Abstract
Background
“Conditional survival probability” is defined as the probability that a patient will survive an additional time, given that the patient has already survived a defined period of time after diagnosis. Such estimates might be more relevant for clinicians and patients during post-diagnosis care, because survival probability projections are based on the patient’s survival to date. Here, we provides the first population-based estimates of conditional survival probabilities by histology for brain cancer in Canada.
Methods
Canadian Cancer Registry data were accessed for patients diagnosed with primary brain cancers during 2000–2008. Kaplan–Meier survival probabilities were estimated by histology. Conditional survival probabilities at 6 months (short-term, denoted scs) and 2 years (long-term, denoted lcs) were derived from the Kaplan–Meier survival estimates for a range of time periods.
Results
Among the 20,875 patients who met the study criteria, scs increased by a margin of 16–18 percentage points from 6-month survivors to 2-year survivors for the three most aggressive brain cancers. The lcs for 2-year survivors was 66% or greater for all tumour groups except glioblastoma. The lcs for 4-year survivors was 62% or greater for all histologies. For glioblastoma and diffuse astrocytoma, the lcs increased each year after diagnosis. For all other histologies, the lcs first increased and then plateaued from 2 years after diagnosis. The lcs and scs both worsened with increasing older age at diagnosis.
Summary
We report histologically specific conditional survival probabilities that can have value for clinicians practicing in Canada as they plan the course of follow-up for individual patients with brain cancer.
Keywords: Conditional survival, survival, brain neoplasms, brain tumours, brain cancer
INTRODUCTION
Survival probabilities for malignant brain tumours by histology in Canada have only recently been described1. Those estimates reflect observed survival from the time of diagnosis and are useful for group comparisons; however, those common statistics have a time origin at the date of diagnosis and do not accurately describe prognosis for individuals who continue to survive for months or years after diagnosis. In contrast, conditional survival probabilities are clinically relevant and provide more informative estimates for surviving patient populations, because the probability of survival changes with increasing survival time2,3.
“Conditional survival probability” is defined as the probability that patients will survive for an additional period given that those patients have already survived for a defined period of time after diagnosis3. Estimates of conditional survival probabilities have a straight-forward interpretation and are easy to understand both for clinicians and for patients seeking relevant prognostic information at the population level.
Conditional survival probabilities for primary malignant brain tumours were first published for U.S. patients in 19992. Estimates have since been extended to include primary nonmalignant brain tumours, thereby capturing all primary brain tumours3,4. Additionally, smaller-scale studies have also examined conditional survival probabilities for selected histologies, particularly glioblastoma multiforme (gbm) and astrocytic tumours5–7. Most recently, conditional survival probabilities were calculated for periods before and after 2005, to examine potential differences in brain tumour survival attributable to changes in treatment protocols4,8.
Using Canadian Cancer Registry (ccr) data, we estimated—for the benefit of patients and their families, and for clinicians and researchers—conditional survival probabilities that reflect the experience of the surviving brain cancer patient population diagnosed during 2000–2008 in Canada.
METHODS
The survival data used in this study come from the ccr, 2012 release. The ccr is a population-based census database that maintains the national cancer incidence and survival data at an individual level. Starting in 1992, the ccr began compiling cancer registry data from each province and territory, and the quality of the data has been improving. According to the North American Association of Central Cancer Registries, the Alberta Cancer Registry achieved gold certification for data quality as of 2000, and the Manitoba and Saskatchewan registries achieved gold certification for data quality as of 20089. In 2014, the association had awarded all but two provinces (Ontario and Quebec) either gold or silver certification.
Our study cohort consisted of Canadian patients with primary brain tumours [codes C70.0–C72.9, C75.1, and C75.3 in the International Classification of Diseases for Oncology (icd-o) 2nd and 3rd edition topography] who were diagnosed during 2000–2008. Patients whose brain tumours were nonmalignant (icd-o behavior codes 0–2) and whose chronologic sequence numbers for multiple primaries exceeded 1 (ccr code TD2), which indicates a prior cancer diagnosis, were excluded. For patients microscopically confirmed (MC), icd-o diagnosis codes were assigned based on radiography or clinical diagnosis (ccr codes T25 and T11). Those patients were included together with the MC cases. The final cohort consisted of 20,875 patients. Patients without a death date were right-censored at 31 December 2008, which is the earliest death clearance cut-off date for all provinces and territories.
Major histology types were defined according to the icd-o codes outlined by the Central Brain Tumor Registry of the United States10. Of all histology types, 7 were associated with 300 or more deaths: gbm, anaplastic astrocytoma, diffuse astrocytoma, anaplastic oligodendroglioma, oligoastrocytic tumour, oligodendroglioma, and glioma not otherwise specified [hereinafter referred to as glioma (nos)]. All other histology types are collapsed into the “all other tumours” category. The overall conditional survival estimate for all brain cancers combined was not reported because histology-specific conditional survival probabilities for the tumours provide more relevant clinical and epidemiologic information.
We report the observed survival estimates rather than relative survival estimates for two reasons. First, observed survival is more clinically relevant for clinicians and patients because it refers to the actual survival experience. That is, clinicians are interested in knowing how long a patient might need treatment and care, and patients are interested in knowing how long an individual with their condition is estimated to live. Second, the fatality rate is high for most brain cancers. In patients with brain cancer who were 15 years of age and older and who were diagnosed during 2006–2008, the two values are very close: 25% (relative survival) compared with 24% (observed survival) at 5 years after diagnosis11.
Unconditional Kaplan–Meier survival probabilities were estimated at 1-year intervals from year 1 to year 6 after diagnosis for each histology group. Conditional survival probabilities were derived from the Kaplan–Meier estimates. Let T denote the survival time and S(t) denote the Kaplan–Meier survival estimate at time t—that is, S(t) ≡ Pr(T > t). The conditional probability of surviving an additional 2 years (long-term) given that patients have already survived x years since diagnosis—that is, LCS[x]—is calculated by
For example, to obtain the long-term (additional 2 years) conditional survival probability estimate (denoted as LCS[4]) for patients who have survived 4 years after diagnosis, the survival estimate at 4 + 2 years, S(6), is divided by the survival estimate at 4 years, S(4). That is, LCS[4] represents conditional survival to 6 years for the population of 4-year survivors.
Short-term conditional survival (an additional 6 months) for a population of x-year survivors (denoted as SCS[0.5]) can be calculated by dividing S(x) into S(x+0.5). For example, to estimate the 6-month short-term conditional survival probability for patients who have survived 0.5 years after diagnosis, the survival estimate at 0.5 + 0.5 = 1 year, S(1), is divided by the survival estimate at 0.5 years, S(0.5)—that is, conditional survival to 1.0 year, given survival to 0.5 years after diagnosis.
Survival probability estimates were not calculated whenever the at-risk group dropped below 20 patients. Thus, given the sample size limitation, the analysis of age-related patterns was limited to gbm and diffuse astrocytoma. Data for patients with gbm who were 20 years of age and younger, and for patients with diffuse astrocytoma who were 65 years of age and older were suppressed in the age-related analyses because of the small number of patients in each of those groups who survived for more than 2 years after diagnosis.
In the study cohort, median follow-up time as estimated by the Kaplan–Meier method for the various histology types ranged from 0.5 years (gbm) to 3.4 years (oligodendroglioma), and the 99th percentile follow-up time ranged from 6.3 years (gbm) to 9 years (oligodendroglioma). As a result, survival probabilities were estimated for up to 6 years after diagnosis for all histology types. Confidence intervals were calculated as described by Davis et al.2, using a variation of the “exponential” Greenwood formula. Analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.) and R (version 3.1.3: The R Foundation for Statistical Computing, Vienna, Austria). All frequencies and proportions presented were subject to rounding in accordance with Statistics Canada requirements, which rounds frequencies to end with 5 or 10.
Our study was approved by Statistics Canada and by the ethics board at the University of Alberta.
RESULTS
Table i summarizes the demographic and clinical characteristics of the study cohort of patients with a first primary brain cancer. The number of incidents and deaths were tabulated by sex, age group at diagnosis, region, selected histology groups, and MC status. Men made up the larger proportion of the patients (56.1% vs. 43.9% women). Patients were mainly (71.9%) middle-aged and older adults (≥45 years of age). Most patients resided in Ontario (38.7%) and Quebec (26.0%), with patient counts reflecting regional population size. Together, gbm (40.1%) and diffuse astrocytoma (11.1%) accounted for more than half the brain cancers diagnosed in Canada.
TABLE I.
Cohort characteristicsa
| Characteristic | All cases [n (% column)] | Deaths [n (% row)] |
|---|---|---|
| Overall | 20,875 (100) | 14,895 (71.4) |
| Sex | ||
| Men | 11,710 (56.1) | 8,495 (72.5) |
| Women | 9,165 (43.9) | 6,405 (69.9) |
| Age group at diagnosis | ||
| ≤20 Years | 2,125 (10.2) | 600 (28.2) |
| 21–44 Years | 3,750 (18.0) | 1,625 (43.3) |
| 45–64 Years | 7,235 (34.7) | 5,520 (76.3) |
| ≥65 Years | 7,760 (37.2) | 7,150 (92.1) |
| Region | ||
| Ontario | 8,085 (38.7) | 5,170 (63.9) |
| Alberta | 1,825 (8.7) | 1,330 (72.9) |
| British Columbia | 2,575 (12.3) | 2,000 (77.7) |
| Atlantic | 1,640 (7.9) | 1,265 (77.1) |
| Quebec | 5,425 (26.0) | 4,100 (75.6) |
| Saskatchewan and Manitoba | 1,300 (6.2) | 1,020 (78.5) |
| Territories | 30 (0.1) | 10 (33.3) |
| Histology | ||
| Glioblastoma multiforme | 8,365 (40.1) | 7,765 (92.8) |
| Anaplastic astrocytoma | 610 (2.9) | 470 (77.0) |
| Diffuse astrocytoma | 2,310 (11.1) | 1,795 (77.7) |
| Anaplastic oligodendroglioma | 555 (2.7) | 325 (58.6) |
| Oligoastrocytoma | 720 (3.4) | 380 (52.8) |
| Oligodendroglioma | 955 (4.6) | 315 (33.0) |
| Glioma (NOS) | 1,050 (5.0) | 650 (61.9) |
| All others | 6,305 (30.2) | 3,200 (50.8) |
| Diagnostic confirmation | ||
| Microscopic | 15,975 (76.5) | 11,310 (70.8) |
| Other | 4,895 (23.4) | 3,585 (73.2) |
Because of rounding, in accordance with Statistics Canada requirements, numbers and percentages reported here do not add up to the total frequencies and 1.
NOS = not otherwise specified.
Overall, the proportion of mc cancers was 76.5%. Cross-tabulation by tumour group revealed that the proportion of mc disease varied by histology. In the 6 major histology groups, fewer than 15% of cancers were non-mc (gbm, 12%; anaplastic astrocytoma, 5%; diffuse astrocytoma, 10%; anaplastic oligodendroglioma, 4%; oligoastrocytic tumours, 3%; oligodendroglioma, 7%). However, for glioma (nos) and “all other tumors”, 61% and 44% respectively were non-mc cancers. During the first 2 years after diagnosis, substantial survival differences were observed between the patients with mc and non-mc cancers in the glioma (nos) and “all other tumours” histology groups, such that the survival probabilities were higher for mc than for non-mc cancers. Therefore, for those two groups, the Kaplan–Meier survival estimates are reported (Table ii), but the conditional survival estimates are not. For the other 6 major histology types, the survival experiences of the patients with mc and non-mc cancers are virtually identical (data not shown); thus, the conditional survival estimates combine the mc and non-mc cancers to improve the generalizability of the results.
TABLE II.
Observed brain cancer survival probabilities by diagnostic method and histology
| Category | Pts (n) | Survival probability (95% CL) by years since diagnosis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | 6 Years | ||
| Overall | 20,875 | 0.47 (0.46, 0.48) | 0.34 (0.33, 0.35) | 0.30 (0.29, 0.30) | 0.27 (0.26, 0.27) | 0.25 (0.24, 0.25) | 0.23 (0.22, 0.24) |
| Diagnostic confirmation | |||||||
| Microscopic | 15,975 | 0.51 (0.50, 0.51) | 0.35 (0.34, 0.35) | 0.30 (0.29, 0.30) | 0.26 (0.26, 0.27) | 0.24 (0.23, 0.25) | 0.22 (0.21, 0.23) |
| Other | 4,895 | 0.36 (0.34, 0.37) | 0.31 (0.30, 0.33) | 0.29 (0.28, 0.30) | 0.28 (0.26, 0.29) | 0.26 (0.25, 0.27) | 0.25 (0.23, 0.26) |
| Histology | |||||||
| Glioblastoma multiforme | 8,365 | 0.29 (0.28, 0.30) | 0.10 (0.09, 0.11) | 0.06 (0.05, 0.06) | 0.04 (0.04, 0.05) | 0.03 (0.03, 0.04) | 0.03 (0.02, 0.03) |
| Anaplastic astrocytoma | 610 | 0.50 (0.46, 0.54) | 0.34 (0.30, 0.38) | 0.27 (0.23, 0.31) | 0.22 (0.19, 0.26) | 0.17 (0.14, 0.21) | 0.14 (0.11, 0.18) |
| Diffuse astrocytoma | 2,310 | 0.45 (0.43, 0.47) | 0.31 (0.29, 0.33) | 0.26 (0.24, 0.27) | 0.22 (0.20, 0.24) | 0.19 (0.18, 0.21) | 0.17 (0.15, 0.19) |
| Anaplastic oligodendroglioma | 555 | 0.74 (0.70, 0.78) | 0.56 (0.52, 0.60) | 0.48 (0.44, 0.53) | 0.42 (0.38, 0.47) | 0.36 (0.32, 0.41) | 0.33 (0.28, 0.38) |
| Oligoastrocytoma | 720 | 0.76 (0.72, 0.79) | 0.60 (0.57, 0.64) | 0.54 (0.50, 0.58) | 0.48 (0.44, 0.53) | 0.41 (0.37, 0.46) | 0.35 (0.31, 0.40) |
| Oligodendroglioma | 955 | 0.88 (0.86, 0.90) | 0.82 (0.79, 0.84) | 0.78 (0.75, 0.81) | 0.73 (0.70, 0.76) | 0.66 (0.63, 0.70) | 0.60 (0.57, 0.65) |
| Glioma (NOS) | 1,050 | 0.52 (0.49, 0.55) | 0.43 (0.40, 0.47) | 0.40 (0.37, 0.43) | 0.38 (0.35, 0.41) | 0.35 (0.32, 0.38) | 0.32 (0.29, 0.36) |
| All others | 6,305 | 0.60 (0.59, 0.61) | 0.55 (0.54, 0.56) | 0.51 (0.50, 0.52) | 0.48 (0.47, 0.50) | 0.46 (0.45, 0.47) | 0.45 (0.43, 0.46) |
CL = confidence limits.
Table ii shows estimates of Kaplan–Meier survival probabilities for the 8 brain cancer histology groups (all patients) from year 1 to year 6 at 1-year intervals. The large differences in survival probability observed between the mc and non-mc groups in the 1st and 2nd years after diagnosis are, as already noted, attributable to the substantial differences in survival probability for the glioma (nos) and “all other tumours” groups. Glioblastoma is the most aggressive brain cancer type, with the worst prognosis; median survival duration is 6 months, and at 2 years after diagnosis, the survival estimate is 10%. The survival experiences for patients with anaplastic astrocytoma and diffuse astrocytoma were similar, the median survival duration being about 1 year, and the survival probability being 20% at 5 years after diagnosis. Anaplastic oligodendroglioma and oligoastrocytic tumours were also similar in overall prognosis, with median survival durations of about 3 years. Oligodendroglioma has the best prognosis: 88% of patients survived the 1st year, and 60% were alive at 6 years after diagnosis.
Surviving an additional period of time after diagnosis resulted in improved and favourable short- and long-term conditional survival probabilities in almost all 6 histology types analyzed (Tables iii and iv). Short-term conditional survival probabilities (survival for an additional 6 months) were estimated for patients who had already survived for 0.5 years up to 2 years, by histology (Table iii). Patients diagnosed with relatively less-aggressive histology types, including anaplastic oligodendroglioma and oligoastrocytic tumour, had short-term conditional survival probabilities that were about 85% or better if they had already survived 0.5 to 2 years after diagnosis. For gbm, anaplastic astrocytoma, and diffuse astrocytoma, the short-term conditional survival probabilities increased by a margin of 16–18 percentage points from the 6-month survivors to the 2-year survivors—for example, 56%–74% for gbm and 72%–90% for diffuse astrocytoma. For oligodendroglioma patients, the short-term conditional survival probabilities were always better than 90% during the first 2 years after diagnosis.
TABLE III.
Short-term (6-month) conditional survival (SCS) probabilities by selected histologies
| Histology | Pts (n) | Conditional survival probability (95% CL) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| SCS[0] | SCS[0.5] | SCS[1] | SCS[1.5] | SCS[2] | ||
| Glioblastoma multiforme | 8365 | 0.51 (0.50, 0.52) | 0.56 (0.54, 0.58) | 0.55 (0.53, 0.57) | 0.63 (0.60, 0.65) | 0.74 (0.71, 0.78) |
| Anaplastic astrocytoma | 610 | 0.67 (0.64, 0.71) | 0.74 (0.70, 0.78) | 0.79 (0.73, 0.83) | 0.86 (0.80, 0.90) | 0.90 (0.84, 0.94) |
| Diffuse astrocytoma | 2310 | 0.63 (0.61, 0.65) | 0.72 (0.70, 0.75) | 0.79 (0.76, 0.81) | 0.87 (0.84, 0.89) | 0.90 (0.88, 0.93) |
| Anaplastic oligodendroglioma | 555 | 0.84 (0.81, 0.87) | 0.88 (0.84, 0.90) | 0.85 (0.81, 0.88) | 0.89 (0.85, 0.92) | 0.90 (0.85, 0.93) |
| Oligoastrocytoma | 720 | 0.85 (0.83, 0.88) | 0.88 (0.86, 0.91) | 0.88 (0.85, 0.91) | 0.91 (0.88, 0.93) | 0.95 (0.92, 0.97) |
| Oligodendroglioma | 955 | 0.93 (0.91, 0.95) | 0.95 (0.93, 0.96) | 0.96 (0.94, 0.97) | 0.97 (0.95, 0.98) | 0.98 (0.96, 0.99) |
CL = confidence limits.
TABLE IV.
Long-term (2-year) conditional survival (LCS) probabilities by selected histologies
| Histology | Pts (n) | Conditional survival probability (95% CL) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| LCS[0] | LCS[1] | LCS[2] | LCS[3] | LCS[4] | ||
| Glioblastoma multiforme | 8365 | 0.10 (0.09, 0.11) | 0.20 (0.18, 0.22) | 0.42 (0.38, 0.46) | 0.58 (0.53, 0.64) | 0.67 (0.60, 0.74) |
| Anaplastic astrocytoma | 610 | 0.34 (0.30, 0.38) | 0.54 (0.47, 0.60) | 0.66 (0.57, 0.73) | 0.63 (0.53, 0.72) | 0.62 (0.49, 0.72) |
| Diffuse astrocytoma | 2310 | 0.31 (0.29, 0.33) | 0.56 (0.53, 0.59) | 0.71 (0.67, 0.75) | 0.76 (0.71, 0.80) | 0.79 (0.73, 0.83) |
| Anaplastic oligodendroglioma | 555 | 0.56 (0.52, 0.60) | 0.65 (0.60, 0.70) | 0.76 (0.69, 0.81) | 0.76 (0.68, 0.82) | 0.77 (0.69, 0.84) |
| Oligoastrocytoma | 720 | 0.60 (0.57, 0.64) | 0.71 (0.67, 0.75) | 0.81 (0.75, 0.84) | 0.76 (0.70, 0.82) | 0.73 (0.65, 0.80) |
| Oligodendroglioma | 955 | 0.82 (0.79, 0.84) | 0.88 (0.85, 0.90) | 0.89 (0.86, 0.92) | 0.85 (0.81, 0.88) | 0.83 (0.79, 0.87) |
CL = confidence limits.
Long-term conditional survival probabilities (survival for an additional 2 years) were estimated for patients who had survived 1–4 years, by histology (Table iv). The long-term conditional survival probability in oligodendroglioma was better than 80% for each of the 4 years already survived after diagnosis. For other histologies, the long-term conditional survival probabilities increased in the first 2 years already survived after diagnosis, becoming greater than 60%—except in the case of gbm. The long-term conditional survival probabilities for patients with gbm and diffuse astrocytoma continued to increase in the first 4 years after diagnosis, indicating that the hazard for death decreased with increasing time already survived. Anaplastic astrocytoma, anaplastic oligodendroglioma, and oligoastrocytic tumours and oligodendroglioma showed improvements in long-term conditional survival in the first 2 years already survived after diagnosis, but long-term conditional survival probabilities either plateaued or slightly decreased at 3 years already survived after diagnosis. After patients had survived for 4 years after diagnosis, the long-term conditional survival probability ranged from 62% to 83% for all histologies.
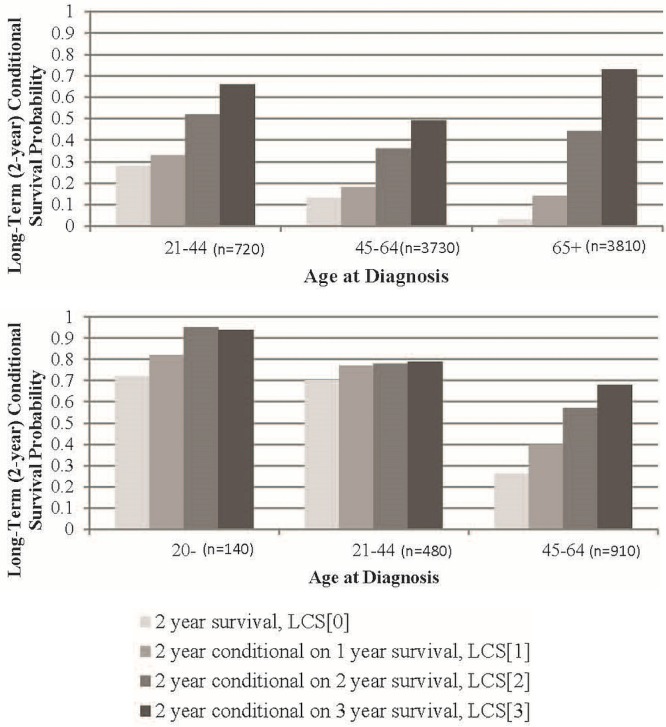
Age-related conditional survival patterns were investigated in gbm and diffuse astrocytoma (Figure 1). For gbm, the group of patients 20 years of age and younger was suppressed because of a small number of patients. Gains in long-term conditional survival for adult patients with gbm were notable for those who had already survived 2 or more years after diagnosis in all age groups examined, but were most pronounced for patients 65 years of age and older. The long-term conditional survival for age groups 21–44, 45–64, and 65 years of age or older immediately after diagnosis was 28%, 13%, and 3% respectively, and for patients who had already survived 2 years after diagnosis, it increased to 52%, 36%, and 44% respectively. It is interesting to note that, among patients who had already survived 2 or more years, senior survivors with gbm had similar or better conditional survival probabilities than did their younger counterparts.
FIGURE 1.
Long-term (2-year) conditional survival (LCS[x], where x is time in years since diagnosis), stratified by age group, for (A) glioblastoma multiforme (age group ≤20 years supressed because of a small number of patients) and (B) diffuse astrocytoma (age group ≥65 years suppressed because of a small number of patients).
In diffuse astrocytoma, the small number of 3-year survivors in the 65-plus age group prevented the analysis of that age group. In the remaining age groups, the long-term conditional survival probabilities were greater for younger patients than for older patients for all time points after diagnosis. Gains in long-term conditional survival for childhood survivors (20 years of age and younger) were substantial, with probabilities increasing from 72% in newly diagnosed children to 94% in children who had already survived 2 years after diagnosis. In contrast, the longer-term conditional survival probabilities for patients 21–44 years of age immediately after diagnosis were similar at 70% and became stable at approximately 78% for patients who have already survived 1, 2, and 3 years after diagnosis. Those long-term conditional survival probabilities were worse for adults than for childhood cancer patients. Gains in long-term conditional survival were greatest for middle-aged patients (ages 45 and 64), who had an initial longer-term survival probability of 26%, which then increased almost linearly to 40%, 57%, and 68% for those who have already survived 1, 2, and 3 years after diagnosis respectively. That pattern differs from the gbm pattern, in which the greatest gains in long-term conditional survival occurred in seniors (age 65 and older), who had an initial long-term survival probability of 3% and a long-term conditional survival probability of 73% after 3 years of post-diagnosis survival.
DISCUSSION
Analysis of Canadian data suggests two primary trends for gbm and diffuse astrocytomas: first, that short-and long-term conditional survival both improve as time from diagnosis increases (up to 4 years after diagnosis); and second, that as the patient’s age increases, the conditional probability of survival tends to decrease. Both results accord with previously reported conditional survival estimates2–4. In those data, conditional survival also increases for most other tumour groups in the first 2 years, but plateaus or decreases thereafter.
Our study has several strengths. The conditional survival probability estimates reported are more representative of the experience of the general population of patients with brain cancer because they include patients with non-mc disease, who tend not to be included in clinical studies. With respect to the conditional survival probabilities reported for the 6 histology types, 85% or more of the individual diagnoses in the histologic groups were mc.
Another strength is that the ccr dataset reflects all provinces and territories in Canada and therefore captures all known cases of malignant brain tumour, yielding a patient cohort with minimum selection bias and large numbers with which to estimate outcomes for rare cancers, and results that are representative of the Canadian population. The ccr dataset undergoes data validation and correlation edits, helping to prevent data errors by verifying the validity of each entry and checking for appropriate relationships between patient and tumour data12. The data validation lends confidence to the quality of survival estimates presented here.
Our study has limitations. Prior studies in the United States have published conditional survival probabilities for patients with malignant brain tumours, reporting patterns by age, histology, tumour behaviour, treatment, sex, and ethnicity2–4. Although Canada has mandated the reporting of data about nonmalignant brain tumours, those data are incomplete in most provinces (Shaw A, Woods R, Semenciw R, Megyesi J. cns tumours in Canada: who are we missing? Presented at: North American Association of Central Cancer Registries 2014 Annual Conference: Capitalizing on Cancer Surveillance Data for Improved Cancer Control; Ottawa, ON; 21–26 June 2014), and estimates were therefore restricted to malignant cases. Efforts are currently underway to improve the reporting of nonmalignant brain tumours. Patient ethnicity and treatment information are not available in the ccr dataset. In our report, the conditional survival estimates are restricted to selected histologies of malignant brain tumours and, in two histology groups, to selected age groups. The estimates directly reflect the experiences of Canadian patients with brain cancer. To compare estimates from Canada with those from other countries, an effort to obtain standardized estimates based on the same referent distribution is needed.
Survival estimates provided in the present study were limited to 6 years after diagnosis, which is a shorter time-frame than was used in several U.S. studies3,4. Extended conditional survival estimates could not be calculated because of the shorter follow-up time provided in the ccr and limited patient numbers, a reflection of Canada’s smaller population relative to the United States. Of course, the validity of any estimate of conditional survival depends on the accuracy of the underlying survival probability estimates. If the registries and death clearance processes miss a case, the count obtained from the ccr will be underestimated, and the survival probabilities could be biased. Given that the same number of deaths would constitute a larger proportion of rare diseases than of common diseases, such an error would have more impact on estimates for rare diseases such as histologically specific brain cancers. That issue is a consideration at Statistics Canada and is more likely to be important for provincial reports of rare tumours than for the national data reported here.
Our estimates are based on information available up to 2008 (the most recent available through the Statistics Canada Research Data Centres) and reflect the survival experience of patients diagnosed almost a decade ago. Data up to 2015 are available in provincial cancer registries, but the death data are undergoing internal reviews at Statistics Canada for accuracy. Once new data have been released through the Research Data Centres for survival analysis, our estimates will be updated. We anticipate that the lag time for future survival data will be shortened (Ellison J. Personal communication, 2016).
In the available data, misclassification of histology is suggested by the similarities of the survival probabilities for diffuse and anaplastic astrocytoma and for anaplastic oligodendrogliomas and oligoastrocytic tumour, which are not reported elsewhere2–4. The data for the gbm histology are considered those most likely to be accurately classified in population databases13. Given that the classification of brain tumours is changing with the emergence of molecular tools, misclassification is likely to be present in most population studies until biomarkers are systematically incorporated into cancer registries14.
CONCLUSIONS
Our histologically specific conditional survival probability estimates are the first to be published for the population of Canadian patients with brain cancer. It should be noted that the estimates reflect the experience of groups of patients and do not apply directly to individuals. However, for clinicians planning the course of treatment and follow-up for individual patients, the information about relevant conditional survival estimates for patients surviving varying times from their date of diagnosis has value. Although the present estimates are likely conservative because they reflect 2000–2008 experiences, they are generally positive for all brain cancer survivors and their families who are searching for prognostic information.
ACKNOWLEDGMENTS
This research was funded in part by the Brain Tumour Foundation of Canada. It was also supported by funds to the Canadian Research Data Centre Network from the Social Sciences and Humanities Research Council, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and Statistics Canada.
The authors thank cancer registry staff and cancer patients for their willingness to make these data available to Statistics Canada and to researchers through the Research Data Centre public use dataset. Although the research and analysis in this work are based on data from Statistics Canada, the opinions expressed do not represent the views of Statistics Canada.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Yuan Y, Shi Q, Li M, Nagamuthu C, Andres E, Davis FG. Canadian brain cancer survival rates by tumour type and region: 1992–2008. Can J Public Health. 2016;107:e37–42. doi: 10.17269/cjph.107.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: Surveillance, Epidemiology, and End Results (seer) data. Cancer. 1999;85:485–91. doi: 10.1002/(SICI)1097-0142(19990115)85:2<485::AID-CNCR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Porter KR, McCarthy BJ, Merbaum ML, Davis FG. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology. 2011;36:230–9. doi: 10.1159/000327752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farah P, Blanda R, Kromer C, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Conditional survival after diagnosis with malignant brain and central nervous system tumor in the United States, 1995–2012. J Neurooncol. 2016;128:419–29. doi: 10.1007/s11060-016-2127-8. [DOI] [PubMed] [Google Scholar]
- 5.Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Conditional probability of survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2011;29:4175–80. doi: 10.1200/JCO.2010.32.4343. [Erratum in: J Clin Oncol 2011;29:4847] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SL, Yang YH, Lieu AS, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neurooncol. 2000;50:257–64. doi: 10.1023/A:1006484220764. [DOI] [PubMed] [Google Scholar]
- 7.Lin CL, Lieu AS, Lee KS, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. 2003;60:402–6. doi: 10.1016/S0090-3019(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118:5608–13. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 9.North American Association of Central Cancer Registries (naaccr) Certified registries [Web resource] Springfield, IL: NAACCR; 2016. [Available at https://www.naaccr.org/certifiedregistries/; cited 18 August 2017] [Google Scholar]
- 10.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 12.Statistics Canada . Statistics Canada > Definitions, data sources and methods > Surveys and statistical programs > Canadian Cancer Registry (CCR) [Web page] Ottawa, ON: Statistics Canada; 2015. [Available online at http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207; cited 18 February 2016.] [Google Scholar]
- 13.Davis FG, Malmer BS, Aldape K, et al. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17:484–9. doi: 10.1158/1055-9965.EPI-07-0725. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, Perry A, Burger P, et al. on behalf of the International Society of Neuropathology–Haarlem International Society of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24:429–35. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]