Abstract
Rocky Mountain spotted fever, caused by Rickettsia rickettsii, is a potentially fatal tick-borne disease spread from North America to Argentina. The major vectors of R. rickettsii in the United States are Dermacentor andersoni Stiles and Dermacentor variabilis (Say). It is generally believed that vector ticks serve as major reservoirs of R. rickettsii in nature; however, the ability of ticks to support the indefinite perpetuation of R. rickettsii has been challenged by reports of deleterious effects of rickettsial infection on D. andersoni. To better elucidate the relationship of the pathogen with D. variabilis, we assessed the effects of R. rickettsii on the survival, fertility, and fecundity of D. variabilis. We used an isolate of R. rickettsii (Di-6), originally acquired from an opossum caught in Virginia, and ticks from a laboratory colony established from adult D. variabilis also collected in Virginia. Overall, infection with R. rickettsii protracted the feeding periods of all life stages of ticks. Infected nymphal and adult ticks experienced a slight decrease in feeding success compared with the uninfected colony, but neither larval nor nymphal molting success was affected. Infected females reached smaller engorgement weights, were less efficient in conversion of bloodmeal into eggs, and produced smaller egg clutches with a lower proportion of eggs hatching. However, no sudden die-off was observed among infected ticks, and longevity was not decreased due to R. rickettsii infection in any stage. Although infection with the studied isolate of R. rickettsii caused slight decrease in fecundity in sympatric vector ticks, no obvious deleterious effects were observed.
Keywords: Rickettsia, Rickettsia rickettsii, Rocky Mountain spotted fever, Dermacentor variabilis, Dermacentor
Rocky Mountain spotted fever (RMSF) is a potentially fatal tick-borne disease spread from North America to Argentina (Paddock et al. 2008). Dermacentor andersoni Stiles—the Rocky Mountain wood tick, and Dermacentor variabilis (Say)—the American dog tick, are regarded as primary vectors of Rickettsia rickettsii in the United States (Burgdorfer 1975, McDade and Newhouse 1986). In addition, several other tick species from genera Amblyomma and Rhipicephalus have been reported as competent vectors throughout the vast geographical range of this pathogen (Demma et al. 2005, Labruna et al. 2008, Parola et al. 2013). It is generally believed that vector ticks also serve as major reservoirs of R. rickettsii in nature, due to efficient vertical transmission—both transstadial and transovarial (Ricketts 1909, Wolbach 1919, Burgdorfer and Brinton 1975).
However, the ability of Dermacentor ticks to support indefinite perpetuation of R. rickettsii in nature was challenged by reports of decreased survival and fecundity in naturally and experimentally infected ticks. First, Burgdorfer and Briton (1975) observed “unusually high mortality among engorged females” of both D. andersoni and D. variabilis transovarially infected with several strains of R. rickettsii as well as decreased fecundity in the surviving ticks. Later, Niebylski and colleagues (1999) reported that R. rickettsii infection caused lethal effects on all developmental stages of D. andersoni. Since the publication of those reports, it has been commonly presumed that most if not all tick-borne pathogenic rickettsiae negatively affect the survival of their respective vectors. This overarching point of view had been reinforced by the demonstrated decrease in fecundity and survival of brown dog ticks, Rhipicephalus sanguineus (Latreille), as a result of infection with Rickettsia conorii (Matsumoto et al. 2005, Levin et al. 2009), although significant differences were noted between effects caused by different isolates of Rickettsia. Surprisingly, since the original report by Burgdorfer and Briton (1975), there has been no data published on the relationship between R. rickettsii and D. variabilis throughout the tick’s developmental cycle. In order to better elucidate the effects of R. rickettsii on this major tick vector, we compared a Rickettsia-infected D. variabilis colony to the pathogen-free colony by quantifying feeding and molting success, survival, and longevity of each developmental stage as well as fertility and fecundity of adult ticks.
Materials and Methods
An uninfected colony of D. variabilis is maintained in the Medical Entomology laboratory at CDC by feeding all developmental stages upon specific pathogen-free New Zealand white rabbits (Oryctolagus cuniculus) as previously described (Troughton and Levin 2007). This colony originated from adult ticks collected from vegetation in Virginia in 2009. Between feedings ticks were held in incubators at 22 ± 1°C with a photoperiod of 16:8 (L:D) h and 90% relative humidity (RH). The Rickettsia-free status of this colony is routinely monitored and confirmed in every generation by quantitative polymerase chain reaction (qPCR) in representative samples of larvae from progeny of every female and by immunofluorescence assay in serum samples from every animal used for colony maintenance.
An isolate of R. rickettsii (BSF-Di6) was originally acquired from an opossum (Didelphys virginiana) also caught in Virginia. The isolate was propagated and stored in yolk-sack culture for five passages and then expanded in Vero cells. The BSF-Di6 isolate has demonstrated pathogenicity in domestic dogs (Levin et al. 2014). All stages of D. variabilis ticks infected with R. rickettsii (isolate BSF-Di6) were derived from the second generation of our infected colony, developed and maintained as described earlier (Levin et al. 2014). In brief, larval and nymphal ticks were acquisition fed upon Hartley guinea pigs needle-inoculated with the second Vero cell passage of isolate BSF-Di6. Following acquisition feeding, the R. rickettsii -infected tick colony was maintained by feeding upon tick- and pathogen-naive New Zealand white rabbits in parallel with the uninfected colony under identical environmental conditions.
For the purpose of this experiment, we used two 4-mo-old tick-naïve pathogen-free female rabbits. One rabbit was designated for infestation with uninfected D. variabilis (control) and the other for infestation with R. rickettsii-infected D. variabilis. One day before tick placement, each rabbit was outfitted with three tick-feeding bags attached to its shaved dorsum with Kamar adhesive (Kamar, Inc., Steamboat Springs, CO), which is approved for veterinary use (Troughton and Levin 2007). Affixing the feeding bags a day before placement of ticks ensured that the adhesive dried thoroughly and would not interfere with tick engorgement. Larval, nymphal, and adult stages of ticks from either infected or uninfected colonies were placed in separate feeding bags. Each rabbit had the three life stages placed in the same order: the first bag closest to the head held ~3,000 larvae from a single egg clutch, the second bag contained 300 nymphs, and the third bag—furthest from the head—contained 25 male and female pairs. Rabbits were kept in separate cages within the same room at 20°C and 30–50% RH. All animals used in the study were maintained according to the protocols approved by the Institutional Animal Care and Use Committee.
Ticks placed on the rabbits were allowed to feed to repletion. Each bag was checked daily, and engorged ticks were collected. Special care was taken to ensure that uninfected and R. rickettsii-infected ticks were kept separate. Engorged larvae and nymphs were counted, and stored in clearly labeled polystyrene tubes inside the designated incubator. Up to 100 larvae or up to 10 nymphs were stored in each polystyrene tube to prevent overcrowding, which may affect the molting success of ticks. Replete females were collected as soon as they completed engorgement and detached from rabbits. Females were individually weighted and placed in separate numbered tubes for oviposition. Females that died during the feeding were immediately collected and processed for qPCR. All male ticks were removed from both rabbits after detachment of the last engorged female tick and immediately processed for qPCR. Nymphal and adult feeding success was determined as the proportion of ticks that successfully engorged in relation to the total number of ticks placed in feeding bags. Larval feeding success was not accessed due to impossibility to count individual hungry larvae without jeopardizing their survival. Feeding duration for individual ticks, excluding males, was calculated as the average number of days it took for the ticks to feed to repletion from the day of placement on rabbits.
Developmental progress of the engorged ticks was monitored daily. Molting success was determined for engorged larvae and nymphs as a percent of ticks successfully molting to the next developmental stage. Twenty-five of the freshly molted nymphs, as well as 10 females and 10 males from the infected and uninfected cohorts, were individually tested by qPCR for the presence of rickettsial DNA. The remaining molted ticks from both cohorts were stored in groups of 50 nymphs or 10 adults (males and females kept separately) per tube and monitored for longevity.
For every engorged female, the duration of the preovipostion and incubation periods was determined as the number of days from detachment to the beginning of oviposition and from the beginning of oviposition to the hatching of the first larva, respectively. The weight of every egg mass was measured upon completion of oviposition, and the bloodmeal conversion index (BMCI—a measure of tick’s ability to convert its bloodmeal into eggs) was calculated as a percentage of the egg mass weight to the weight of the corresponding engorged female at the time of its drop-off from the rabbit. Larval eclosion in each individual egg batch was monitored three times per week for 12 wk, and the hatchability—percentage of hatching eggs—in each batch was determined as C/(E + C) × 100, where: C = eggshells, E = unhatched eggs (Abbott 1925). Following assessment of hatchability, a pool of ~100 larvae from the progeny of each female in both cohorts was also tested by qPCR for the presence of rickettsial DNA.
DNA extraction and qPCR procedures were carried out in separate facilities as described (Levin et al. 2012, Zemtsova et al. 2015). DNA was extracted from ticks and blood samples using the Qiagen DNEasy Blood and Tissue kit (Qiagen Inc., Valencia, CA) according to manufacturer’s protocols. The presence of rickettsial DNA was detected using primers RR190-547F and RR190-701R to amplify a 154-bp fragment of the rompA gene of Rickettsia (Roux et al. 1996, Eremeeva et al. 2003). All samples were tested in duplicates with negative samples included in all extraction and qPCR rounds. Water was used as a no-template negative control.
Statistical differences between uninfected and R. rickettsii-infected cohorts in feeding, survival, and reproductive parameters were analyzed using chi-square (χ2) and analysis of variance (ANOVA) tests. Percentage indices were arcsine transformed for statistical analyses. Variables were considered significantly different when the P value was ≤ 0.05. Survival of flat nymphs was analyzed using linear regression.
Results
Overall, feeding, molting, and fecundity parameters were measured for 1,304 larvae, 285 nymphs, and 22 pairs of adults from the R. rickettsii-infected colony of D. variabilis. These were compared with data from 1,677 larvae, 299 nymphs, and 25 pairs of adults from the uninfected colony (Table 1).
Table 1.
Summary of averages of biological parameters of D. variabilis ticks infected with R. rickettsii and uninfected control groups with 95% confidence intervals
| Biological parameter | Uninfected | Infected |
|---|---|---|
| Larval feeding duration (d) | 4.9 ± 0.01 | 5.1 ± 0.4* |
| Larval molting success | 48.7 ± 2.41% | 65.9 ± 2.61%* |
| Nymphal feeding duration (d) | 5.1 ± 0.02 | 5.9 ± 0.06* |
| Nymphal feeding success | 99.7 ± 0.650% | 95.0 ± 2.47%* |
| Nymphal molting success | 99.3 ± 1.02% | 99.2 ± 1.06% |
| Female feeding duration (d) | 7.5 ± 0.3 | 9.3 ± 0.6* |
| Female feeding success | 100% | 88 ± 13%* |
| Male feeding success | 100% | 84 ± 15%* |
| Female engorgement weight (g) | 0.628 ± 0.049 | 0.537 ± 0.047* |
| Preoviposition period (d) | 8.9 ± 0.6 | 9.2 ± 0.8 |
| Clutch weight (g) | 0.298 ± 0.032 | 0.213 ± 0.043* |
| Bloodmeal conversion index | 0.472 ± 0.026 | 0.389 ± 0.058* |
| Incubation (d) | 53.4 ± 0.9 | 53.5 ± 0.8 |
| Percentage hatched | 83 ± 12% | 60 ± 9.5%* |
Values of D. variabilis ticks infected with R. rickettsii are significantly different than uninfected D. variabilis ticks (P ≤ 0.05).
D. variabilis larvae from the R. rickettsii-infected cohort fed for an average of 5.13 d, while uninfected D. variabilis larvae had an average feeding duration of 4.87 d. Although this delay in larval engorgement was relatively small, the observed difference is statistically significant (P[ANOVA] < 0.001). On the other hand, engorged larvae in the infected colony had a higher molting success (65.91%) than their uninfected counterparts (48.73%; P[x2] < 0.00001).
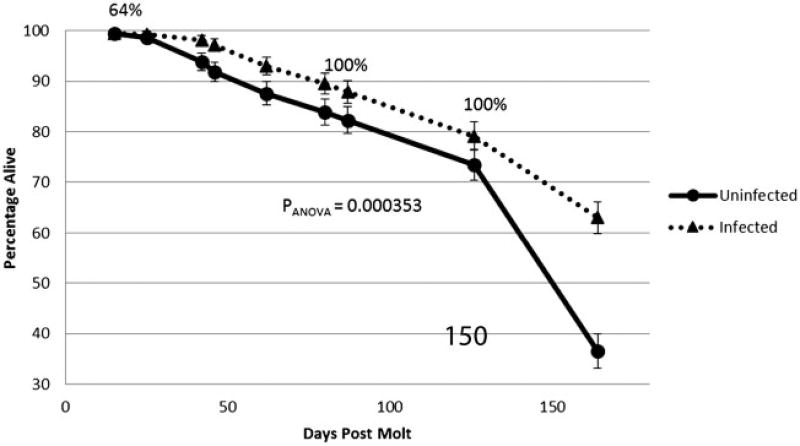
When representative samples of nymphal ticks from the uninfected cohort were tested by qPCR for the presence of rickettsial DNA, all were negative as expected. QPCR testing of representative samples among nymphs in the R. rickettsii-infected cohort showed 64% prevalence of rickettsial DNA immediately after molting and 100% when tested 126 d post molt (Fig. 1). Correspondingly, 63% of unfed nymphs from the R. rickettsii-infected cohort remained alive at 164 d post molt, while in the uninfected cohort only 36.5% of nymphs survived to that point (P[x2] < 0.00001).
Fig. 1.
Survival of D. variabilis nymphs in R. rickettsii-infected (blue line) and uninfected (red line) cohorts without a bloodmeal. (Vertical bars—95% confidence intervals; numbers—prevalence of rickettsial DNA in the infected cohort.)
The nymphal feeding success in the R. rickettsii-infected cohort (95%) was significantly lower than that in the uninfected cohort (99.7%; P[x2] = 0.004), and infected D. variabilis nymphs took a significantly longer time to fully engorge, with the average feeding duration of 5.85 d compared with an average of 5.14 d for uninfected nymphs (P[ANOVA] = 0.001; Table 1). However, there was no difference observed in the molting success between infected and uninfected D. variabilis nymphs, with >99% of ticks in both cohorts successfully molting into the adult stage. The prevalence of rickettsial infection in the adult stage decreased with age from 95% among freshly molted ticks to only 45% after 126 d of starvation. Adult tick longevity, on the other hand, did not appear to be affected by rickettsial infection, as all of the infected and uninfected unfed adults were still alive at 164 d post molt.
Out of 25 adult pairs (25 males and 25 females) of D. variabilis from the infected colony only 86%—22 females and 21 males— successfully fed, and females required on average 9.27 ± 0.61 d to complete engorgement. On the other hand, 100% of uninfected adults fed to repletion, and females engorged within 7.47 ± 0.25 d (Table 1). Similar to the immature stages, the duration of feeding was increased (P[ANOVA] = 7.01E-05) and the feeding success— decreased (P[x2] = 0.006) in the R. rickettsii-infected adult cohort compared with the uninfected adults. Moreover, R. rickettsii-infected females had an average engorgement weight of 0.537 g, whereas uninfected females had an average engorgement weight of 0.628 g (Table 1). Thus, the infected females appeared to acquire a smaller bloodmeal (P[ANOVA] = 0.017151) despite feeding longer than their uninfected counterparts. QPCR testing confirmed that in the control cohort none of the engorged males or females, tested after oviposition, contained rickettsial DNA. Within the infected cohort of D. variabilis adults, 95.45 ± 8.91% of the postoviposition females and 64 ± 19.2% of the fed males contained R. rickettsii DNA.
Both infected and uninfected engorged females commenced oviposition ~9 d after repletion. In the uninfected cohort, all 25 engorged females produced egg clutches, whereas in the Rickettsia-infected cohort 2 of the 22 fed ticks died without producing any eggs. In addition, BMCI of infected D. variabilis females was lower than in uninfected females (Table 1). As a result, egg clutches of infected females (on average 0.213 g) were significantly smaller than those produced by uninfected ticks (on average 0.298 g; P[ANOVA] = 0.008). Rickettsial DNA was detected in samples from only nine progenies of the 19 infected females (one batch of eggs was lost to fungal infection and discarded). The duration of egg incubation did not seem affected by the presence of R. rickettsii, and progenies of both infected and uninfected females began hatching within 52–54 d after commencement of oviposition or 60–65 d after female repletion. However, egg hatchability was lower (60.0 ± 9.5%) in clutches laid by infected females versus those produced by uninfected ticks (83.2 ± 11.7%). This difference was also statistically significant (P[ANOVA] = 0.006), resulting in the smaller numbers of live larvae, even though only half of the infected females succeeded in transmitting R. rickettsii transovarially.
Discussion
Two previous studies reported that several isolates of R. rickettsii may negatively affect the survival of its Dermacentor tick vectors. In one, authors observed large numbers of transovarially infected engorged females failing to produce eggs (Burgdorfer and Brinton 1975). This effect was noted only starting with the fifth filial generation of transovarially infected ticks maintained in separate lines and, thus, might have been caused by tick inbreeding (Troughton and Levin 2007) rather than by the infection itself. Niebylski, on the other hand, reported lethal effects of R. rickettsii infection on all life stages of D. andersoni immediately after feeding uninfected ticks upon needle-inoculated guinea pigs (Niebylski et al. 1999). Whether the observed effects were due to high virulence of the particular isolate, the initial infectious dose, or the intrinsic incompatibility between the specific isolate and the particular population of ticks remains unclear. Endeavoring to avoid the same pitfalls in our experiments, we assessed effects of R. rickettsii in ticks infected transovarially, rather than by an artificially inoculated host, but limited observations to the second generation—before the survival and fecundity of ticks could be affected by inbreeding.
Thus, we evaluated how natural infection with R. rickettsii affects survival and propagation of D. variabilis ticks. Since different strains of a pathogen may have disparate effects on tick vectors from different populations (Levin et al. 2009), we purposely infected our ticks (derived from Virginia) with a R. rickettsii isolate that originated from the same geographical region. Effects of rickettsial infection were assessed starting with F2 larvae infected transovarially. These were evaluated by comparison with an uninfected tick colony maintained in exact parallel, on the same schedule, under the same laboratory conditions, and fed on the same species of vertebrate hosts. Contrary to the above-mentioned reports, we did not observe outright deleterious effects of a sympatric isolate of R. rickettsii on the survival or vitality of D. variabilis. Moreover, data suggest that effects of rickettsial infection vary between tick life stages, and the pathogen negatively affect some aspects of the life cycle but not the others.
Overall, results of this study demonstrate that in D. variabilis ticks infected with a sympatric isolate of R. rickettsii, length of the feeding period was extended at all life stages, with the biggest difference observed in adult ticks. This delay in repletion can be seen as a negative effect because under natural conditions extended attachment may result in more ticks being removed or damaged by the host’s grooming. Although these differences in the mean duration of feeding between infected and uninfected ticks were significant from the statistical point, they were not substantial and fell within the range previously reported for uninfected D. variabilis under standardized laboratory conditions (Troughton and Levin 2007). Both nymphal and adult stages of infected ticks experienced a measurable decrease in their feeding success when compared with the uninfected cohorts, though not to a detrimental extent, as 95% of nymphs and 84% of adults in the R. rickettsii-infected colony fed to repletion. However, neither larval nor nymphal molting success was affected by R. rickettsii infection, illustrating that infected ticks, which survived the feeding, were able to reach their next developmental stage.
Interestingly, unfed nymphal ticks in our study appeared to benefit from R. rickettsii infection as their ability to endure long starvation increased. This resulted in a significantly larger percentage of nymphs in the infected cohort surviving for extended periods of time without a bloodmeal. Moreover, within the infected cohort, the prevalence of rickettsial infection increased from 64% among freshly molted nymphs to 100% among unfed nymphs remaining alive at 126 d after the molt. This rise in the prevalence of infection within the same cohort through the 3-mo starvation period may suggest either postmolt proliferation of rickettsiae inside infected ticks above the detection threshold, or a greater longevity among infected individuals. The later statement is seemingly supported by the larger proportion of nymphs in the infected cohort surviving for extended periods of time without a bloodmeal than in the uninfected cohort. Contrary to the previous studies, we did not observe any unusual mortality among naturally infected D. variabilis adults either, as none of the unfed adult ticks in either Rickettsia-infected or uninfected cohorts had died at 164 d after molting.
In spite of the prolonged feeding period, R. rickettsii-infected females had a decreased engorgement weight, clutch weight, BMCI, and hatchability, which all resulted in a smaller progeny size. These results are similar to those observed in Haemaphysalis leporispalustris (Packard) females infected with R. rickettsii, with the exception of the reduced BMCI (Freitas et al. 2009). Interestingly, the noted negative effects on tick fecundity took place regardless whether samples of their individual progenies tested PCR-positive for rickettsial DNA. It is also noteworthy that we did not observe any sudden die-offs in any larval batches in the infected cohort, and the longevity of unfed larvae was not decreased due to R. rickettsii infection.
These relatively minor effects of R. rickettsii infection may hypothetically be due to the reported ability of the pathogen to transform to a dormant phase—“degeneration of R. rickettsii structures” within the ticks (Hayes and Burgdorfer 1982). During this degeneration, which has been observed during the host-seeking phase of the tick life cycle, R. rickettsii seems to become dormant, possibly allowing the pathogen to have a decreased effect on the fitness of the vector (Socolovschi et al. 2009). It has been suggested that this transformation to the dormant phase may be triggered by starvation, drops in temperature, or hormonal changes within the tick (Gilford and Price 1955, Hayes and Burgdorfer 1982, Socolovschi et al. 2009).
Our observation of mildly negative effects of rickettsial infection on some biological parameters of D. variabilis and the seeming improvement in others are in agreement with studies in H. leporispalustris and Rh. sanguineus ticks infected with R. rickettsii (Freitas et al. 2009, Piranda et al. 2011). While the overall performance of infected ticks in both reports was somewhat decreased, some parameters showed improvement in comparison with uninfected ticks. It is noteworthy that neither study reported deleterious effects of R. rickettsii on the respective tick vector (Freitas et al. 2009, Piranda et al. 2011).
Thus, a decrease in fecundity of infected females appears to be the only notable negative effect of R. rickettsii infection on the sympatric D. variabilis observed in this study. No obvious lethal effects were observed. Our results demonstrate that the previously reported lethal effects caused by a specific isolate of R. rickettsii in a specific laboratory colony of D. andersoni should not be taken as an axiom and presumptively expanded to D. variabilis, or other tick vectors of R. rickettsii. On the other hand, in the light of this and other recently published studies, we propose that sympatric populations of tick vectors and rickettsial pathogens should be able to adjust to each other through the process of coadaptation and survive without causing mutual extermination.
Acknowledgments
We would like to express our appreciation to Lindsay Killmaster and Galina Zemtsova for their help and support, and also to Scott Dahlgren for his insight on statistical analysis. Lauren Schumacher is supported by the CDC Foundation and Alyssa Snellgrove is supported by the Oak Ridge Institute for Science and Education (ORISE).
References Cited
- Abbott W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:256–257. [Google Scholar]
- Burgdorfer W. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 1975;13:269–278. doi: 10.1093/jmedent/12.3.269. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Brinton LP. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann. N.Y. Acad. Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Zaki SR, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME, Dasch GA, Silverman DJ. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group Rickettsiae. J. Clin. Microbiol. 2003;41:5466–5472. doi: 10.1128/JCM.41.12.5466-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas LHT, Faccini LH, Labruna MB. Experimental infection of the rabbit tick, Haemaphysalis leporispalustris, with the bacterium Rickettsia rickettsii, and comparative biology of infected and uninfected tick lineages. Exp. Appl. Acarol. 2009;47:321–345. doi: 10.1007/s10493-008-9220-4. [DOI] [PubMed] [Google Scholar]
- Gilford GH, Price WH. Virulent-a virulent conversions of Rickettsia rickettsii in vitro. Proc. Natl. Acad. Sci. USA. 1955;41:870–873. doi: 10.1073/pnas.41.11.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SF, Burgdorfer W. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: An ultrastructural analysis. Infect. Immun. 1982;37:779–785. doi: 10.1128/iai.37.2.779-785.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Ogrzewalska M, Martins TF, Pinter A, Horta MC. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J. Med. Entomol. 2008;45:1156–1159. doi: 10.1603/0022-2585(2008)45[1156:csolso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova G, Grant D, Mumcuoglu KY, Eremeeva ME, Dasch GA. Incongruent effects of two isolates of Rickettsia conorii on the survival of Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 2009;48:347–359. doi: 10.1007/s10493-009-9268-9. [DOI] [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE. Domestic dogs (Canis familiaris) as reservoir hosts for Rickettsia conorii. Vector Borne Zoonotic Dis. 2012;12:28–33. doi: 10.1089/vbz.2011.0684. [DOI] [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, Langham G. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS ONE. 2014;9:e115105. doi: 10.1371/journal.pone.0115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Brouqui P, Raoult D, Parola P. Experimental infection models of ticks of the Rhipicephalus sanguineus group with Rickettsia conorii. Vector Borne Zoonotic Dis. 2005;5:363–372. doi: 10.1089/vbz.2005.5.363. [DOI] [PubMed] [Google Scholar]
- McDade JE, Newhouse VF. Natural history of Rickettsia rickettsii. Annu. Rev. Microbiol. 1986;40:287–309. doi: 10.1146/annurev.mi.40.100186.001443. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl. Environ. Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Fernandez S, Echenique GA, Sumner JW, Reeves WK, Zaki R, Remondegui CE. Rocky mountain spotted fever in Argentina. Am. J. Trop. Med. Hyg. 2008;78:687–692. [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piranda EM, Faccini JLH, Pinter A, Pacheco RC, Cancçado PHD, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 2011;11:29–36. doi: 10.1089/vbz.2009.0250. [DOI] [PubMed] [Google Scholar]
- Ricketts HT. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. The Wesley M. Carpenter lecture of the New York Academy of Medicine. Med. Record. 1909;76:843–855. [Google Scholar]
- Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovschi C, Mediannikov O, Raoult D, Parola P. The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet. Res. 2009;40:34. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton DR, Levin ML. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol. 2007;44:732–740. doi: 10.1603/0022-2585(2007)44[732:lcosit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wolbach SB. Studies on Rocky Mountain spotted fever. J. Med. Res. 1919;41:1–197. [PMC free article] [PubMed] [Google Scholar]
- Zemtsova GE, Montgomery M, Levin ML. Relative sensitivity of conventional and real-time PCR assays for detection of SFG Rickettsia in blood and tissue samples from laboratory animals. PLoS ONE. 2015;10:e0116658. doi: 10.1371/journal.pone.0116658. [DOI] [PMC free article] [PubMed] [Google Scholar]