Abstract
Background
Recent studies show a mechanistic link between intestinal microbial metabolism of dietary phosphatidylcholine and coronary artery disease pathogenesis. Concentrations of a pro-atherogenic gut microbe-generated metabolite, trimethylamine N-oxide (TMAO), predict increased incident cardiovascular disease risks in multiple cohorts. TMAO concentrations are increased in patients with type 2 diabetes mellitus (T2DM), but their prognostic value and relation to glycemic control are unclear.
Methods
We examined the relationship between fasting TMAO and two of its nutrient precursors, choline and betaine, versus 3-year major adverse cardiac events and 5-year mortality in 1,216 stable patients with T2DM who underwent elective diagnostic coronary angiography.
Results
TMAO (4.4 µmol/L [interquartile range 2.8–7.7µmol/L] vs. 3.6[2.3–5.7µmol/L]; P<0.001) and choline concentrations were higher in individuals with T2DM versus healthy controls. Within T2DM patients, higher plasma TMAO was associated with a significant 3.0-fold increased 3-year major adverse cardiac events risk (P<0.001) and a 3.6-fold increased 5-year mortality risk (P<0.001). Following adjustments for traditional risk factors and high sensitivity C-reactive protein, glycated hemoglobin and estimated glomerular filtration rate, increased TMAO concentrations remained predictive of both major adverse cardiac events and mortality risks in T2DM patients (e.g. Quartiles 4 vs. 1, hazard ratio 2.05[95%CI 1.31–3.20], P<0.001; and 2.07[95%CI 1.37–3.14], P<0.001, respectively).
Conclusion
Fasting plasma concentrations of the pro-atherogenic gut microbe-generated metabolite TMAO are higher in diabetic patients and portend higher major adverse cardiac events and mortality risks independent of traditional risk factors, renal function, and relationship to glycemic control.
Keywords: trimethylamine N-oxide, diabetes mellitus, glycemic control, intestinal microbiota
INTRODUCTION
Choline is an essential nutrient that is both synthesized endogenously and obtained from dietary sources, especially phosphatidylcholine (lecithin), in a variety of animal and plant products (1). Animal model studies reveal adequate levels of choline are required for appropriate formation of phospholipid membranes and the neurotransmitter acetylcholine, with choline deficiency, producing neurological impairment (2). The direct oxidation product of choline is betaine, which is a methyl donor in homocysteine remethylation, and also plays an important role in maintaining cellular volume and stability (3). Together, choline and betaine have been shown to provide hepatoprotection and improve insulin resistance in non-alcoholic fatty liver mouse models(4).
Recent studies indicate a pathophysiological contribution of gut microbiota to cardiometabolic diseases with mechanistic links to gut microbial choline metabolism. Specifically, the gut microbiome has been shown to serve as an important modulator of host energy balance and metabolism in Type 2 diabetes mellitus (T2DM) (5–7). Our group discovered plasma concentrations of three metabolites of dietary phosphatidylcholine – choline, betaine, and trimethylamine N-oxide (TMAO) – are associated with atherosclerotic cardiovascular risks in patients, and mechanistically linked to atherosclerosis development in animal models (8). TMAO formation in both animal models and humans requires gut microbes (8–11), and proceeds via gut microbe metabolism of either choline, betaine or other trimethylamine nutrients to initially form trimethylamine (TMA), which is delivered to the liver via the portal circulation where it is rapidly converted by host hepatic flavin monooxygenase 3 into TMAO (8, 12–14). TMAO itself promotes pro-atherosclerotic biological activities (8, 11, 15), and studies in a large independent cohort revealed the adverse prognostic value of increased plasma choline and betaine concentrations is driven by their nutrient precursor→product relationship with TMAO (14). Interestingly, recent animal model studies using the liver specific insulin receptor null mouse in the low-density lipoprotein (LDL) receptor null background, an insulin resistance mouse model of atherosclerosis, showed that suppression of hepatic flavin monooxygenase 3 and TMAO levels inhibited both hyperglycemia and atherosclerosis development (16). Moreover, in recent studies, development of an inhibitor of gut microbial formation of trimethylamine, the precursor of TMAO, was shown to inhibit diet induced atherosclerosis. Previous studies have suggested that patients with T2DM may have increased TMAO concentrations compared to patients without T2DM (17); however whether or not this relationship was confounded by glycemic control or renal function is unclear. We hypothesize that fasting plasma concentrations of the pro-atherogenic gut microbe-generated metabolite TMAO portend higher major adverse cardiac events and mortality risks independent of traditional risk factors, renal function, and relationship to glycemic control.
METHODS
We prospectively enrolled two cohorts for this study. The first was comprised of sequential stable patients at Cleveland Clinic undergoing elective, non-urgent, coronary angiographic evaluation between the years 2001–2007. We excluded those who had recently experienced acute coronary syndrome within the preceding 30 days (cardiac troponin I ≤0.03 ng/ml). In this analysis, we analyzed glucose and glycated hemoglobin (HbA1c), along with available laboratory results and medication use in the electronic medical record, and identified patients with a history of T2DM (defined as documented HbA1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL (6.99 mol/L), oral glucose tolerance test with 2-hour post-load glucose ≥ 200 mg/dL (11.1 mmol/L), and/or a history of T2DM with appropriate glucose lowering drug use). The second cohort examined was an independent set of 300 prospectively recruited, apparently healthy individuals ≥ 21 years old without known T2DM or cardiac diseases from health screens in the Cleveland area, solely for the purposes of providing reference interval values of these metabolites. Both studies were approved by the Cleveland Clinic Institutional Review Board.
After informed consent, fasting plasma blood samples were collected using ethylenediaminetetraacetic acid tubes, and immediately processed and frozen at −80°C until analysis. Quantification of fasting plasma TMAO concentrations was performed utilizing stable isotope dilution liquid chromatography with on-line tandem mass spectrometry (LC/MS/MS) on an AB Sciex API 5500 triple quadrupole mass spectrometer (Applied Biosystems) using d9(trimethyl)TMAO (d9-TMAO) as internal standards and detection of precursor product ion transitions at m/z 76→58, as previously described (18). The assay described shows good inter- and intra-day reproducibility (all CVs <7%), accuracy (>98.5% across low, mid and high values), and with good stability with freeze-thaw cycles (≥5, intercycle CV% <9)(18). Plasma choline and betaine can also be measured simultaneous using this method.
High-sensitivity C-reactive protein (hsCRP), fasting lipid panel, HbA1c, glucose, insulin, and serum creatinine were measured using the Roche Cobas 4000 analyzer platform (Roche Diagnostics). Estimated glomerular filtration rate (eGFR) was calculated by the Modified Diet in Renal Diseases equation (19). Adverse events were tracked prospectively with direct contact for 3 years for major adverse cardiac events (death, non-fatal myocardial infarction, non-fatal stroke) using a combination of postcard and scripted telephone follow-up, and manual chart review, as well as all-cause mortality for 5 years by electronic chart review and Social Security Death Index up to 2011 and confirmed by telephone interviews, official hospital record, or death certificates.
Continuous variables are summarized as mean ± standard deviation if normally distributed and median [interquartile range, IQR] if non-normally distributed. The Student t test or Wilcoxon-rank sum test for continuous variables and chi-square test for categorical variables were employed to examine between group differences. Spearman correlation was used to examine the associations between choline, betaine, TMAO and other laboratory measurements. Kaplan-Meier survival plots were calculated from baseline to time of adverse event and compared using the log-rank test. Cox proportional hazards analysis were used to determine hazard ratio (HR) and 95% CI for all-cause mortality stratified according to choline, betaine, or TMAO according to tertiles. Adjustments were made for individual traditional risk factors including age, gender, history of cardiovascular disease, history of heart failure, systolic blood pressure, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, smoking, as well as log-transformed body mass index, log-transformed hsCRP, HbA1c, and log-transformed eGFR. We used category-free net reclassification improvement (NRI) and Integrated Discrimination Improvement (IDI) to quantify improvement in model performance, and compared c-statistics between the two models with vs without TMAO. Statistical analyses were performed using SAS software (version 9.3, SAS Institute) and R software (Version 3.1.2).
RESULTS
Baseline characteristics of our study cohort of 1,216 patients with T2DM undergoing elective diagnostic coronary angiography are shown in Table 1, stratified by tertiles of plasma TMAO. A significant proportion of patients had a history of cardiovascular disease, with nearly half having a history of prior myocardial infarction and roughly a third having a history of revascularization via coronary artery bypass graft, and/or percutaneous intervention. Participants with increased HbA1c levels tended to be younger and a higher proportion were female. In addition, individuals with increased HbA1c were more likely to have lower HDL, higher hsCRP, higher choline, and be on either an ACE inhibitor or angiotensin receptor blocker (Table 1). Apart from correlations between TMAO and choline with eGFR, the majority of correlations between the three metabolites and laboratory measurements were weak (Supplemental Table 1).
Table 1.
Baseline Characteristics of Diabetic Cohort Stratified According to TMAO Tertiles
| Total (n=1216) |
Tertile 1 (n=401) | Tertile 2 (n=414) |
Tertile 3 (n=401) |
P value | |
|---|---|---|---|---|---|
| Age (years) | 64.4±10.2 | 61.1±9.7 | 65.2±9.9 | 66.9±10.1 | <0.001 |
| Male (%) | 58 | 64 | 57 | 53 | 0.004 |
| Hypertension (%) | 79 | 74 | 76 | 88 | <0.001 |
| Smoking (%) | 63 | 65 | 60 | 65 | 0.355 |
| History of MI (%) | 47 | 43 | 46 | 51 | 0.067 |
| History of stroke (%) | 8 | 5 | 7 | 12 | 0.002 |
| History of CABG (%) | 34 | 28 | 33 | 41 | <0.001 |
| History of PCI (%) | 35 | 36 | 34 | 35 | 0.796 |
| LDL cholesterol (mg/dL)* | 95 (76–115) | 96 (77–114) | 95 (79–118) | 93 (73–114) | 0.203 |
| HDL cholesterol (mg/dL)* | 33 (27–40) | 35 (28–41) | 33 (28–39) | 32 (26–38) | <0.001 |
| hs-CRP (mg/L) | 3.3 (1.3–8.3) | 2.9 (1.2–8.2) | 3.2 (1.4–7.9) | 4.0 (1.4–8.8) | 0.155 |
| Estimated GFR (ml/min/1.73 m2) | 82 (62–94) | 92 (83–100) | 80 (65–93) | 61 (45–82) | <0.001 |
| ACE inhibitor/ARB (%) | 59 | 55 | 60 | 63 | 0.038 |
| Statins (%) | 64 | 67 | 64 | 62 | 0.335 |
| Beta blockers (%) | 66 | 66 | 66 | 66 | 0.982 |
| Aspirin (%) | 75 | 76 | 78 | 72 | 0.112 |
| Glucose-lowering drugs (%) | 55 | 46 | 54 | 64 | <0.001 |
| TMAO (umol/L) | 4.4 (2.8–7.7) | 2.3 (1.7–2.8) | 4.4 (3.7–5.3) | 9.7 (7.8–14.9) | <0.001 |
| Choline (umol/L) | 10.6 (8.4–13.5) | 9.2 (7.7–11.1) | 10.4 (8.6–13.2) | 12.6 (10.0–16.2) | <0.001 |
| Betaine (umol/L) | 39 (30.7–49.6) | 37.6 (30.6–47) | 38.8 (30.1–49.7) | 40.7 (31.7–51.9) | 0.022 |
Abbreviations: MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; LDL, low density lipoprotein; HDL, high density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; TMAO, trimethylamine N-oxide;
To convert mg/dL to mmol/L multiply cholesterol by 0.02586.
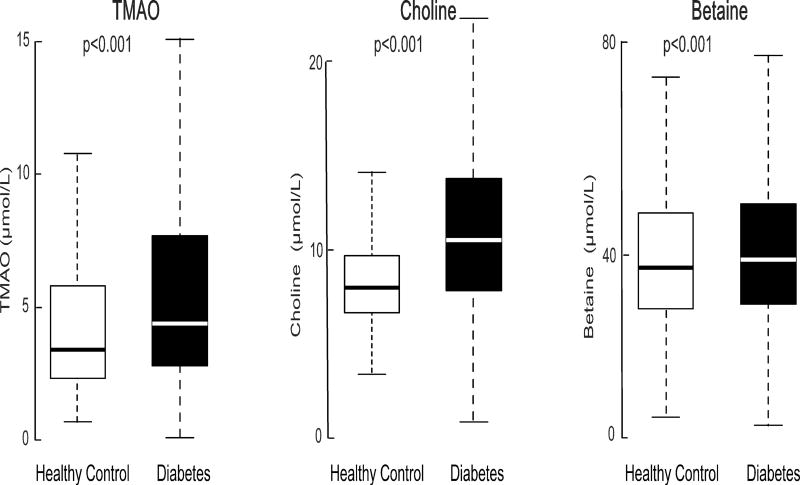
We first performed a cross-sectional comparison of TMAO concentrations between this study cohort with an independent set of 300 prospectively recruited, apparently healthy individuals without known cardiac diseases or T2DM from a health screening program at various locations across Cleveland, Ohio (baseline characteristics in Supplemental Table 2). We observed that the median [IQR] plasma concentrations for TMAO (4.4 [2.8–7.7] µmol/L vs. 3.4 [2.3–5.8] µmol/L, P<0.001), choline (10.6 [8.4–13.5] µmol/L vs. 8.2 [7.0–9.7] µmol/L, P<0.001), and betaine concentrations (39 [30.7–49.6] µmol/L vs. 37.7 [29.8–47.9] µmol/L, P=0.028) were each significantly higher in patients with T2DM compared to the healthy control cohort (Figure 1).
Figure 1.
Comparisons in fasting TMAO, choline and betaine concentrations between individuals with T2DM (n=1,216) and healthy, non-diabetic controls (n=300).
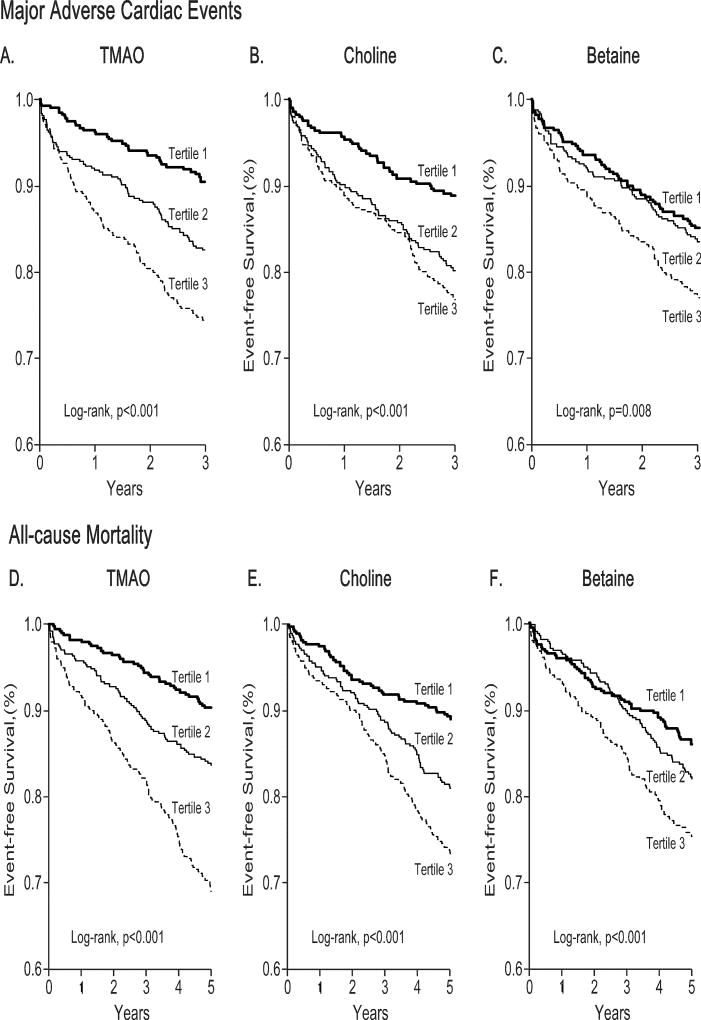
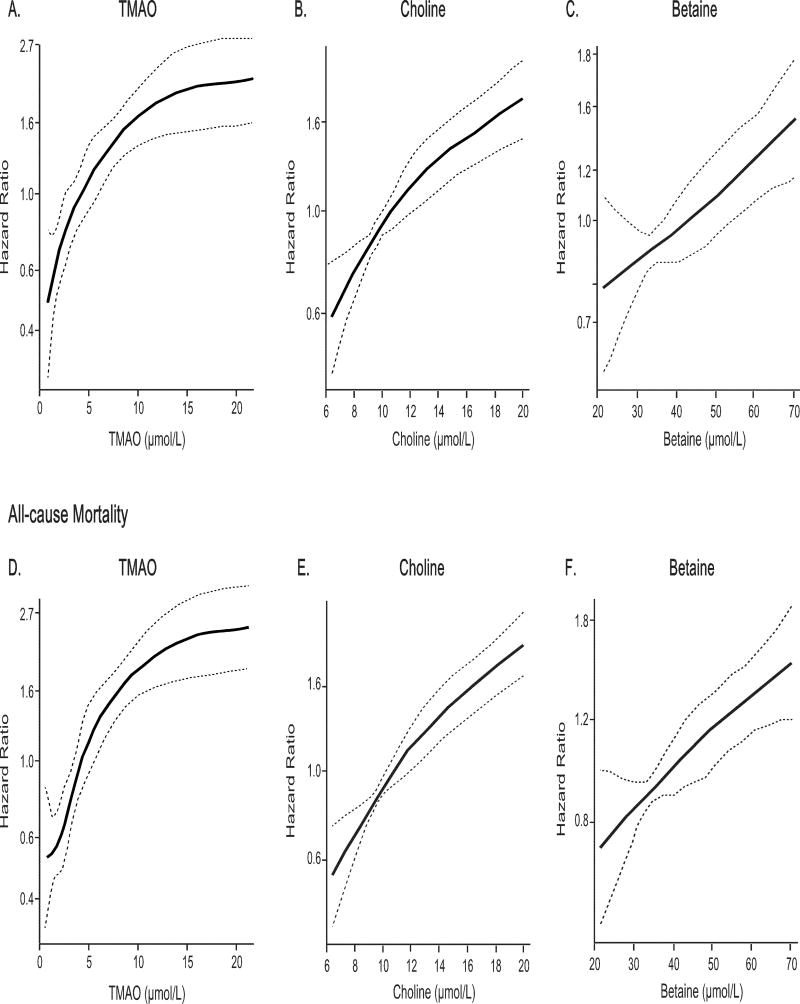
In our study cohort with 1,216 T2DM patients, a total of 209 major adverse cardiac events occurred over the initial 3 years following enrollment, and 227 deaths occurred over the ensuing 5 years following enrollment. Kaplan-Meier analyses for both major adverse cardiac events and mortality outcomes are shown in Figure 2, with plasma concentrations of TMAO, choline, and betaine each stratified by tertiles. We observed that increased fasting TMAO concentrations were associated with increased risk for 3-year major adverse cardiac events (Tertiles 3 vs 1, HR=3.03, 95%CI 2.08–4.42, P<0.001) and 5-year all-cause mortality (Tertiles 3 vs 1, HR=3.63, 95%CI 2.53–5.21, P<0.001). Similarly, increased fasting choline concentrations and betaine concentrations conferred increased mortality risks in both cohorts (Supplemental Table 2). After adjustments for traditional risk factors, hsCRP, HbA1c, history of cardiovascular disease, and eGFR, increased concentrations of fasting TMAO (adjusted HR=2.07 [95%CI 1.37–3.14], P<0.01), choline (adjusted HR=1.52, 95%CI 1.03–2.26, P<0.05), and betaine (adjusted HR=1.59, 95%CI 1.11–2.26, P<0.05) all remained independently predictive of increased risk for all-cause mortality (Supplemental Table 2). Cubic spline curves of Hazard ratios for major adverse cardiac events and all-cause mortality showed progressively increased risk with higher concentrations of TMAO, choline, and betaine (Figure 3). Similar but somewhat attenuated dose-dependent relationships were observed between systemic choline and betaine concentrations and major adverse cardiac event risks in patients. Furthermore, the highest mortality risk cohorts for choline and betaine stratification were also confined to those with increased TMAO concentrations (Supplemental Figure 1). When all 3 metabolites were included into the same adjusted model, the prognostic value of TMAO remained statistically significant (MACE: HR=1.86, 95%CI 1.19–2.92, P<0.01; all-cause mortality: HR=1.78, 95%CI 1.15–2.73, P<0.01). The inclusion of TMAO as a covariate resulted in a significant improvement in risk estimation over traditional risk factors for MACE (NRI, 25.8 %, [P<0.001]; IDI, 2%, [P=0.01]) and for all-cause mortality (NRI, 35.6 %, [p<0.001]; IDI, 2%, [p=0.02]), although neither saw a statistically significant increment in c-statistics (MACE: AUC from 69.6% to 71.2%, P=0.11; all-cause mortality: from 74.4% to 75.3%, P=0.186).
Figure 2.
Kaplan-Meier survival analysis of 3-year risk of major adverse cardiac events and all-cause mortality stratified according to fasting TMAO (A, D), choline (B, E) and betaine (C, F) tertiles.
Figure 3.
Cubic spline curves of hazard ratios for major adverse clinical events (death, non-fatal myocardial infarction, and stroke) at 3years and all-cause mortality at 5 years with fasting concentrations of TMAO (A, B), choline (C, D) and betaine (E, F).
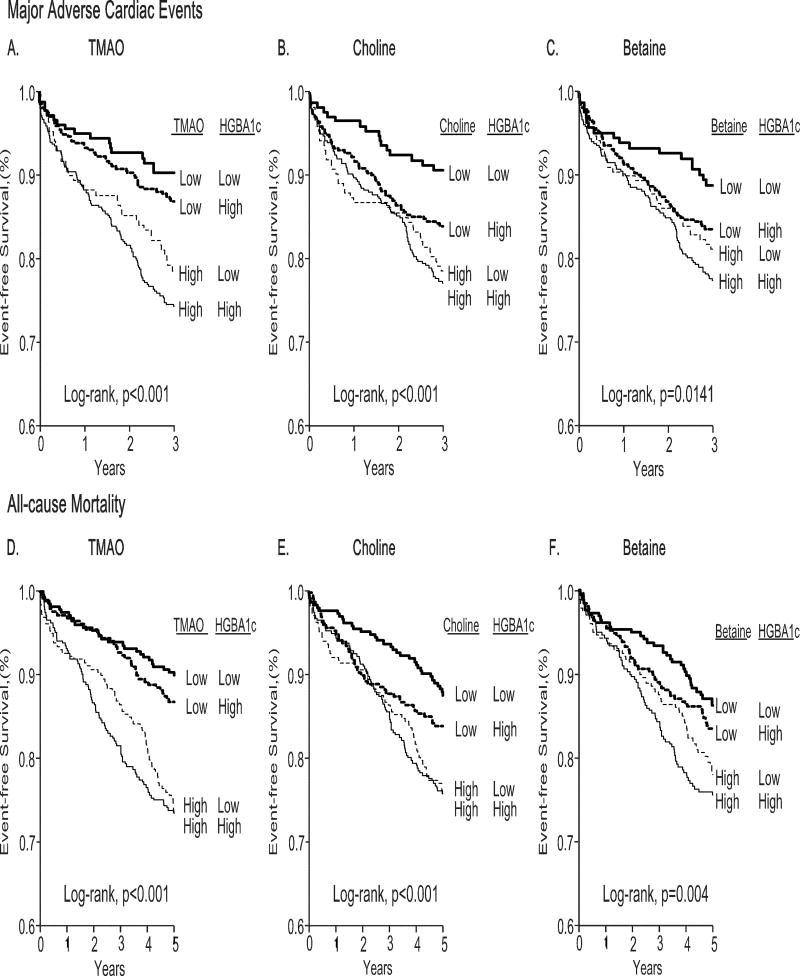
We further investigated the association between phosphatidylcholine metabolite (i.e. TMAO, choline and betaine) concentrations with glycemic status, as quantified by HbA1c levels, within our T2DM cohort. Overall, we observed a modest but statistically significant correlation between choline and HbA1c (r=0.08, P=0.004), while TMAO and HbA1c (r=0.02 P=0.527) and betaine and HbA1c (r=−0.03, P=0.36) did not show significant correlations (Supplemental Table 1). T2DM subjects were further divided according to median phosphatidylcholine metabolite concentrations and further stratified according to glycemic status (above and below HbA1c of 6.5%). We observed that stratification by TMAO, choline, and betaine concentrations conferred stronger MACE risk and mortality risk compared to glycemic status (Figure 4). These results were similar when stratified by HbA1c cut-off of 7.5% (data not shown). Specifically, T2DM subjects with high concentrations of phosphatidylcholine metabolites but good glycemic control had higher mortality risk than those with low concentrations of phosphatidylcholine metabolites but poor glycemic control. Compared to those with low phosphatidylcholine metabolite concentrations and good glycemic control, T2DM patient with increased fasting phosphatidylcholine metabolite concentrations and with concomitantly poor glycemic control had the highest mortality risk (i.e. TMAO [HR=3.2, 95%CI 1.88–5.44, P<0.001]; betaine [HR=1.67, 95%CI 1.08–2.6, P=0.021]; choline [HR=2.14, 95% CI 1.34–3.42, P=0.001]).
Figure 4.
Kaplan-Meier survival analysis of 3-year risk of major adverse cardiac events (A–C) and all-cause mortality (D–F) stratified according to median fasting TMAO (4.4µmol/L), choline (10.6µmol/L) and betaine (39µmol/L) concentrations with glycated hemoglobin levels (6.5%).
DISCUSSION
In this study, we tested the hypothesis that plasma concentrations of the gut microbe-generated metabolite TMAO and two of its nutrient precursors, choline and betaine, were associated with increased long-term mortality risk in patients with T2DM. Several key findings were noted in this analysis. First, plasma TMAO, choline and betaine were each dose-dependently associated with greater 3-year major adverse cardiac events risk and 5-year mortality risk among stable patients with T2DM. Further, the increased 3-year major adverse cardiac events risk remained robust in the TMAO and betaine groups after adjusting for traditional risk factors, inflammation (hsCRP), glycemic status (HbA1c), history of cardiovascular disease and kidney function (eGFR). Moreover, both TMAO and betaine also remained significant for 5-year mortality risk after adjustments in the fully loaded model. Second, we confirmed previous reports (21, 22) that suggested patients with T2DM had higher TMAO, choline, and betaine concentrations than apparently-healthy controls. Third, increased fasting TMAO, choline, and betaine concentrations were observed to be associated with worse prognosis regardless of glycemic status. Fourth, we observe that the prognostic value of increased choline and betaine are only observed amongst those with increased TMAO.
Participation of the gut microbiome in the pathogenesis of cardiometabolic diseases such as obesity, T2DM, and cardiovascular disease is emerging as an exciting and recent realization (5, 20). Studies assessing microbial composition have begun to identify T2DM enriched microbiota in different populations (21, 22). Similarly, specific gut microbial taxa appear to be enriched within individuals having cardiovascular disease (23). And, microbial transplantation studies reveal that impaired glycemic control and both atherosclerosis susceptibility and TMAO production are transmissible traits (10). Various bacterial enzyme complexes have recently been discovered that can generate the precursor to TMAO, trimethylamine (TMA) (13, 24, 25). Interestingly, many members of the desulfovibrio genus, from which the first microbial choline TMA lyase enzyme was reported (26), represents a choline-degrading genre that can liberate TMA from dietary precursors. This sulfate-reducing bacterium has also been identified as a diabetogenic associated taxa (22). In addition, common intestinal residents such as the clostridium genre have been associated with both T2DM and increased TMAO cohorts (11, 22). However, these associations have not proven to be consistent in all populations (21). Nevertheless, it is tempting to speculate on the possibility of microbial ‘gut signatures’ that may confer altered insulin sensitivity and TMAO production potential in the host, and thus correspond to specific disease states such as T2DM and cardiovascular risk.
Several groups have observed associations between choline and betaine with the prevalence of T2DM (17, 27–29). Moreover, many studies have observed increased concentrations of choline and betaine associated with the increased incidence of acute coronary syndromes, as well as increased blood lipid concentrations (14, 17, 30, 31). These studies suggest a potential link between choline and betaine with T2DM and cardiovascular health. However, the evidence is muddied by the fact that some epidemiological studies that predicted choline and betaine intake based upon food questionnaire data show only limited associations with cardiovascular morbidity that failed to remain significant after adjustments for comorbidities (32). Interestingly, in our study, despite the fact that higher plasma betaine concentrations predicted increased adverse prognosis, the average value of betaine was lower in the higher HbA1c cohort. Betaine is known to play an important role in cell volume regulation and the reduction in homocysteine through remethylation (3). However, the relationships between betaine concentrations and cardiovascular risks are still unknown. Studies on betaine as a marker for cardiovascular disease, T2DM, or metabolic syndrome have resulted in widely contrasting marker levels in association with these disease processes (27, 28, 33). However, in general, these studies suggest that decreased betaine, whether as a result of deficiency or increased excretion, may be linked to cardiometabolic disease. The mechanism(s) for observed reduction in betaine concentrations in the setting of T2DM are unknown, though hypotheses may include increased betaine-homocysteine methyltransferase activity or betaine efflux dysregulation (17, 34).
Indeed, choline and betaine have been linked to changes in insulin resistance with increased choline bacterial conversion to TMA and TMAO associated with impaired glucose homeostasis in a non-alcoholic fatty liver animal model (4). Recent studies report that dietary TMAO enhances the impaired glucose tolerance observed in mice fed a high fat diet (35). Moreover, manipulation of TMAO concentrations in mice through inhibition in host flavin monooxygenase 3 (FMO3), the key host enzyme responsible for converting TMA into TMAO (12), demonstrates the pathway exerts broad effects on glucose metabolism (12, 36). Thus, some studies suggest mechanistic links between the TMAO pathway and T2DM. Consistent with this, in several clinical cohorts, including individuals undergoing elective diagnostic cardiac catheterization, as well as among patients with chronic systolic heart failure, we have observed in cross section an increased prevalence of T2DM among individuals with increased TMAO (9, 14, 37).
In this study, while individuals with T2DM had higher TMAO, choline and betaine concentrations relative to controls, we did not observe a strong relationship between the concentrations of TMAO, choline, or betaine and glycemic status in this T2DM population. Although choline was modestly correlated with HbA1c levels, TMAO and betaine were not. Nonetheless, evidence of associations between increased TMAO, choline and betaine concentrations and increased mortality across glycemic status suggest that these metabolites, especially TMAO, may serve as an important risk marker for adverse prognosis in T2DM, and be increased in T2DM by a common metabolic alteration. Furthermore, the dependence of TMAO on gut microbiota composition and its dietary relationship with choline and betaine lead us to believe that gut microbial processes generating TMAO from choline or betaine may represent a potential pathogenic link with both cardiovascular disease development and T2DM status. Whether targeting the gut microbial TMAO pathway pharmacologically can impact cardiovascular disease (and T2DM) risks in patients is not yet known. However, it is noteworthy that in recent studies, non-lethal pharmacological targeting of gut microbial TMA lyase activity was effective at both inhibiting TMA and TMAO production in vivo, and attenuating diet induced atherosclerosis in an animal model (38). Moreover, the TMA lyase inhibitor used was also found to be a natural product present in abundance in some cold-pressed extra virgin olive oils and grape seed oils (38). Consumption of extra virgin olive oil is a critical component of the Mediterranean diet, and adherence to a Mediterranean diet has been associated with both reduced concentrations of TMAO (39)and reduction in risk for both cardiovascular disease and T2DM development (40). Further studies on the relationship between the gut microbial TMAO pathway and both T2DM and cardiovascular risks are warranted.
Study Limitations
As a single-center observational cohort, these findings need to be replicated in other validation cohorts. Furthermore, we do not have adequate information regarding the type and/or drug dosing of various glucose-lowering agents used and some drugs may be temporarily withheld or reduced in dosages in the setting of scheduled elective diagnostic coronary angiography. We also do not have information regarding dietary patterns, duration of diabetes/insulin use, or different types and severity of diabetic complications associated with microvascular dysfunction (neuropathy, nephropathy, and retinopathy). Similarly, no information was available regarding causes of death or non-prescriptive dietary supplements that may have been utilized by patients at the time of study. Regardless, the present studies suggest that further investigations into the role of modulating gut microbiota to modify cardiovascular risks in patients with T2DM are of interest in this vulnerable patient population.
CONCLUSION
The plasma concentration of TMAO, a pro-atherogenic compound formed by gut microbe-dependent metabolism of choline, is increased in individuals with T2DM and associated with increased risk of major adverse cardiac events and mortality in patients with T2DM independent of glycemic status.
Supplementary Material
Table 2.
Relationship between plasma concentrations of TMAO, choline or betaine and cardiovascular and mortality risks
| Hazard ratio |
Major Adverse Cardiac Events at 3 years | All-Cause Mortality at 5 years | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| TMAO (µM) | <3.2 | 3.2–6.3 | ≥6.3 | <3.2 | 3.2–6.3 | ≥6.3 |
|
| ||||||
| Unadjusted Model | 1 | 1.95 (1.31–2.9)** | 3.03 (2.08–4.42)*** | 1 | 1.77 (1.19–2.64)** | 3.63 (2.53–5.21)*** |
| Adjusted Model | 1 | 1.75 (1.15–2.66)** | 1.94 (1.23–3.05)** | 1 | 1.46 (0.96–2.22) | 1.85 (1.21–2.84)** |
| Events | 36/401 | 71/414 | 102/401 | 38/401 | 66/414 | 123/401 |
|
| ||||||
| Choline (µM) | <9.1 | 9.1–12.3 | ≥12.3 | <9.1 | 9.1–12.3 | ≥12.3 |
|
| ||||||
| Unadjusted Model | 1 | 1.86 (1.28–2.71)** | 2.2 (1.52–3.17)*** | 1 | 1.81 (1.24–2.63)** | 2.64 (1.85–3.77)*** |
| Adjusted Model | 1 | 1.55 (1.03–2.33)* | 1.38 (0.89–2.13) | 1 | 1.41 (0.95–2.09) | 1.36 (0.91–2.05) |
| Event | 42/401 | 78/412 | 89/403 | 43/401 | 77/412 | 107/403 |
|
| ||||||
| Betaine (µM) | <33.2 | 33.2–45.7 | ≥45.7 | <33.2 | 33.2–45.7 | ≥45.7 |
|
| ||||||
| Unadjusted Model | 1 | 1.11 (0.78–1.59) | 1.62 (1.16–2.27)** | 1 | 1.3 (0.92–1.84) | 1.88 (1.35–2.63)** |
| Adjusted Model | 1 | 1.08 (0.74–1.58) | 1.41 (0.97–2.03) | 1 | 1.32 (0.92–1.91) | 1.57 (1.09–2.26)* |
| Event | 56/400 | 65/415 | 88/401 | 55/400 | 74/415 | 98/401 |
Adjusted for traditional risk factors include age, gender, history of cardiovascular disease, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, hsCRP, log-transformed HbA1c, log-transformed eGFR, log-transformed body mass index, and history of heart failure;
P < 0.05,
P < 0.01,
P < 0.001.
Acknowledgments
Funding Support: This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000). The GeneBank study has been supported by NIH grants P01HL076491, P01HL098055, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr. Wang was partially supported by an American Heart Association Scientist Development Grant. Dr. Hazen is also partially supported by a gift from the Leonard Krieger endowment. Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Innovations Award by AB SCIEX.
Dr. Wang and Dr. Hazen are named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion and P&G. Dr. Hazen reports receiving research funds from Astra Zeneca, P&G, Pfizer Inc., Roche and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Siemens, Esperion, Frantz Biomarkers, LLC.
Footnotes
Financial Disclosure: All other authors have no relationships to disclose.
Author contributions were as follows: W.H.W.T. and S.L.H conceived and designed the experiments. Z.W., X.L. performed the experiments. Y.F. and Y.W. performed the statistical analyses. W.H.W.T., D.S.L. and S.L.H. wrote the manuscript. All authors critically reviewed the manuscript.
References
- 1.Zeisel SH, da Costa KA. Choline: An essential nutrient for public health. Nutr Rev. 2009;67:615–23. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paoletti L, Elena C, Domizi P, Banchio C. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life. 2011;63:714–20. doi: 10.1002/iub.521. [DOI] [PubMed] [Google Scholar]
- 3.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43:732–44. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1068–77. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Microbiota and diabetes: An evolving relationship. Gut. 2014;63:1513–21. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–60. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, et al. Trimethylamine n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, et al. Gamma-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to tmao. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-n-oxide. Eur Heart J. 2014;35:904–10. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, et al. The tmao-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Betaine and trimethylamine-n-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: An observational study. PLoS One. 2014;9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-n-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–11. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in european women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 23.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–8. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craciun S, Marks JA, Balskus EP. Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem Biol. 2014;9:1408–13. doi: 10.1021/cb500113p. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Jameson E, Crosatti M, Schafer H, Rajakumar K, Bugg TD, Chen Y. Carnitine metabolism to trimethylamine by an unusual rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111:4268–73. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109:21307–12. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–20. doi: 10.1093/jn/138.5.914. [DOI] [PubMed] [Google Scholar]
- 28.Lever M, Slow S, McGregor DO, Dellow WJ, George PM, Chambers ST. Variability of plasma and urine betaine in diabetes mellitus and its relationship to methionine load test responses: An observational study. Cardiovasc Diabetol. 2012;11:34. doi: 10.1186/1475-2840-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schartum-Hansen H, Ueland PM, Pedersen ER, Meyer K, Ebbing M, Bleie O, et al. Assessment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One. 2013;8:e69454. doi: 10.1371/journal.pone.0069454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danne O, Lueders C, Storm C, Frei U, Mockel M. Whole blood choline and plasma choline in acute coronary syndromes: Prognostic and pathophysiological implications. Clin Chim Acta. 2007;383:103–9. doi: 10.1016/j.cca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Lever M, George PM, Atkinson W, Molyneux SL, Elmslie JL, Slow S, et al. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS One. 2011;6:e21666. doi: 10.1371/journal.pone.0021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: The atherosclerosis risk in communities (aric) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lever M, George PM, Elmslie JL, Atkinson W, Slow S, Molyneux SL, et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7:e37883. doi: 10.1371/journal.pone.0037883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35:1175–9. doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- 35.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine n-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-n-oxide in patients with heart failure: Refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–95. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–21. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 40.Salas-Salvado J, Bullo M, Babio N, Martinez-Gonzalez MA, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the mediterranean diet: Results of the predimed-reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–9. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.