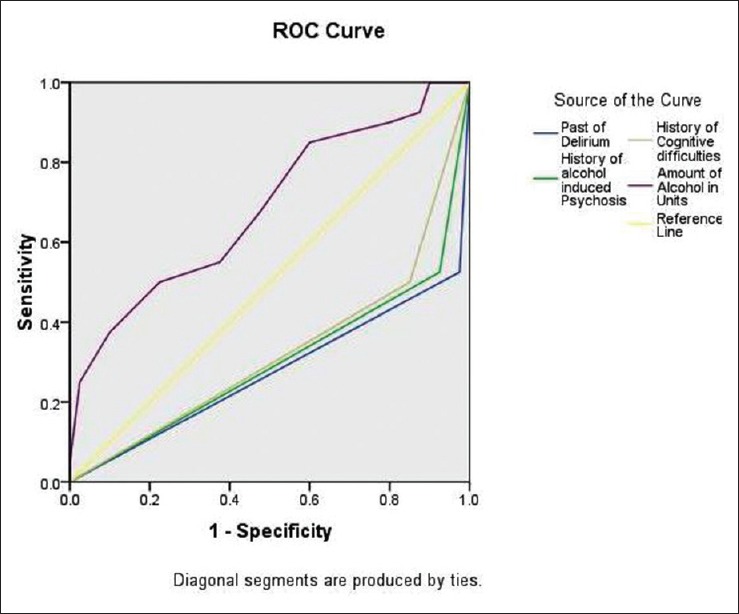
A receiver operating characteristic curve analysis between androgen deprivation therapy with delirium tremens and androgen deprivation therapy without delirium tremens group
Table 5.
Receiver operating characteristic (ROC) curve analysis showing the area under curve of the predictors of Delirium Tremens
DISCUSSION
Our study design had some methodological advantages over previous similar studies.[6,7,8,9,10] In the present study, the experimental group was selected after detailed evaluation of patients following their admission. Two subgroups of patients were taken, one who got admitted with AWD and another who developed AWD after admission. In both groups, detailed evaluation was done to rule out other causes of delirium and a homogenous group was selected. Most previous studies have considered cases that became delirious after admission into de-addiction center. Since our hospital is a tertiary care referral hospital, most of the cases were referred from other centers and presented with delirium. Only eight cases developed delirium after getting admitted for simple withdrawal. Furthermore, the control group was age and sex matched to patients in the experimental group. Moreover, the control group was followed up for 2 weeks to check for emergence of delirium, and if so, the patient was reassigned to the experimental group. Most of the previous studies in this are aware of retrospective chart reviews,[15,16,17] but in our study, we directly interviewed the patient as well as their informants. Furthermore, patients who could not initially give a proper history were later interviewed when out of delirium. Assessment of withdrawal symptoms, delirium, and cognitive functioning was done using standardized tools which gave more strength to the study. The sociodemographic and clinical pro forma was meticulously prepared selecting all the relevant parameters from literature which pose as potential risk factors for the development of delirium.
The mean age of patients in both the groups was comparable; however, patients with DT had a later age of onset of alcoholism and also had a lesser duration of dependence. However, the amount of alcohol consumed per day was significantly higher in the DT group than in the non-DT group. Quantity of alcohol consumed per day is also a significant predictor of delirium in this population. Larger quantities of alcohol disrupt the neuronal stability and thus create a higher vulnerability for developing delirium.[15] Since chronic exposure to high quantity of alcohol results in upregulation of N-methyl-D-aspartate (NMDA) receptors, therefore abrupt cessation of alcohol can lead to severe hyperexcitability of brain resulting in delirium.[1]
Furthermore, our study highlights the fact that binge drinking pattern might have some effects on the restoration of the normalcy of cerebral depression, thus reducing delirium, in contrast to chronic continuous drinking of alcohol where cerebral depression persists. However, the concept of kindling used to explain repeated detoxification leading to worsening of subsequent alcohol withdrawal state producing delirium later, is still a debated one.[18]
In the present study, statistically significant differences were observed between the delirium and nondelirium groups with respect to clinical factors such as past history of withdrawal fits, delirium, alcohol-induced psychosis, and the presence of cognitive deficits. Further, logistic regression analysis showed that factors such as past history of delirium, history of alcohol-induced psychosis, and the presence of cognitive deficits to be important predictors of AWD. These findings corroborate with results of previous studies which have found past history of DT and withdrawal fits to be consistent predictors of DT.[6,7] Previous studies have also found other predictors of AWD, i.e., thrombocytopenia,[10,19,20] hypertension,[15,16] medical comorbidity,[9,16,17] hypokalemia,[20,21] tachycardia,[7,17] hyperthermia,[15] and hypertension[15,16,22] and also association of DT with high blood homocysteine level and low blood level of pyridoxine in a recent study by Kim et al.[19] In most of the earlier studies, the risk factors were more laboratory based[6,9,19] which might not be useful where such facilities are not available like in a primary health-care system. Our study focused on the importance of a relevant clinical history in predicting delirium, and thus early screening of patients with SAWS may help to prevent them from developing subsequent withdrawal delirium.
In our study, the significant predictors of delirium which emerged (delirium, induced psychosis, and cognitive deficits) perhaps imply that pre-existing brain insults may lead to future episodes of delirium. A similar finding was reported in a study by Eyer et al.,[20] which showed the presence of structural brain lesion to be one of the predictors of delirium. Thus, apart from their clinical relevance, these findings can also be understood from a neurobiological perspective. In delirium, there is a disruption in gamma-aminobutyric acid (GABA), glutamate, and dopaminergic systems, which is also the case in psychosis as well as in patients having cognitive dysfunction.[1,23,24] Therefore, DT can be viewed as brain pathology occurring in a preexisting disturbance of brain neurotransmitter systems.
The present study highlights the importance of a focused and detailed clinical history taking from both patients as well as their informants in the treatment of alcohol withdrawal syndrome. A patient with SAWS with heavy consumption of alcohol must be screened for past injuries to the brain in terms of withdrawal delirium or psychosis and presence of cognitive deficits. Thereafter, this group of patients should be treated with higher doses of benzodiazepines in an inpatient setting.[25] Close monitoring should be done for the initial week after stopping alcohol for emergence of features suggestive of delirium. Neuropsychological evaluation can be helpful in this group of patients after detoxification, and strict abstinence should be enforced.
This study also provides an understanding about the neurobiological aspect of DT. It is well known that alcohol depresses the brain by its effect on the GABA-A receptors in the brain. Chronic exposure to high doses of alcohol results in compensatory reduction in GABA-A receptors response to GABA, i.e., the downregulation of GABA receptors. On the other hand, there is an upregulation of NMDA neuroreceptors during heavy exposure to alcohol.[26,27,28] Thus, with a preexisting destabilized neurotransmitter system in this group of patients, an abrupt cessation of alcohol leads to a burst of NMDA activity which increases the hyperexcitable state many times producing withdrawal fits or delirium. Furthermore, a recent study by Graef et al.[29] illustrated that abnormal burst activity of T-type calcium channel in the medial thalamus and hypothalamus causes disruption of normal rhythm producing complications such as seizures, disturbed sleep-wake cycle, electroencephalogram abnormalities, and subsequent delirium. This phenomenon is more likely to occur in patients with past history of delirium or psychosis because of highly sensitized thalamic burst activity in them.[23,29] A recent study[30] has also found an association of dopamine transporter gene with withdrawal seizure and DT which further strengthens the understanding of our findings about how the presence of psychosis has a role to play in the development of DT.
Our study being a hospital based one, most DT cases were already delirious when included in the study, and patients who developed delirium after hospitalization were few in number. Hence, a comparison between the two was not possible. Although cognitive function was assessed using a highly recommended and sensitive screening tool like MMSE, a detailed neuropsychological assessment could have been more comprehensive and informative. Unlike some previous studies, our study did not include any laboratory parameters since the study design did not permit predelirium testing, while laboratory parameters would have been disturbed during the delirium state.
CONCLUSION
DT is a fatal complication of severe alcohol dependence syndrome. Due to its high mortality and various associated complications, prevention of the condition is preferred more than treatment of this condition. In the present study, past history of DT, history of alcohol-induced psychosis, and the presence of cognitive deficits emerged as significant clinical predictors of delirium in this group of patients with heavy consumption of alcohol. Therefore, goal of treatment in such cases should be prevention of emerging delirium by identifying these risk factors followed by close monitoring in a hospital setup; along with a high dosage of benzodiazepine titrated according to the units of alcohol consumed per day. Our findings also provide insight into the neurobiological underpinnings of AWD by suggesting that patients with preexisting brain dysfunction are at a greater risk for developing DT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bayard M, McIntyre J, Hill KR, Woodside J., Jr Alcohol withdrawal syndrome. Am Fam Physician. 2004;69:1443–50. [PubMed] [Google Scholar]
- 2.Grover S, Ghormode D, Ghosh A, Avasthi A, Chakrabarti S, Mattoo SK, et al. Risk factors for delirium and inpatient mortality with delirium. J Postgrad Med. 2013;59:263–70. doi: 10.4103/0022-3859.123147. [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med. 2008;15:788–90. doi: 10.1111/j.1553-2712.2008.00187.x. [DOI] [PubMed] [Google Scholar]
- 4.Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90:253–61. doi: 10.1093/qjmed/90.4.253. [DOI] [PubMed] [Google Scholar]
- 5.Finucane TE. Management of withdrawal delirium (delirium tremens) N Engl J Med. 2015;372:580. doi: 10.1056/NEJMc1415679. [DOI] [PubMed] [Google Scholar]
- 6.Goodson CM, Clark BJ, Douglas IS. Predictors of severe alcohol withdrawal syndrome: A systematic review and meta-analysis. Alcohol Clin Exp Res. 2014;38:2664–77. doi: 10.1111/acer.12529. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Jang MK, Lee JY, Kim SM, Kim KH, Park JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20:1833–7. doi: 10.1111/j.1440-1746.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- 8.Minden SL, Carbone LA, Barsky A, Borus JF, Fife A, Fricchione GL, et al. Predictors and outcomes of delirium. Gen Hosp Psychiatry. 2005;27:209–14. doi: 10.1016/j.genhosppsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson JA, Suelzer CJ, Eckert GJ, Zhou XH, Dittus RS. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11:410–4. doi: 10.1007/BF02600188. [DOI] [PubMed] [Google Scholar]
- 10.Berggren U, Fahlke C, Berglund KJ, Blennow K, Zetterberg H, Balldin J. Thrombocytopenia in early alcohol withdrawal is associated with development of delirium tremens or seizures. Alcohol Alcohol. 2009;44:382–6. doi: 10.1093/alcalc/agp012. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 12.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Monte R, Rabuñal R, Casariego E, Bal M, Pértega S. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20:690–4. doi: 10.1016/j.ejim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Fiellin DA, O'Connor PG, Holmboe ES, Horwitz RI. Risk for delirium tremens in patients with alcohol withdrawal syndrome. Subst Abus. 2002;23:83–94. doi: 10.1080/08897070209511478. [DOI] [PubMed] [Google Scholar]
- 17.Palmstierna T. A model for predicting alcohol withdrawal delirium. Psychiatr Serv. 2001;52:820–3. doi: 10.1176/appi.ps.52.6.820. [DOI] [PubMed] [Google Scholar]
- 18.Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28:549–85. doi: 10.1016/j.ccc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Kim DW, Kim HK, Bae EK, Park SH, Kim KK. Clinical predictors for delirium tremens in patients with alcohol withdrawal seizures. Am J Emerg Med. 2015;33:701–4. doi: 10.1016/j.ajem.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Eyer F, Schuster T, Felgenhauer N, Pfab R, Strubel T, Saugel B, et al. Risk assessment of moderate to severe alcohol withdrawal – Predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol. 2011;46:427–33. doi: 10.1093/alcalc/agr053. [DOI] [PubMed] [Google Scholar]
- 21.Wetterling T, Kanitz RD, Veltrup C, Driessen M. Clinical predictors of alcohol withdrawal delirium. Alcohol Clin Exp Res. 1994;18:1100–2. doi: 10.1111/j.1530-0277.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 22.Burapakajornpong N, Maneeton B, Srisurapanont M. Pattern and risk factors of alcohol withdrawal delirium. J Med Assoc Thai. 2011;94:991–7. [PubMed] [Google Scholar]
- 23.Perälä J, Kuoppasalmi K, Pirkola S, Härkänen T, Saarni S, Tuulio-Henriksson A, et al. Alcohol-induced psychotic disorder and delirium in the general population. Br J Psychiatry. 2010;197:200–6. doi: 10.1192/bjp.bp.109.070797. [DOI] [PubMed] [Google Scholar]
- 24.Monte-Secades R, Rabuñal-Rey R, Guerrero-Sande H. Inpatient alcohol withdrawal syndrome. Rev Clin Esp. 2015;215:107–16. doi: 10.1016/j.rce.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Daeppen JB, Gache P, Landry U, Sekera E, Schweizer V, Gloor S, et al. Symptom-triggered vs. fixed-schedule doses of benzodiazepine for alcohol withdrawal: A randomized treatment trial. Arch Intern Med. 2002;162:1117–21. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 26.Ramos R, Mallet T, Divittis A, Cohen R. Predictors of severity of alcohol withdrawal in hospitalized patients. J Clin Med Res. 2013;5:376–80. doi: 10.4021/jocmr1514w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mennecier D, Thomas M, Arvers P, Corberand D, Sinayoko L, Bonnefoy S, et al. Factors predictive of complicated or severe alcohol withdrawal in alcohol dependent inpatients. Gastroenterol Clin Biol. 2008;32:792–7. doi: 10.1016/j.gcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Mainerova B, Prasko J, Latalova K, Axmann K, Cerna M, Horacek R, et al. Alcohol withdrawal delirium – Diagnosis, course and treatment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:44–52. doi: 10.5507/bp.2013.089. [DOI] [PubMed] [Google Scholar]
- 29.Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW. Disrupted thalamic T-type Ca2+ channel expression and function during ethanol exposure and withdrawal. J Neurophysiol. 2011;105:528–40. doi: 10.1152/jn.00424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorwood P, Limosin F, Batel P, Hamon M, Adès J, Boni C. The A9 allele of the dopamine transporter gene is associated with delirium tremens and alcohol-withdrawal seizure. Biol Psychiatry. 2003;53:85–92. doi: 10.1016/s0006-3223(02)01440-3. [DOI] [PubMed] [Google Scholar]


