Abstract
Background
Radiotherapy (rt) has been the standard treatment for early oropharyngeal cancer, achieving excellent outcomes, but with significant toxicities. Transoral robotic surgery (tors) has emerged as a promising alternative. A decision aid (da) can help to establish patient treatment preferences.
Methods
A da was developed and piloted in 40 healthy adult volunteers. Assuming equal oncologic outcomes of the treatments, participants indicated their preference. The treatment trade-off point was then established, and participant perceptions were elicited.
Results
More than 80% of participants initially selected tors for treatment, regardless of facilitator background. For all participants, the treatment trade-off point changed after an average 15% cure benefit. Treatment toxicities, duration, novelty, and perceptions all influenced treatment selection. All subjects valued the da.
Conclusions
A da developed for early oropharyngeal cancer treatment holds promise in the era of shared decision-making. Assuming equal cure rates, tors was preferred over rt by healthy volunteers.
Keywords: Oropharyngeal cancer, decision aids, shared decision-making, transoral robotic surgery, radiation therapy
INTRODUCTION
Definitive radiation therapy (rt) in early oropharyngeal squamous cell carcinoma (opscc) carries a significant burden of toxicity, which is becoming more of a concern as the rates of incidence and survival both rise1. The introduction of transoral robotic surgery (tors)—which offers patients with newly diagnosed opscc an innovative alternative to the standard of treatment with rt, potentially providing superior functional outcomes—is currently being used for most patients with early opscc in the United States2,3. The uptake of tors is increasing despite a lack of clear evidence about its oncologic or quality-of-life outcomes3,4. A randomized trial is currently underway to evaluate those questions by directly comparing tors with rt5.
In the setting of early disease, patient preference with respect to treatment modality is unknown. Given distinct toxicity profiles and a lack of randomized data to guide the choice of tors or rt in clinical practice, patients and clinicians face a challenging decision.
During the wait for randomized data, tors and rt are likely to both remain the main treatment options for opscc. That situation parallels the situation for other primary cancers in which multiple “gold standard” treatments, contrasting in both their approach and potential side effects, are available6. In such settings of clinical equipoise, shared decision-making can allow for individualized treatment recommendations that best reflect the patient’s wishes. Shared decision-making is regarded as the pinnacle of patient-centred care, but it is known to be difficult to achieve in standard clinical encounters because of the clinician’s inability to predict patient values and the patient’s limited knowledge, unrealistic outcome expectations, and decisional conflict7,8.
The use of decision aids (das) has been shown to facilitate the process of shared decision-making by increasing patient knowledge, enhancing patient choice congruent with their values, reducing decisional conflict, and increasing overall patient satisfaction9,10. In this pilot study, an interactive Web-based da was created to enhance the shared decision-making process between patient and clinician when navigating the complicated process of deciding between tors and rt as primary treatment in early opscc. The intention is to supplement the traditional clinical encounter with visual descriptions of the available treatments and to encourage patients to make a treatment choice based on their values.
The goal of the present study was thus to develop, pilot, and test, in a cohort of health volunteers, a new online da for early opscc patients.
METHODS
In this research ethics board–approved study, a da was developed on an interactive multimedia Web platform. The da provides a visual description of tors and rt treatments, including their respective timelines, and photographs of treatment-related equipment. It then further details the potential risks and side effects of each treatment, categorizing them according to their relative frequencies (Figure 1). The side-effect frequencies were derived from the patient letter of information used in a randomized trial comparing tors with rt (https://clinicaltrials.gov/ct2/show/NCT01590355), appraised using relevant literature, and reviewed with a multidisciplinary tumour board to ensure accuracy and completeness2,11–13.
FIGURE 1.
Sample visual from the decision aid for patients diagnosed with early oropharyngeal squamous cell carcinoma.
Healthy adult volunteers (18–80 years of age) were recruited to pilot-test the online module and confirm its psychometric properties with a trained researcher having a background in surgery (GMS) or radiation oncology (JSL). Participants were provided with a verbal introduction to the hypothetical diagnosis of early opscc and were guided through the da to gain understanding of the treatment options. Appendix a presents a complete script of the scenario used.
Participants were then asked to indicate their preferred treatment based on the assumption of equal oncologic outcomes, colloquially described as “cure rates.” After the participant made an initial selection, the da proceeded to raise the cure rate for the alternative treatment by 5%, 10%, 15%, 20%, and finally 50%, until the participant’s treatment preference changed. That process established the treatment trade-off point—that is, the point at which the enhanced oncologic control of the alternative treatment outweighed the perceived benefits of the original treatment of choice. To confirm the psychometric properties of the da, it was expected that all participants would accept a treatment trade-off point at one of the percentages offered in the da. Finally, the participants were asked to indicate the aspects of treatment that guided their initial treatment selection, their perceived utility of the da itself, and their suggestions for improvements to the da.
RESULTS
The 40 healthy adult volunteers (18 men, 22 women) who agreed to participate in the study had a mean age of 36 years (range: 18–75 years). None had a prior diagnosis of cancer. Table i lists additional participant characteristics.
TABLE I.
Demographics of the 40 participants
| Variable | Value |
|---|---|
| Age (years) | |
| Mean | 36 |
| Range | 18–75 |
| Age ≥60 years [n (%)] | 4 (10) |
| Sex [n (%)] | |
| Men | 18 (45) |
| Women | 22 (55) |
| Marital status [n (%)] | |
| Married | 19 (47.5) |
| Widowed or divorced | 3 (7.5) |
| Single | 18 (45) |
| Education [n (%)] | |
| High school | 9 (22.5) |
| Postsecondary studies | 23 (57.5) |
| Postgraduate studies | 8 (20) |
| Employment status [n (%)] | |
| Student | 12 (30) |
| Employed | 24 (60) |
| Unemployed | 1 (2.5) |
| Retired | 3 (7.5) |
With respect to treatment side-effect profiles, the concerns most commonly identified for tors were bleeding, death, stroke, and aspiration pneumonia. The toxicities of rt most commonly identified as concerning were tooth decay, requirement for a feeding tube, and risk of secondary malignancy (Table ii). Other factors influencing treatment selection included treatment duration, psychological impact of having the tumour physically removed, and the potential unknowns of tors (because of its novelty). As for the da itself, all 40 participants perceived utility in its use and indicated that they would value having a similar tool available if they found themselves in a similar situation. The tool was felt to positively supplement, but not to be able to replace, the clinician–patient encounter.
TABLE II.
Decision aid survey results
| Variable | Therapy choice [n (%)] | |
|---|---|---|
|
| ||
| Radiation therapy | Transoral robotic surgery | |
| Initial selection | 7 (18) | 33 (82) |
| Participant sex | ||
| Men | 5 (28) | 13 (72) |
| Women | 2 (9) | 20 (91) |
| Interviewer background | ||
| Radiotherapy (n=17) | 3 (18) | 14 (82) |
| Surgery (n=23) | 4 (17) | 19 (83) |
| Usefulness of decision aid | 7 (100) | 33 (100) |
| Most concerning side effects | Tooth decay Feeding tube Second malignancy |
Bleeding Death Stroke Aspiration pneumonia |
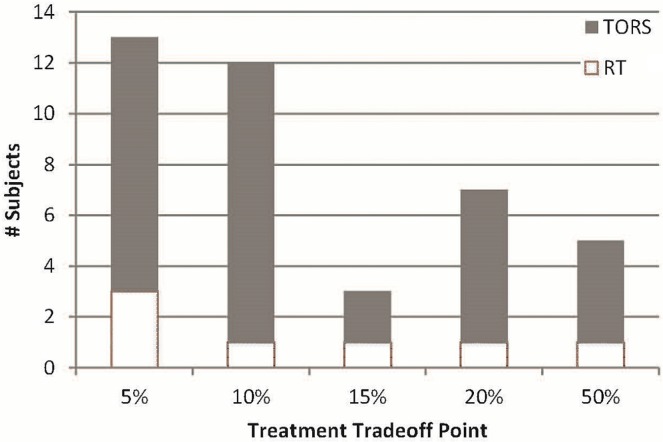
Most of the healthy volunteers (82%) selected tors as their preferred treatment option—a result that was consistent regardless of the interviewer’s background (surgery or radiation oncology, Table ii). All participants switched to the alternative treatment at one of the trade-off points offered, confirming the expected psychometric properties of the da. Analysis of the trade-off switches revealed that participants required an average of a 15% increased cure rate (range: 5%–50%) with the alternative therapy to abandon their initial treatment choice, which was true regardless of the treatment initially selected (Figure 2).
FIGURE 2.
Treatment trade-off point by initial treatment selection. TORS = transoral robotic surgery; RT = radiotherapy.
DISCUSSION
We report a novel da for the choice of rt compared with tors in early opscc that was found to be of value in a cohort of healthy volunteers. In this pilot study, tors was preferred over definitive rt provided that the treatments had equivalent oncologic control rates. Oncologic control was valued over other treatment-related factors, reflected by the finding that all subjects would change their treatment preference for an increased cure rate at an average treatment trade-off point of 15% regardless of the initial treatment choice. The background of the da administrator did not appear to influence the initial treatment choice, as would be expected if specialty bias had been present.
To our knowledge, our study is the first to develop a da to navigate the treatment options for early opscc. Decision aids are available throughout the field of medicine, in both cancer and non-cancer populations (examples at https://decisionaid.ohri.ca/AZlist.html). Within oncology, das have been reported in the context of cancer screening and in the selection of treatment in the adjuvant and definitive settings9,14–16. The hallmarks of the cancer das include facilitating communication, aiding in the decision-making process, and enhancing patient satisfaction9,10. Another reported benefit of das is to minimize the influence of specialty bias, whereby specialists are more likely to recommend treatments within their own specialty and to minimize the benefits of treatments that they do not provide17. A da is therefore an ideal resource to be made available to patients newly diagnosed with early opscc, helping them to choose between tors and rt in a manner congruent with their own values, while minimizing external bias.
Despite the aforementioned benefits and utility of our da, our pilot study has limitations. First, the tool was piloted in healthy volunteers, and thus our findings might not be generalizable to an early opscc population. Possible differences between our sample and early opscc patients include age, education level, socioeconomic status, technologic literacy, medical comorbidities, and fitness for surgery. Second, the results are derived from a single institution in southwestern Ontario, where inherent biases, such as those related to language and cultural and religious beliefs, might be present in the population. Finally, although the side-effect profiles for the two treatments were derived from the available literature, more data about toxicities arising from treatment are available for rt than for tors. Inherent publication bias might therefore affect the profile for the latter treatment, given the mostly retrospective nature of the data published at single-institution academic centres of excellence.
Given the foregoing limitations, future work within our group will include testing the da in patients previously treated for head-and-neck cancer. Once any pertinent modifications are made to the da after that phase of development, our ultimate aim is to use the final da with newly diagnosed opscc patients in our multidisciplinary head-and-neck oncology clinic to facilitate shared decision-making with the treating oncologists.
CONCLUSIONS
We successfully developed an online da that was found to be useful in a population of healthy volunteers, who, after use of the da, indicated a strong preference for tors. Further work is underway to test the da in patients with oropharyngeal cancer and ultimately to provide the da as part of routine clinical practice.
ACKNOWLEDGMENTS
Financial support for this research was provided by a London Regional Cancer Program Catalyst Grant.
APPENDIX A. NARRATIVE TO ACCOMPANY THE DECISION AID CONSULTATION
We created a decision aid to help cancer patients decide between their treatment options, and would like to gain your perspective on it.
Please click on “Start Decision Aid.”
The hypothetical situation is that you have been diagnosed with an early oropharyngeal cancer—this is a tumour that sits at the back of the throat. There are two treatments available to treat this cancer:
■ radiation potentially with chemotherapy, or
■ transoral robotic surgery.
Both methods are believed to have the same cure rate.
The first treatment option I will discuss is radiation therapy.
■ For this, you’ll have a planning appointment called a ct simulation.
■ At this appointment, a custom-made plastic mask is made for you to wear during treatments to ensure you’re in the same position every time and that there’s minimal movement during each treatment.
■ Wearing the mask, you will receive a ct scan with iv contrast.
■ Using the ct, we mark out the areas that we want to treat and the areas that we want to avoid to create the treatment plan.
■ The treatments are daily for 7 weeks, Monday to Friday for a total of 35 treatments, usually with you as an outpatient living at home.
■ For each treatment, you lie on the table with your mask on, and the machine moves around you. You won’t feel anything—similar to getting an X-ray or ct done. The appointments take about 20 minutes, with checking your positioning and the treatment time.
I will now discuss the side effects of treatment, which are listed on the decision aid you’re holding and are categorized by the likelihood they will occur. The side effects are largely related to the tissues that the radiation beams travel through to reach the tumour target.
Thinking anatomically, those that occur whilst on treatment and shortly after include
■ fatigue;
■ skin changes, like a temporary sunburn: redness, dryness, peeling, itchiness, pain;
■ hair loss, which can be permanent;
■ ear pain or irritation;
■ a dry mouth with thickened saliva;
■ altered taste or smell sensations, which can be permanent, contributing to a lack of appetite;
■ voice hoarseness, which can be permanent;
■ pain or difficulty when swallowing, which may require pain medication and might lead to weight loss, where if it’s substantial, you might need to have a feeding tube inserted into your stomach;
■ difficulty breathing due to the tumour blocking the airway, which may require inserting a tube in your neck to secure the airway; and
■ irritation of the nerves along the mouth, neck or shoulders, causing numbness, weakness, or pain.
Side effects that can occur in the long run include
■ permanent skin changes, include thickening or tightening, skin tone changes, prominent blood vessels;
■ tooth decay, minimized with daily fluoride treatments for life;
■ an underactive thyroid gland, which can be managed with a daily thyroid hormone tablet;
■ nerve damage causing numbness, weakness, pain, or electric shock sensations of the arm;
■ bone pain or injury of the jaw bone;
■ stroke;
■ serious injury to any structure in the radiation field, which could require major surgery;
■ secondary malignancies, which is a cancer caused by the radiation treatment itself.
If chemotherapy is given, it can
■ worsen the mouth pain and inflammation;
■ cause nausea;
■ lower blood counts, putting you at increased risk of infection;
■ cause hearing loss;
■ cause kidney damage; and
■ carry a 1% risk of death
If, after completing the course of radiation, there are large lymph nodes or other findings suggesting a poor response to treatment, a salvage surgery might have to be done to remove tissues suspicious for having active cancer.
Surgery is the other option, which is done using a robot-assisted approach through the mouth.
■ You need to have a medical and anesthesiology assessment done beforehand. On the day of surgery, you come into the hospital.
■ The surgery is done under a general anesthetic, so you will be asleep. A tube is initially put into your airway to help you breath, and your mouth is held open using a retractor.
■ The robot is positioned so that the instruments and a 3D camera can be placed within your mouth, which is a fairly small space. The surgeon sits at a separate console, where everything is magnified, and he or she has control of the robotic arms.
■ The tumour is cut out with a 1 cm margin of healthy tissue taken and sent to pathology for analysis.
■ For the lymph node sampling, called a neck dissection, an incision is made in your neck to expose the lymph nodes. You will also require a temporary tracheotomy, a plastic tube inserted in your airway through a cut made in your neck and possibly a nasogastric tube, which is a plastic feeding tube inserted into the nose and tunneled into the stomach.
■ The surgery takes about 2–4 hours, but you are kept in hospital 5–7 days for monitoring, to make sure your body recovers from the anesthetic and to assess for any potential complications.
I will now discuss the side effects of the surgery, which are also listed on the decision aid you’re holding and are categorized by the likelihood they will occur. The side effects are largely related to the tissues that are encountered when cutting out the tumour tissue and to the general anesthetic itself.
Thinking anatomically, those that occur during and shortly after the surgery include
■ damage to the teeth, lips, tongue, mouth.
■ bleeding, bruising, or infection along the surgical sites.
- ■ swelling and irritation of the eating passage.
- ■ You will require iv fluids whilst in hospital.
- ■ If impaired swallowing is severe, a gastric tube might need to be inserted for feeds, which can be permanent.
- ■ swelling and irritation of the airway.
- ■ We attempt to minimize these complications by inserting the breathing tube in your neck, but if they last, the tube might need to remain longer. It is usually removed before discharge, but can, in rare cases, be permanent
- ■ nerves that are stunned or cut, causing
- ■ pain;
- ■ numbness;
- ■ weakness of shoulder, tongue, or lips; or
- ■ vocal changes.
You will have scars.
Events more specific to the general anesthetic are
■ aspiration pneumonia;
■ DVT formation;
■ stroke or heart attack;
■ anaphylaxis, malignant hyperthermia; and
■ death.
Depending on the findings from the pathology assessment of the tumour, there may be a need for postoperative radiation, potentially with chemotherapy. This would be to account for any suggestion that there are microscopic or macroscopic cancer cells left behind. This would potentially occur if there was more-advanced disease or incompletely resected disease, a close or positive margin, high nodal disease, or extracapsular extension, which is when the tumour is trying to escape the lymph node capsule.
Take your time on this page, and feel free to select “More info” under either treatment image to learn more, or ask me any questions.
When you feel you have a grasp of the two options, let me know which one you would select and click on the corresponding door. [Wait for treatment selection.]
Initially, we stated that the two treatment options had an equal chance of cure. Would your decision change if you found out the other treatment had a 5% higher cure rate? [Repeat until patient chooses “Yes.” The options are 5%, 10%, 15%, 20%, 50%.]
What were the 3 most concerning side effects that kept you from choosing this treatment initially? [Record responses.]
If you were found to be in a similar situation, would you like to have a decision aid such as this available to you? [Record response.]
Thank you for your time.
Footnotes
These authors contributed equally to the present work.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–19. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Leonhardt FD, Quon H, Abrahao M, O’Malley BW, Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. 2012;34:146–54. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 3.Cracchiolo JR, Baxi SS, Morris LG, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016;122:1523–32. doi: 10.1002/cncr.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh DH, Tam S, Fung K, et al. Transoral robotic surgery vs. radiotherapy for management of oropharyngeal squamous cell carcinoma—a systematic review of the literature. Eur J Surg Oncol. 2015;41:1603–14. doi: 10.1016/j.ejso.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Nichols AC, Yoo J, Hammond JA, et al. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. trans-oral robotic surgery (orator)—study protocol for a randomized phase ii trial. BMC Cancer. 2013;13:133. doi: 10.1186/1471-2407-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Fort Washington, PA: NCCN; 2016. Ver. 3.2016. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (free registration required); cited 28 October 2016] [Google Scholar]
- 7.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 8.Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58:293–304. doi: 10.3322/CA.2008.0006. [DOI] [PubMed] [Google Scholar]
- 9.Whelan TJ, Levine MN, Gafni A, et al. Breast irradiation postlumpectomy: development and evaluation of a decision instrument. J Clin Oncol. 1995;13:847–53. doi: 10.1200/JCO.1995.13.4.847. [DOI] [PubMed] [Google Scholar]
- 10.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2010;120:2218–22. doi: 10.1002/lary.21144. [DOI] [PubMed] [Google Scholar]
- 12.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–80. doi: 10.1002/cncr.10567. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein GS, O’Malley BW, Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133:1220–6. doi: 10.1001/archotol.133.12.1220. [DOI] [PubMed] [Google Scholar]
- 14.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening: a randomized, controlled trial. Ann Intern Med. 2000;133:761–9. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 15.Levine MN, Gafni A, Markham B, MacFarlane D. A bedside decision instrument to elicit a patient’s preference concerning adjuvant chemotherapy for breast cancer. Ann Intern Med. 1992;117:53–8. doi: 10.7326/0003-4819-117-1-53. [DOI] [PubMed] [Google Scholar]
- 16.Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59:379–90. doi: 10.3322/caac.20039. [DOI] [PubMed] [Google Scholar]
- 17.Sah S, Fagerlin A, Ubel P. Effect of physician disclosure of specialty bias on patient trust and treatment choice. Proc Natl Acad Sci U S A. 2016;113:7465–9. doi: 10.1073/pnas.1604908113. [DOI] [PMC free article] [PubMed] [Google Scholar]