Abstract
Purpose
To describe the risk of early‐ and late‐onset preeclampsia across pregnancies exposed to antidepressants and to evaluate the impact of timing and length of gestational exposure to antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), on preeclampsia.
Methods
The Norwegian Mother and Child Cohort, a prospective population‐based study, and the Medical Birth Registry of Norway provided information on antidepressant exposure, depression, and anxiety symptoms in pregnancy, preeclampsia diagnoses, and important covariates. Within a pregnancy cohort of depressed women, we compared the risk of late‐onset preeclampsia between SSRI‐exposed and nonmedicated pregnancies using marginal structural models (weighted) and modified Poisson regression models.
Results
Of the 5887 pregnancies included, 11.1% were exposed at any time before week 34 to SSRIs, 1.3% to serotonin‐norepinephrine reuptake inhibitors, 0.4% to tricyclic antidepressants, and 0.5% to other antidepressants. The risks of early‐ and late‐onset preeclampsia by exposure status in pregnancy were 0.3% and 3.6% (nonmedicated), 0.4% and 3.7% (SSRIs), 1.5% and 4.1% (serotonin‐norepinephrine reuptake inhibitors), and 7.1% and 10.0% (tricyclic antidepressants). Compared with nonmedicated pregnancies, SSRI‐exposed in mid and late gestation had adjusted relative risks for late‐onset mild preeclampsia of 0.76 (95% confidence interval, 0.38‐1.53) and 1.56 (0.71‐3.44) (weighted models), respectively. There was no association between SSRI exposure in pregnancy and severe late‐onset preeclampsia.
Conclusions
We have provided evidence that SSRI use in early and midpregnancy does not substantially increase the risk of late‐onset preeclampsia.
Keywords: antidepressant, MoBa, pharmacoepidemiology, preeclampsia, pregnancy, SSRI, The Norwegian Mother and Child Cohort Study
1. INTRODUCTION
During pregnancy, up to 20% of women have depressive symptoms,1 and 1% to 8% are treated with antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs).2, 3 It has been suggested that SSRIs may increase the risk of preeclampsia.4, 5, 6, 7 Preeclampsia is a major complication affecting 3% to 5% of all pregnancies and is cause of maternal‐infant morbidity and mortality.8
Toh et al4 were the first to show an association between use of SSRI beyond the first trimester and increased risk of preeclampsia compared to no use (4.9‐fold magnitude). While some studies replicated this association, albeit with smaller effect sizes (16‐60% increased risk),5, 6, 9, 10 other studies did not.7, 11 Three of these7, 9, 11 found that women prenatally exposed to serotonin‐norepinephrine reuptake inhibitors (SNRIs) or tricyclic antidepressants (TCAs) had an elevated risk of preeclampsia.
Antidepressants and maternal mood disorders may via different pathways trigger pathogenic vascular processes leading to preeclampsia,12, 13 and this poses challenges in disentangling the effect of pharmacotherapy from that of the underlying depression. Although some prior studies have compared the risk of preeclampsia in depressed women treated versus untreated with antidepressants and adjusted for proxy measures of depression severity (eg, number of outpatient and inpatient depression diagnoses or other antidepressant [OAD] indications),5, 9, 10, 11 none of them could directly measure depression severity and account for its possible fluctuation at 2 time points in pregnancy.
In a pregnancy cohort of depressed women, we aimed to (1) describe the risk of early‐ and late‐onset preeclampsia across women prenatally exposed to various antidepressants and (2) closely examine the association between timing and length of gestational exposure to SSRIs and late‐onset preeclampsia, accounting for time‐varying exposure and severity of depressive and anxiety symptoms during pregnancy.
KEY POINTS.
This study extends the literature by considering 2 measures of depressive and anxiety symptom severity in pregnancy.
SSRI exposure in early and midpregnancy did not substantially increase the risk of late‐onset preeclampsia.
Our study could rule out a 2‐fold increased risk of late‐onset preeclampsia following SSRI exposure in late pregnancy, although we were unable to confirm or refute whether a smaller increased risk exists.
The crude risk of early‐ and late‐onset preeclampsia was higher among SNRI‐ and TCA‐exposed pregnancies than among depressed nonexposed.
2. METHODS
This study is based on The Norwegian Mother and Child Cohort Study (MoBa) linked to the Medical Birth Registry of Norway (MBRN) via women's personal identification number. The MoBa is a prospective population‐based pregnancy cohort study conducted by the Norwegian Institute of Public Health.14 Participants were recruited from all over Norway in 1999 to 2008 through a postal invitation in connection with a routine ultrasound at gestational weeks (GWs) 17 and 18. Data were gathered prospectively from 2 self‐completed questionnaires at GWs 17 (Q1) and 30 (Q3). Women consented to participation in 41% of the pregnancies, and among these, the response rate was 95% for Q1 and 92% for Q3.15 The cohort now includes 114 500 children, 95 200 mothers, and 75 200 fathers. The current study is based on version 8 of the quality‐assured data files released for research including women who delivered between 1999 and 2009. The establishment and data collection in MoBa has obtained a license from the Norwegian Data Inspectorate and approval from The Regional Committee for Medical Research Ethics. All participants gave their written informed consent prior to participation.
The MBRN is based on compulsory notification of all live births, stillbirths, and induced abortions after GW 12 (after week 16 up to 2002) since 1967.16 This study included pregnancies within women with a medicated depression in the 6 months prior to pregnancy and/or with depression during pregnancy, as self‐reported in Q1 and/or Q3. Both questionnaires included a list of previous and/or concurrent illnesses, including depression, and were completed at a mean GW of 17.5 (SD, 2.8) and 30.7 (SD, 1.7). The exclusion criteria and flowchart to achieve the final analysis cohort are outlined in Figure 1.
Figure 1.
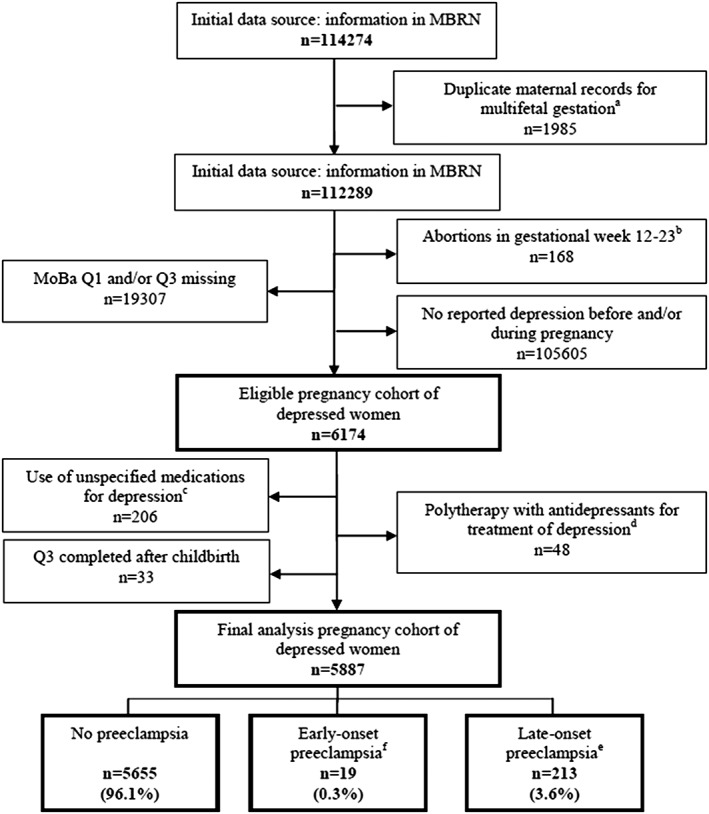
Flowchart to achieve the final analysis cohort*. MBRN, Medical Birth Registry of Norway; MoBa, The Norwegian Mother and Child Cohort Study; Q1, MoBa questionnaire 1; Q3, MoBa questionnaire 3. *Conditions may overlap. aWomen with multifetal gestation have one pregnancy record per fetus; a single pregnancy record was retained in the final eligible cohort. bIncludes both induced and spontaneous abortions. cWomen who did not report any medication for treatment of depression but checked the boxes related to timing of use of medication either before or during pregnancy. dWomen on therapy with antidepressants belonging to different antidepressant groups (eg, selective serotonin reuptake inhibitor and tricyclic antidepressant). ePreeclampsia with onset after gestational week 34, also including eclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome postdelivery. fPreeclampsia with onset between gestational weeks 20 and 34
2.1. Depression and anxiety symptoms in pregnancy
The short versions of The Hopkins Symptom Checklist–25 (SCL‐25), ie, the 5‐ (SCL‐5) and 8‐item (SCL‐8) scales, were used to measure severity of depressive and anxiety symptoms at GWs 17 and 30, respectively.15, 16 Both scales measure symptoms in the last 2 weeks prior to completion and are highly correlated to the SCL‐25,17 a reliable screening instrument for depression as defined by the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.17 For each SCL item, a score from 1 to 4 can be assigned. The sum score was calculated and divided by the number of items in each instrument.
To reflect the temporality of data collection in MoBa, as well as the time points in gestation when on average Q1 and Q3 were completed, and the SCL measurement validity time span, we considered 3 time points: T0 (GWs 0‐16), T1 (GWs 17‐28), and T2 (GWs 29‐34) (Figure 2).
Figure 2.
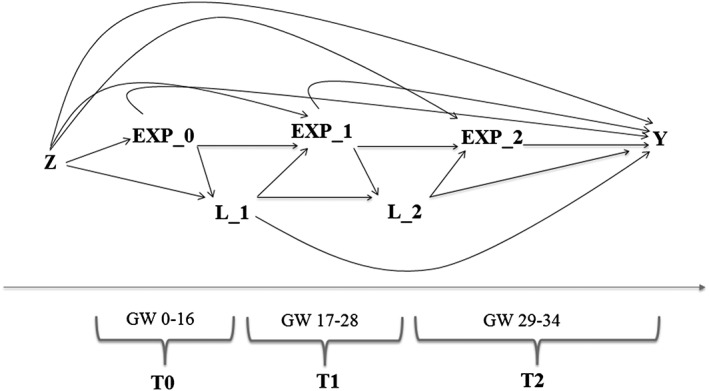
Directed acyclic graph for the outcome late‐onset preeclampsia. GW, gestational week. Z is a vector of baseline covariates; EXP_0, EXP_1, and EXP_2 represent antidepressant exposure, specifically selective serotonin reuptake inhibitors in GW 0‐16, 17‐28, and 29‐34; L_1 is a vector of time‐varying confounders, including comedication with analgesics during weeks 0‐16 and depressive/anxiety symptoms measured at GW 17; L_2 is a vector of time‐varying confounders, including comedication with analgesics during weeks 17‐28 and depressive/anxiety symptoms measured at GW 30. Y is the outcome measure, late‐onset preeclampsia
2.2. Exposure
The Q1 and Q3 provided information about antidepressant exposure. Women reported the name of the medication taken along with timing of use (6 months before pregnancy and during pregnancy by 4‐week intervals). Drug classification was based on the Anatomical Therapeutic Chemical (ATC) Classification System.18 Exposure to antidepressants was defined as exposure to a drug belonging to the ATC group N06A, subdivided into SSRIs (ATC: N06AB), SNRIs (venlafaxine and duloxetine), TCAs (ATC: N06AA), and OADs (mianserin, nefazodone, mirtazapine, bupropion, and reboxetine). Women on antidepressant polytherapy, ie, exposed to multiple antidepressant groups in pregnancy, were excluded (Figure 1).
The primary exposure windows were early (T0), mid (T1), and late (T2) pregnancy, with the addition of any time in pregnancy (T0‐T2). The rationale behind this exposure definition is described in Appendix 1.
Length of antidepressant exposure was defined according to how many 4‐week intervals were checked in the questionnaires: 0, 1, 2 to 4, and 5 to 8 intervals. Women were classified as exposed if they reported use of SSRIs, SNRIs, TCAs, or OADs during these periods. When more than one medication was reported for the same indication, we assumed that all medications were taken at the intervals checked. Based on previous studies,4, 7, 9 we also defined, among the pre‐pregnancy SSRI exposed, continuers beyond the first trimester and discontinuers within the same period. We expanded this approach to later gestation, ie, continuers in first half of third trimester versus discontinuers among midpregnancy SSRI users.
2.3. Outcome
The main outcome variable was late‐onset preeclampsia (any severity, mild, and severe), ie, preeclampsia developing after GW 34 as registered in the MBRN. Information provided in the registry is based on forms completed by midwives/obstetricians at delivery and during prenatal checkups. The main outcome variable included any diagnosis of late‐onset preeclampsia (mild, severe, or unspecified); hemolysis, elevated liver enzymes, and low platelet count; and eclampsia. Severe preeclampsia included the following diagnoses: severe preeclampsia; hemolysis, elevated liver enzymes, and low platelet count syndrome; or eclampsia. Mild preeclampsia diagnosis was used when given alone. Early‐onset preeclampsia was included as secondary outcome, ie, developing between GWs 20 and 34. Late‐ and early‐onset preeclampsia were treated as mutually exclusive outcomes since they are generally regarded as subtypes of preeclampsia.19, 20 Details about the diagnostic criteria for preeclampsia in Norway21 are outlined in Appendix 1. The validity of preeclampsia registration in the MBRN for MoBa participants has been assessed against antenatal records/hospital discharge codes, yielding a positive predictive value of 84%; upon generalization to the whole eligible MoBa population, the sensitivity was 43% and the specificity 99%.22
2.4. Covariates
Relevant confounding factors were identified with the aid of directed acyclic graphs.23 Maternal age, parity, education, income, body mass index at conception, smoking status during pregnancy until week 30, depressive and anxiety symptoms in pregnancy, and comedication with analgesics (ie, opioids and nonsteroidal anti‐inflammatory drugs) or other psychotropics (ie, antipsychotics, anxiolytics, hypnotics, and sedatives) in pregnancy were all ascertained from Q1 and Q3. The MoBa Q1 also captured information about lifetime event of major depression via utilization of the “The Life Time History of Major Depression” instrument,24 comprising 6 items, which closely correspond to the DSM‐III criteria for lifetime major depression. Obstetric risk factors for preeclampsia were ascertained from the MBRN (ie, multiple gestation, history of preeclampsia, chronic hypertension, gestational diabetes, and infertility treatment) and Q1 (ie, maternal blood pressure at the first antenatal visit).
2.5. Statistical analysis
All statistical analyses were performed by using STATA version 14. Details about the study power in relation to SSRIs/late‐onset preeclampsia are given in Appendix 1. Our study was underpowered for the SNRI‐, TCA‐, and OAD‐exposed group, as well as for the outcome early‐onset preeclampsia, for which only descriptive statistics are presented.
To estimate the relative risks (RRs) of late‐onset preeclampsia (overall and by severity) along with their corresponding 95% confidence interval (CI) in relation to SSRI exposure, 2 sets of analyses were performed. First, we used modified Poisson regression models within the generalized linear model framework (unweighted models). A robust variance estimator was applied to account for women's participation with more than one pregnancy in MoBa. We computed crude and adjusted RRs (aRRs) with 95% CI for the exposure windows; adjustment was done for a sufficient set of confounders (ie, maternal age, parity, body mass index, education, income, smoking status and weight gain, comedication with analgesics and other psychotropics, depressive symptoms as average of the standardized z‐scores at each time point, and lifetime event of major depression).
In the second set (weighted models), we fit marginal structural models to account for time‐varying SSRI exposure and time‐varying confounders (depressive and anxiety symptoms in pregnancy and comedication with analgesics) (Figure 2).25 We estimated the probability of SSRI treatment using a pooled logistic regression in which the outcome was current SSRI treatment. The covariates were maternal baseline factors, time‐varying confounders at T1 and T2, time‐fixed confounders, and SSRI history treatment (T0 exposure) (Appendix 2). We derived stabilized inverse probability of treatment weights (IPTW) for each pregnancy at each time point. We then fit a modified Poisson regression model with robust standard errors applying the IPTW. To avoid violation of the positivity assumption, we only included pregnancies of depressed women in the analysis. Data are presented as crude and aRR with 95% CI in both weighted and unweighted models.
Missing values on confounders ranged from 1% to 15%. Under the assumption that data were missing at random, we imputed missing values via multiple imputation (20 replications). Imputed data were used in both analysis sets. Details about missing data are provided in Appendix 1.
2.6. Sensitivity and subanalyses
A number of sensitivity and subanalyses were undertaken. We considered in the main analyses major obstetric risk factors for preeclampsia and baseline maternal blood pressure by adjustment and restriction. Since parity is a major risk factor for preeclampsia, we run subanalyses among nulliparous women and explored any effect modification by parity. We also repeated the analysis restricted to the first pregnancy recorded in case of multiple participations in MoBa to limit selection bias by healthier maternal status. For comparison purposes with prior research,4, 5, 9, 10, 11 we explored in the unweighted models the effect of early SSRI exposure on preeclampsia as an aggregated outcome measure and compared SSRI‐exposed pregnancies in the specific time windows to the depressed, never medicated counterpart. We examined the sensitivity of the findings in the weighted models by applying different model specifications including obstetric risk factors for preeclampsia and baseline maternal blood pressure or excluding covariates thought to lead to more extreme stabilized weights (cf Appendix 2). To explore the bias‐precision trade‐off, we progressively truncated the IPTW at 1st/99th and 5th/95th percentile.25 We conducted complete‐case analyses and considered in the analysis only SCL imputed values whenever more than a half of the items were completed with exclusion of pregnancies with missing information on the other important covariates. We conducted probabilistic sensitivity analyses to correct for exposure and outcome misclassification, unmeasured confounding and random error simultaneously using bias parameters (10 000 simulations) stemming from existing studies.22, 26, 27 The latter validation study27 showed a specificity of 99.9% and a sensitivity of 81.7% for SSRI use in mid/late pregnancy as reported in MoBa. To address unmeasured confounding by nutrition (ie, adherence to the Nordic diet, consisting of healthy foods native to a Nordic climate), we used prevalence estimates for high diet adherence ranging from 0.30 to 0.5028 and assumed that high adherence to the Nordic diet was half as prevalent in SSRI users than in nonusers. We allowed the association between high diet adherence and late‐onset preeclampsia to vary between 0.50 and 0.80.28
3. RESULTS
The final analysis cohort included 5887 pregnancies ending with a live‐born (99.8%) or stillborn (0.2%, >GW 34) infant (Figure 1). Lost to follow‐up (ie, Q3 not completed) occurred more often within younger, more disadvantaged women with more severe early gestation depressive symptoms compared to women remaining in the study (ie, both Q1 and Q3 completed). Lost to follow‐up pregnancies presented higher rates of early‐ but not of late‐onset preeclampsia.
Baseline sociodemographic, lifestyle, and health characteristics of the 5887 pregnancies in women with depression by antidepressant treatment status are shown in Table 1. The SSRI monotherapy (hereafter, SSRI) was the most common antidepressant exposure at any time in pregnancy before GW 34 (654/5887; 11.1%), with rates of use ranging from 10.1% in early to 4.1% and 3.4% in mid and late pregnancy, respectively. Few pregnancies were exposed to monotherapy with SNRIs (1.3%), TCAs (0.4%), or OADs (0.5%) at any time before GW 34 (Table 1).
Table 1.
Sociodemographic, lifestyle, and health characteristics of the sample by antidepressant use status during pregnancy before gestational week 34 (n = 5887)
| Depression, Nonmedicated | Depression, SSRI Monotherapy | Depression, SNRI Monotherapy | Depression, TCA Monotherapy | Depression, OAD Monotherapy | |
|---|---|---|---|---|---|
| n = 5106 | n = 654 | n = 75 | n = 21 | n = 31 | |
| Maternal age, y; mean ± SD | 29.6 ± 5.1 | 30.0 ± 5.0 | 29.2 ± 5.6 | 31.0 ± 5.5 | 30.9 ± 4.3 |
| Multiple gestation; no., % | |||||
| No | 5030 (98.5) | 637 (97.4) | 73 (97.3) | 21 (100.0) | 30 (96.8) |
| Yes | 76 (1.5) | 17 (2.6) | 2 (2.7) | — | 1 (3.2) |
| Gestational age at delivery, wk; mean ± SD | 39.4 ± 1.70 | 39.1 ± 1.81 | 39.2 ± 1.98 | 38.6 ± 1.53 | 39.1 ± 1.88 |
| Parity; no., % | |||||
| 0 | 2410 (47.2) | 357 (54.6) | 38 (50.7) | 10 (47.6) | 12 (38.7) |
| ≥1 | 2696 (52.8) | 297 (45.4) | 37 (49.3) | 11 (52.4) | 19 (61.3) |
| Marital status; no., % | |||||
| Married/cohabiting | 4663 (91.3) | 566 (86.5) | 62 (82.7) | 20 (95.2) | 28 (90.3) |
| Other | 399 (7.8) | 84 (12.8) | 11 (14.7) | 1 (4.8) | 3 (9.7) |
| Educational level; no., % | |||||
| University/college | 2428 (47.6) | 311 (47.6) | 29 (38.7) | 7 (33.3) | 15 (48.4) |
| Lower than university/college | 2326 (45.6) | 301 (46.0) | 43 (57.3) | 14 (66.7) | 16 (51.6) |
| Woman's gross yearly income, NOK; no., % | |||||
| No income | 204 (4.0) | 29 (4.4) | 1 (1.3) | 1 (4.8) | 1 (3.2) |
| <199 999 | 1858 (36.4) | 277 (42.4) | 31 (41.3) | 9 (42.9) | 16 (51.6) |
| 200‐399 999 | 2470 (48.4) | 286 (43.7) | 33 (44.0) | 10 (47.6) | 11 (35.5) |
| >400 000 | 402 (7.9) | 43 (6.6) | 7 (9.3) | 1 (4.8) | 1 (3.2) |
| BMI at conception; mean ± SD | 24.3 ± 4.6 | 24.5 ± 4.9 | 24.5 ± 5.0 | 25.6 ± 4.5 | 22.6 ± 3.7 |
| Weight gain, kg, until GW 30; mean ± SD | 9.8 ± 5.4 | 9.5 ± 5.4 | 10.9 ± 6.0 | 10.0 ± 4.8 | 9.7 ± 6.6 |
| Smoking status at GW 30; no., % | |||||
| No | 3643 (71.4) | 439 (67.1) | 42 (56.0) | 15 (71.4) | 13 (41.9) |
| Yes | 728 (14.3) | 111 (17.0) | 22 (29.3) | 3 (14.3) | 10 (32.3) |
| Stopped in pregnancy | 683 (13.4) | 100 (15.3) | 9 (12.0) | 3 (14.3) | 8 (25.8) |
| Infertility treatment; no., % | |||||
| No | 4831 (94.6) | 623 (95.3) | 72 (96.0) | 20 (95.2) | 28 (90.3) |
| Yes | 275 (5.4) | 31 (4.7) | 3 (4.0) | 1 (4.8) | 3 (9.7) |
| Chronic hypertension and use of antihypertensive before conception; no., % | |||||
| No | 5098 (99.8) | 653 (99.9) | 75 (100.0) | 21 (100.0) | 31 (100.0) |
| Yes | 8 (0.2) | 1 (0.1) | — | — | — |
| Systolic blood pressure, mm Hga; mean ± SD | 113.1 ± 12.5 | 111.7 ± 13.1 | 107.7 ± 9.8 | 114.8 ± 14.4 | 110.0 ± 15.0 |
| Diastolic blood pressure, mm Hga; mean ± SD | 68.1 ± 9.6 | 67.3 ± 9.7 | 66.0 ± 8.2 | 69.4 ± 9.0 | 66.8 ± 9.7 |
| Preexisting diabetes; no., % | |||||
| No | 5181 (99.4) | 650 (99.4) | 75 (100.0) | 21 (100.0) | 31 (100.0) |
| Yes | 34 (0.7) | 4 (0.6) | — | — | — |
| Preeclampsia history; no., % | |||||
| No | 4910 (96.2) | 627 (95.9) | 71 (94.7) | 21 (100.0) | 29 (93.6) |
| Yes | 196 (3.8) | 27 (4.1) | 4 (5.3) | — | 2 (6.5) |
| History of spontaneous abortion(s)b; no., % | |||||
| No | 3480 (68.2) | 466 (71.3) | 53 (70.7) | 13 (61.9) | 18 (58.1) |
| Yes | 1000 (19.6) | 121 (18.5) | 12 (16.0) | 6 (28.6) | 12 (38.7) |
| Lifetime major depressionc; no., % | |||||
| No | 1328 (26.0) | 46 (7.0) | 7 (9.3) | 2 (9.5) | 2 (6.4) |
| Yes | 3491 (68.4) | 582 (89.0) | 66 (88.0) | 19 (90.5) | 29 (93.6) |
| Depressive symptoms at GW 17; z‐score ± SD | −0.03 ± 0.98 | 0.18 ± 1.11 | 0.37 ± 1.15 | 0.14 ± 0.87 | 0.39 ± 1.08 |
| Depressive symptoms at GW 30; z‐score ± SD | −0.02 ± 0.97 | 0.10 ± 1.13 | 0.43 ± 1.39 | 0.31 ± 1.08 | 0.44 ± 1.31 |
| Comedication use in pregnancy; no., % | |||||
| Other psychotropic drugsd | 142 (2.8) | 81 (12.4) | 10 (13.3) | 5 (23.8) | 8 (25.8) |
| NSAIDs and/or opioid analgesics | 450 (8.8) | 85 (13.0) | 11 (14.7) | 3 (14.3) | 6 (19.4) |
Abbreviations: BMI, body mass index; GW, gestational week; NOK, Norwegian Krone (1 NOK=0.13 USD); NSAID, nonsteroidal anti‐inflammatory drugs; OADs, other antidepressants; SNRIs, serotonin‐norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants.
Numbers may not add up to total due to missing values, ranging from 1% to 3% for smoking, BMI, and socioeconomic status, to 5% to 7% for educational level, lifetime history of major depression and depressive, and anxiety symptoms in GW 17, 8% to 10% for depressive and anxiety symptoms in GW 30 and weight gain, and 14% for baseline blood pressure values.
Self‐reported systolic and diastolic blood pressure as measured at first antenatal visit.
Defined as history of spontaneous abortion(s) before GW 12.
Defined as Kendlers lifetime major depression scale score of 3 or more of 6 depressive symptoms of duration of more than 2 weeks.
Other psychotropic drugs include all medications under the Anatomical Therapeutic Chemical code N05, ie, antipsychotics, anxiolytics, hypnotics, and sedatives.
Early‐ and late‐onset preeclampsia complicated 19 (0.3%) and 213 (3.6%) of the pregnancies. The incidence of late‐onset mild, severe, and unspecified preeclampsia was 2.4%, 1.0%, and 0.2%, respectively. The risk of late‐onset preeclampsia by exposure status at any time before GW 34 is outlined in Figure 3A, whereas Figure 3B depicts the risk of early‐preeclampsia according to antidepressant exposure in early pregnancy.
Figure 3.
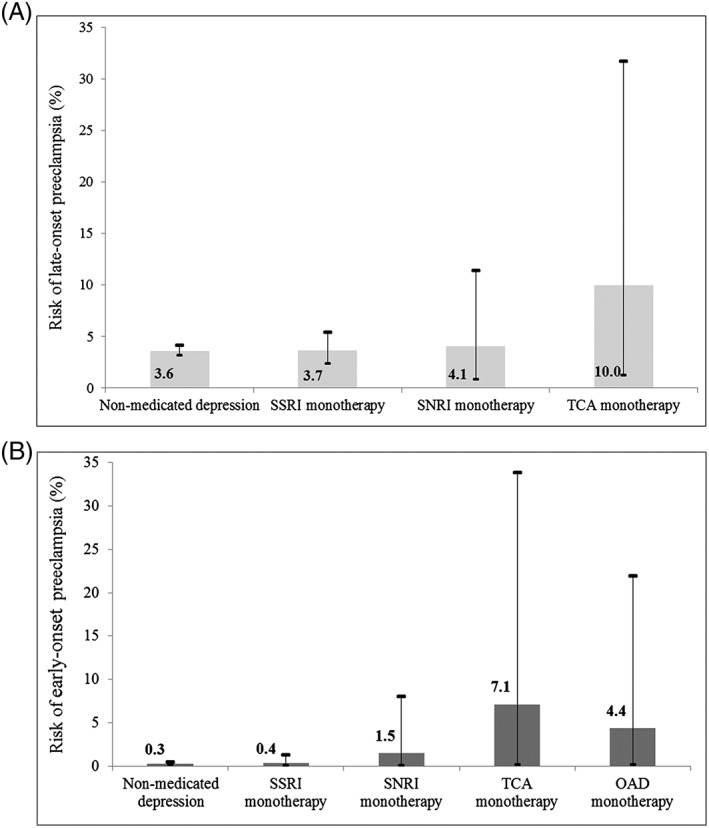
Risk of A, late‐onset (ie, later than week 34) and B, early‐onset (ie, weeks 20‐34) preeclampsia by antidepressant exposure status. SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin‐norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; OAD, other antidepressant. Antidepressant exposure in gestational weeks 0‐34 (within A) and antidepressant exposure in gestational weeks 0‐16 (within B). The Other antidepressant (OAD) exposure group not presented in A, since there were no cases of late‐onset preeclampsia
Five thousand seven hundred forty‐five pregnancies from 5550 women were analyzed in relation to the effect of SSRIs on late‐onset preeclampsia (hereafter, preeclampsia). There was no association between SSRI exposure in early, mid, late, or at any time in pregnancy and preeclampsia of any severity in the unweighted models (Table 2). Adjustment for the necessary set of confounders did not substantially affect the crude estimates, with depressive and anxiety symptoms being the covariates having the largest impact. There was no effect modification by parity. In the weighted model, the effect estimate for SSRI exposure in midpregnancy (aRR, 0.66; CI, 0.33‐1.28) was lower than the one observed in the unweighted model, while larger for the late pregnancy window (aRR, 1.34; CI, 0.61‐2.93); however, the CIs were wide and included the null. Table 2 also outlines the effect estimates for mild preeclampsia according to timing of SSRI exposure.
Table 2.
Risk of late‐onset preeclampsia—overall and by severity—according to timing of SSRI exposure in pregnancy (n = 5745)
| Depressed Cohort | Total | N (%) with Outcome | Crude RR (95% CI) | Unweighted Adjusted RRa (95% CI) | Weighted Adjusted RRb (95% CI) |
|---|---|---|---|---|---|
| Preeclampsia, any severity | |||||
| Nonmedicated, GWs 0‐16 | 5154 | 186 (3.6) | Reference | Reference | |
| SSRI, GWs 0‐16 | 591 | 22 (3.7) | 1.03 (0.67‐1.59) | 0.96 (0.63‐1.46) | — |
| Nonmedicated, GWs 17‐28 | 5505 | 200 (3.6) | Reference | Reference | Reference |
| SSRI, GWs 17‐28 | 240 | 8 (3.3) | 0.92 (0.46‐1.84) | 0.92 (0.48‐1.79) | 0.66 (0.33‐1.28) |
| Nonmedicated, GWs 29‐34 | 5547 | 200 (3.6) | Reference | Reference | Reference |
| SSRIs, GWs 29‐34 | 198 | 8 (4.0) | 1.12 (0.56‐2.24) | 1.08 (0.57‐2.07) | 1.34 (0.61‐2.93) |
| Nonmedicated, GWs 0‐34 | 5093 | 184 (3.6) | Reference | Reference | |
| SSRIs, GWs 0‐34 | 652 | 24 (3.7) | 1.02 (0.67‐1.55) | 0.96 (0.64‐1.45) | — |
| Preeclampsia, mild | |||||
| Nonmedicated, GWs 0‐16 | 5092 | 124 (2.4) | Reference | Reference | |
| SSRI, GWs 0‐16 | 585 | 16 (2.7) | 1.12 (0.67‐1.88) | 1.05 (0.63‐1.75) | — |
| Nonmedicated, GWs 17‐28 | 5438 | 133 (2.5) | Reference | Reference | Reference |
| SSRI, GWs 17‐28 | 239 | 7 (2.9) | 1.20 (0.57‐2.53) | 1.22 (0.59‐2.54) | 0.76 (0.38‐1.53) |
| Nonmedicated, GWs 29‐34 | 5480 | 133 (2.4) | Reference | Reference | Reference |
| SSRI, GWs 29‐34 | 197 | 7 (3.6) | 1.46 (0.69‐3.09) | 1.49 (0.73‐3.04) | 1.56 (0.71‐3.44) |
| Nonmedicated, GWs 0‐34 | 5031 | 122 (2.4) | Reference | Reference | |
| SSRI, GWs 0‐34 | 646 | 18 (2.8) | 1.15 (0.71‐1.87) | 1.10 (0.67‐1.80) | — |
| Preeclampsia, severe | |||||
| Nonmedicated, GWs 0‐34 | 4959 | 50 (1.0) | Reference | Reference | |
| SSRI, GWs 0‐34 | 633 | 5 (0.8) | 0.78 (0.31‐1.96) | 0.72 (0.31‐1.68) | — |
Abbreviations: CI, confidence interval; GW, gestational week; RR, relative risk; SSRI, selective serotonin reuptake inhibitors.
Numbers of severe and mild late‐onset preeclampsia may not add up to total due to cases of unspecified late‐onset preeclampsia. Data are not shown because of low number of cases. Severe late‐onset preeclampsia could only be explored in relation to the exposure window GWs 0‐34 because of low statistical power.
Adjusted for maternal age, body mass index at conception, woman's socioeconomic status, educational level, parity, smoking status in pregnancy, weight gain, comedication with analgesics or other psychotropics, lifetime history of major depression, and average z‐score of depressive and anxiety symptoms at 2 time points in pregnancy.
Marginal structural model weighted with stabilized inverse probability of treatment weighting constructed at each time point using baseline covariates, time‐varying and time‐fixed confounding factors, and SSRI history treatment.
Table 3 summarizes unweighted RR for preeclampsia (any severity) according to length of SSRI exposure and continuation/discontinuation beyond first trimester. No severity‐specific analyses were conducted because of low statistical power. Of the 240 pregnancies exposed to SSRIs in midpregnancy, 194 continued the treatment in late pregnancy and 8 of these (4.1%) developed preeclampsia, compared to none among discontinuers.
Table 3.
Risk of late‐onset preeclampsia (any severity) according to length of SSRI exposure in pregnancy and continuation/discontinuation in early pregnancy (n = 5745)
| Depressed Cohort | Total | N (%) with Outcome | Crude RR (95% CI) | Unweighted Adjusted RRa (95% CI) |
|---|---|---|---|---|
| Preeclampsia, any severity | ||||
| Nonmedicated, ever | 5127 | 186 (3.6) | Reference | Reference |
| SSRI in 1 interval | 220 | 6 (2.7) | 0.75 (0.34‐1.68) | 0.70 (0.32‐1.55) |
| SSRI in 2‐4 intervals | 203 | 8 (3.9) | 1.09 (0.54‐2.17) | 0.96 (0.48‐1.91) |
| SSRI in 5‐8 intervals | 195 | 8 (4.1) | 1.13 (0.57‐2.26) | 1.16 (0.60‐2.24) |
| SSRI in 1 interval | 220 | 6 (2.7) | Reference | Reference |
| SSRI in 2‐4 intervals | 203 | 8 (3.9) | 1.44 (0.51‐4.10) | 1.27 (0.42‐3.82) |
| SSRI in 5‐8 intervals | 195 | 8 (4.1) | 1.50 (0.53‐4.26) | 1.47 (0.56‐3.90) |
| SSRI discontinuersb | 246 | 7 (2.9) | Reference | Reference |
| SSRI continuersc | 208 | 11 (5.3) | 1.86 (0.73‐4.71) | 1.63 (0.66‐4.03) |
Abbreviations: CI, confidence interval; RR, relative risk; SSRI, selective serotonin reuptake inhibitor.
Adjusted for maternal age, body mass index at conception, woman's socioeconomic status, educational level, parity, smoking status in pregnancy, weight gain, comedication with analgesics or other psychotropics, lifetime history of major depression, and average z‐score of depressive and anxiety symptoms at 2 time points in pregnancy.
Defined as SSRI exposed pregnancies before pregnancy, but SSRI treatment discontinued within the first trimester (ie, before gestational week 13).
Defined as SSRI exposed pregnancies before pregnancy and SSRI treatment continued in and beyond the first trimester.
3.1. Sensitivity and subanalyses
The results of the unweighted (not shown) and weighted (Appendix 2) main models accounting for major obstetric risk factors for preeclampsia and maternal baseline blood pressure did not materially change our main findings. Restriction to nulliparous women, aggregation of early/late‐onset preeclampsia in a unique outcome (Appendices 3 and 4), comparison of SSRI‐exposed pregnancies in the specific time windows to the depressed, never medicated counterpart in the unweighted analysis, and alternative model specifications in the weighted (Appendix 2) did not produce substantially different results. The progressive IPTW truncation yielded an SSRI late exposure estimate with larger standard error, and the stabilized weight did not achieve better precision.
Appendices 1, 5, and 6 outline the distribution of key variables in relation to missingness and results of the complete case analyses. The complete case analysis estimates were higher than those found in the imputed data, particularly for SSRI early exposure. The unweighted and weighted results of the complete case analyses expanded to pregnancies with only SCL imputed values did not substantially deviate from the main findings (Appendix 7). Results of probabilistic bias analyses are summarized in Appendices 8 and 9 and supported our main findings.
4. DISCUSSION
This study adds to the conflicting literature on the effect of SSRIs in pregnancy on preeclampsia and has the unique advantage of being able to account for severity of anxiety and depressive symptoms throughout gestation. We found no evidence that exposure to SSRIs in early or midpregnancy increases the risk of late‐onset preeclampsia. Our findings on SSRI midpregnancy exposure are partly in line with one prior study11 but also in contrast with 5 other studies.4, 5, 6, 9, 10 These discrepancies may be caused by several factors: different definitions of timing of exposure and outcome, lack of information on depression severity during gestation in all prior research, and cohort inclusion conditions (ie, live birth only versus live birth and stillbirth pregnancies), which may have introduced collider stratification bias. Missing data handling may have also produced divergent results. Our unweighted complete case analysis produced larger point estimates, which were partly in line with some prior studies.5, 9, 10 The missing data mechanism in our study seemed to be linked to maternal age and to the extent of completion of the SCL items, and as supported by our sensitivity analyses, a complete case analysis approach could have yielded biased estimates.29
When accounting for time‐varying severity of maternal depression and anxiety symptoms, we found that compared to nonexposed, the risk of late‐onset mild preeclampsia was lower among midpregnancy SSRI‐exposed (24% decreased risk) but higher (56% increased risk) among SSRI‐exposed in late pregnancy. Although the estimate CIs were wide and crossed the null effect, these divergent estimates may suggest the importance of timing of SSRI exposure and/or of time‐specific interplay of biological mechanisms in relation to preeclampsia. Depression and anxiety may plausibly increase the risk of preeclampsia via an altered excretion of vasoactive hormones or other neuroendocrine transmitters.30, 31 Treatment with SSRIs seems to normalize the dysfunction of the hypothalamic‐pituitary‐adrenal axis and could thereby reduce the risk of preeclampsia posed by depression through this mechanism.32 It is possible that our findings on late pregnancy SSRI exposure may be ascribed to residual confounding by depression severity or stress at the very end of gestation.
There are however biological mechanisms supporting a role of SSRIs in preeclampsia, which may be relevant during the maternal systemic response to preeclampsia. The SSRIs elicit increased levels of serotonin,12 which in turn enhance vasoconstriction on the umbilical‐placental vessels, and they also inhibit synthesis of nitric oxide, an important vasodilator.33, 34 In late gestation pregnant sheep, for instance, there was a transient reduction of uterine artery blood flow following fluoxetine administration.35 Our point estimate by length of SSRI exposure (5‐8 vs one 4‐week interval) was of similar magnitude to that observed in a prior study,11 although the CIs were wide and crossed the null effect. Palmsten et al11 also found no association between cumulative SSRI dose and preeclampsia, which raises the question of whether timing of exposure may be linked to preeclampsia rather than cumulative dose; this could also be supported by our findings on the extent of preeclampsia in relation to SSRI continuation/discontinuation in the first half of the third trimester. Since maternal susceptibility to vascular damage and factors other than poor placentation are etiologically relevant for late‐onset preeclampsia,36 our findings may provide, together with prior research,37 some hints about the underlying mechanisms of this elusive syndrome.
The elevated risk for preeclampsia observed among TCA‐exposed pregnancies at any time before week 34 aligns with findings of 2 previous studies (10‐11%),9, 11 while the one for SNRI exposure was not as large (6‐9%). Because of their vasoconstrictive effects,12 SNRIs and TCAs may plausibly be linked to preeclampsia, but the elevated risk seen in relation to early preeclampsia would also indicate a potential role of these medications on placentation. The small sample size however impeded us to conduct adjusted analyses and further explore these potential associations.
4.1. Strengths and limitations of the study
There are several strengths and limitations inherent this investigation and the MoBa study. Data collection was conducted prospectively, avoiding the risk of recall bias. Distinction of early‐ from late‐onset preeclampsia reduced the risk of reverse causality. We could account for several health‐related and maternal factors, including severity of depressive and anxiety symptoms in pregnancy via a validated instrument. Although this latter measurement cannot replace a clinical interview and is not designed to measure perinatal mood specifically, it provides a reliable measure of the severity of these psychiatric conditions.17, 38 We accounted for unmeasured confounding by diet quality,28 conducted several sensitivity and subanalyses to explore the robustness of our findings, and also explored the impact of missing information on important covariates on our estimates. However, residual confounding by depression severity or number of cigarette smoked during gestation cannot be ruled out.
One limitation is that antenatal depression was self‐reported in MoBa. However, our analysis cohort was about 5% of the initial data source, which equals estimates of major depression in pregnancy based on structural clinical interviews.1 Depressive and anxiety symptoms were not measured at baseline, but only at 2 time points in pregnancy; however, information about lifetime history of major depression was used in the generation of the stabilized weights. Exposure and outcome misclassification could be an additional concern. Use of antidepressants was self‐reported; however, the impact of SSRI misclassification has been explored and assessed as minimal for mid/late pregnancy exposure.27 Yet the impact of misclassification of SSRI exposure in terms of length and by the individual 4‐week intervals has not been addressed. Information on dosage is not available in the MoBa, and data about duration of exposure are not complete. The positive predictive value for a diagnosis registration of preeclampsia in the MBRN was found to be 84%, and false negative cases seemed to have mild forms of preeclampsia.22 The results of probabilistic bias analyses did not materially change our main conclusions.
The MoBa study has a low response rate (41%), with a possible self‐selection of the healthiest women.15 Its potential for bias has been thoroughly explored by comparing the MoBa with the total Norwegian birthing population,39 and although the prevalence estimates could not necessarily be generalized, the measures of associations tested were valid in the MoBa. We cannot, however, rule out that selection bias and/or lost to follow‐up of more disadvantaged women may have impacted the study results. Our sample size limited the statistical power of the analysis on mid and late pregnancy SSRI exposure, and impeded us to explore antidepressants other than SSRIs, as well as early‐onset preeclampsia.
To conclude, SSRI use in early and midpregnancy does not substantially increase the risk of late‐onset preeclampsia. This information may assist clinicians when evaluating the risk of treatment with SSRIs versus that of nonmedicated depression. The elevated risk of late‐onset preeclampsia (mainly mild) among SSRI‐exposed in late pregnancy needs to be refuted or confirmed by additional studies with greater numbers of exposed cases. Whether SNRIs and TCAs have an etiological role in relation to early‐ and late‐onset preeclampsia needs to be further elucidated in both epidemiological and biological research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix 1: Supplementary information to the main text presenting additional details on the Methods and Results sections
Appendix 2: Specification of various treatment models and generation of the corresponding stabilized weights in relation to the outcome late‐onset preeclampsia (any severity) (n = 5745)
Appendix 3: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy among nulliparous women (n = 2759) analysis
Appendix 4: Risk of preeclampsia (early and/or late‐onset) ‐ overall and by severity – according to SSRI exposure in early pregnancy
Appendix 5: Key variable statistics according to complete or missing information on important covariates (n = 5745)
Appendix 6: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy in the complete case (n = 3913) analysis
Appendix 7: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy in the complete case, but with imputation on SCL items (n = 4361) analysis
Appendix 8: Results of probabilistic bias analyses in relation to late‐onset preeclampsia of any severity by timing of SSRI exposure
Appendix 9: Results of probabilistic bias analyses in relation to late‐onset preeclampsia of mild severity by timing of SSRI exposure
ACKNOWLEDGEMENTS
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no. N01‐ES‐75558) and NIH/NINDS (grant no.1 UO1 NS 047537‐01 and grant no.2 UO1 NS 047537‐06A1). We are grateful to all the participating families in Norway who take part in this ongoing cohort study. A.L.'s postdoc research fellowship is funded through HN's ERC Starting Grant “DrugsInPregnancy” (grant no. 678033).
Lupattelli A, Wood M, Lapane K, Spigset O, Nordeng H. Risk of preeclampsia after gestational exposure to selective serotonin reuptake inhibitors and other antidepressants: A study from The Norwegian Mother and Child Cohort Study. Pharmacoepidemiol Drug Saf. 2017;26:1266–1276. https://doi.org/10.1002/pds.4286
PRIOR POSTINGS AND PRESENTATIONS; Preliminary results of this work have been presented as an abstract (oral presentation) at the ICPE 2016.
REFERENCES
- 1. Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071‐1083. https://doi.org/10.1097/01.AOG.0000183597.31630.db [DOI] [PubMed] [Google Scholar]
- 2. Charlton RA, Jordan S, Pierini A, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population‐based study in six European regions. BJOG. 2015;122(7):1010‐1020. https://doi.org/10.1111/1471‐0528.13143 [DOI] [PubMed] [Google Scholar]
- 3. Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35(3):265‐271. https://doi.org/10.1016/j.genhosppsych.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, Hernandez‐Diaz S. Selective serotonin reuptake inhibitor use and risk of gestational hypertension. Am J Psychiatry. 2009;166(3):320‐328. https://doi.org/10.1176/appi.ajp.2008.08060817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avalos LA, Chen H, Li DK. Antidepressant medication use, depression, and the risk of preeclampsia. CNS Spectr. 2015;20(1):39‐47. https://doi.org/10.1017/S1092852915000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40(10):1723‐1733. https://doi.org/10.1017/S0033291709992194 [DOI] [PubMed] [Google Scholar]
- 7. De Ocampo MP, Araneta MR, Macera CA, Alcaraz JE, Moore TR, Chambers CD. Risk of gestational hypertension and preeclampsia in women who discontinued or continued antidepressant medication use during pregnancy. Arch Womens Ment Health. 2016;19(6):1051‐1061. https://doi.org/10.1007/s00737‐016‐0655‐z [DOI] [PubMed] [Google Scholar]
- 8. Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287(24):3183‐3186. https://doi.org/10.1001/jama.287.24.3183 [DOI] [PubMed] [Google Scholar]
- 9. Palmsten K, Setoguchi S, Margulis AV, Patrick AR, Hernandez‐Diaz S. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988‐997. https://doi.org/10.1093/aje/kwr394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malm H, Sourander A, Gissler M, et al. Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population‐based national register data. Am J Psychiatry. 2015;172(12):1224‐1232. https://doi.org/10.1176/appi.ajp.2015.14121575 [DOI] [PubMed] [Google Scholar]
- 11. Palmsten K, Huybrechts KF, Michels KB, et al. Antidepressant use and risk for preeclampsia. Epidemiology. 2013;24(5):682‐691. https://doi.org/10.1097/EDE.0b013e31829e0aaa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Donnell J, Shelton R. Drug therapy of depression and anxiety disorders In: Chabner B, Brunton L, Knollman B, eds. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw‐Hill; 2011. [Google Scholar]
- 13. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta‐analysis. J Clin Psychiatry. 2013;74(4):e321‐e341. https://doi.org/10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- 14. Magnus P, Birke C, Vejrup K, et al. Cohort profile update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382‐388. https://doi.org/10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 15. Magnus P, Irgens LM, Haug K, et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35(5):1146‐1150. https://doi.org/10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- 16. Norwegian Institute of Public Health . Medical Birth Registry of Norway (MBRN). http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25,7840:1:0:0:::0:0. Accessed March 11, 2015.
- 17. Sandanger I, Moum T, Ingebrigtsen G, Dalgard OS, Sorensen T, Bruusgaard D. Concordance between symptom screening and diagnostic procedure: The Hopkins Symptom Checklist‐25 and the Composite International Diagnostic Interview I. Soc Psychiatry Psychiatr Epidemiol. 1998;33(7):345‐354. https://doi.org/10.1007/s001270050064 [DOI] [PubMed] [Google Scholar]
- 18. WHO Collaborating centre for drugs statistics methodology. ATC/DDD index 2012. http://www.whocc.no/atc_ddd_index/. Accessed 17 March, 2012.
- 19. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. Am J Obstet Gynecol. 2013;209(6):544 e541‐544 e512. https://doi.org/10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 20. von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143‐148. https://doi.org/10.1081/PRG‐120021060 [DOI] [PubMed] [Google Scholar]
- 21. Staff A, Andersgaard AB, Henriksen TB, et al. Veileder i fødselshjelp 2014. Hypertensive svangerskapskomplikasjoner og eklampsi. 2014; http://legeforeningen.no/Fagmed/Norsk‐gynekologisk‐forening/Veiledere/Veileder‐i‐fodselshjelp‐2014/Hypertensive‐svangerskapskomplikasjoner‐og‐eklampsi/. Accessed February 3, 2016.
- 22. Klungsoyr K, Harmon QE, Skard LB, et al. Validity of pre‐eclampsia registration in the Medical Birth Registry of Norway for women participating in The Norwegian Mother and Child Cohort Study, 1999‐2010. Paediatr Perinat Epidemiol. 2014;28(5):362‐371. https://doi.org/10.1111/ppe.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745 https://doi.org/10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- 24. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry. 1993;50(11):863‐870. https://doi.org/10.1001/archpsyc.1993.01820230054003 [DOI] [PubMed] [Google Scholar]
- 25. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656‐664. https://doi.org/10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Springer; 2009. [Google Scholar]
- 27. Skurtveit S, Selmer R, Tverdal A, Furu K, Nystad W, Handal M. Drug exposure: inclusion of dispensed drugs before pregnancy may lead to underestimation of risk associations. J Clin Epidemiol 2013; 66(9):964–972. DOI:https://doi.org/10.1016/j.jclinepi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 28. Hillesund ER, Overby NC, Engel SM, et al. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in The Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol. 2014;29(10):753‐765. https://doi.org/10.1007/s10654‐014‐9948‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moodie EE, Delaney JA, Lefebvre G, Platt RW. Missing confounding data in marginal structural models: a comparison of inverse probability weighting and multiple imputation. The International Journal of Biostatistics. 2008;4(1): Article 13 [DOI] [PubMed] [Google Scholar]
- 30. Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487‐490. https://doi.org/10.1016/s0029‐7844(99)00602‐x [DOI] [PubMed] [Google Scholar]
- 31. Sandman CA, Wadhwa PD, Chicz‐DeMet A, Dunkel‐Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814(1):266‐275. https://doi.org/10.1111/j.1749‐6632.1997.tb46162.x [DOI] [PubMed] [Google Scholar]
- 32. Ruhe HG, Khoenkhoen SJ, Ottenhof KW, Koeter MW, Mocking RJ, Schene AH. Longitudinal effects of the SSRI paroxetine on salivary cortisol in major depressive disorder. Psychoneuroendocrinology. 2015;52:261‐271. https://doi.org/10.1016/j.psyneuen.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 33. Finkel MS, Laghrissi‐Thode F, Pollock BG, Rong J. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull. 1996;32(4):653‐658. [PubMed] [Google Scholar]
- 34. Bolte AC, van Geijn HP, Dekker GA. Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol. 2001;95(1):12‐21. https://doi.org/10.1016/S0301‐2115(00)00367‐5 [DOI] [PubMed] [Google Scholar]
- 35. Morrison JL, Chien C, Riggs KW, Gruber N, Rurak D. Effect of maternal fluoxetine administration on uterine blood flow, fetal blood gas status, and growth. Pediatr Res. 2002;51(4):433‐442. https://doi.org/10.1203/00006450‐200204000‐00007 [DOI] [PubMed] [Google Scholar]
- 36. Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32‐S37. https://doi.org/10.1016/j.placenta.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engel SM, Scher E, Wallenstein S, et al. Maternal active and passive smoking and hypertensive disorders of pregnancy: risk with trimester‐specific exposures. Epidemiology. 2013;24(3):379‐386. https://doi.org/10.1097/EDE.0b013e3182873a73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL‐25, SCL‐10, SCL‐5 and MHI‐5 (SF‐36). Nord J Psychiatry. 2003;57(2):113‐118. https://doi.org/10.1080/08039480310000932 [DOI] [PubMed] [Google Scholar]
- 39. Nilsen RM, Vollset SE, Gjessing HK, et al. Self‐selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597‐608. https://doi.org/10.1111/j.1365‐3016.2009.01062.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Supplementary information to the main text presenting additional details on the Methods and Results sections
Appendix 2: Specification of various treatment models and generation of the corresponding stabilized weights in relation to the outcome late‐onset preeclampsia (any severity) (n = 5745)
Appendix 3: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy among nulliparous women (n = 2759) analysis
Appendix 4: Risk of preeclampsia (early and/or late‐onset) ‐ overall and by severity – according to SSRI exposure in early pregnancy
Appendix 5: Key variable statistics according to complete or missing information on important covariates (n = 5745)
Appendix 6: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy in the complete case (n = 3913) analysis
Appendix 7: Risk of late‐onset preeclampsia ‐ overall and by severity – according to SSRI exposure in pregnancy in the complete case, but with imputation on SCL items (n = 4361) analysis
Appendix 8: Results of probabilistic bias analyses in relation to late‐onset preeclampsia of any severity by timing of SSRI exposure
Appendix 9: Results of probabilistic bias analyses in relation to late‐onset preeclampsia of mild severity by timing of SSRI exposure


